Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy
Abstract
1. Introduction
2. Materials and Methods
- Observation of the surface and the cross-section of the sample by means of an electron scanning microscope HITACHI S-3000N.
- Determination of the chemical composition of the coating with surface element distribution by the EDS method, by means of the NORAN INSTRUMENTS system cooperating with an electron scanning microscope HITACHI S-3000N.
- Chemical structure of the synthesized coatings was determined using inVia confocal micro-Raman spectrometer (Renishaw), working with 532 nm wavelength and power of 2.5 mW.
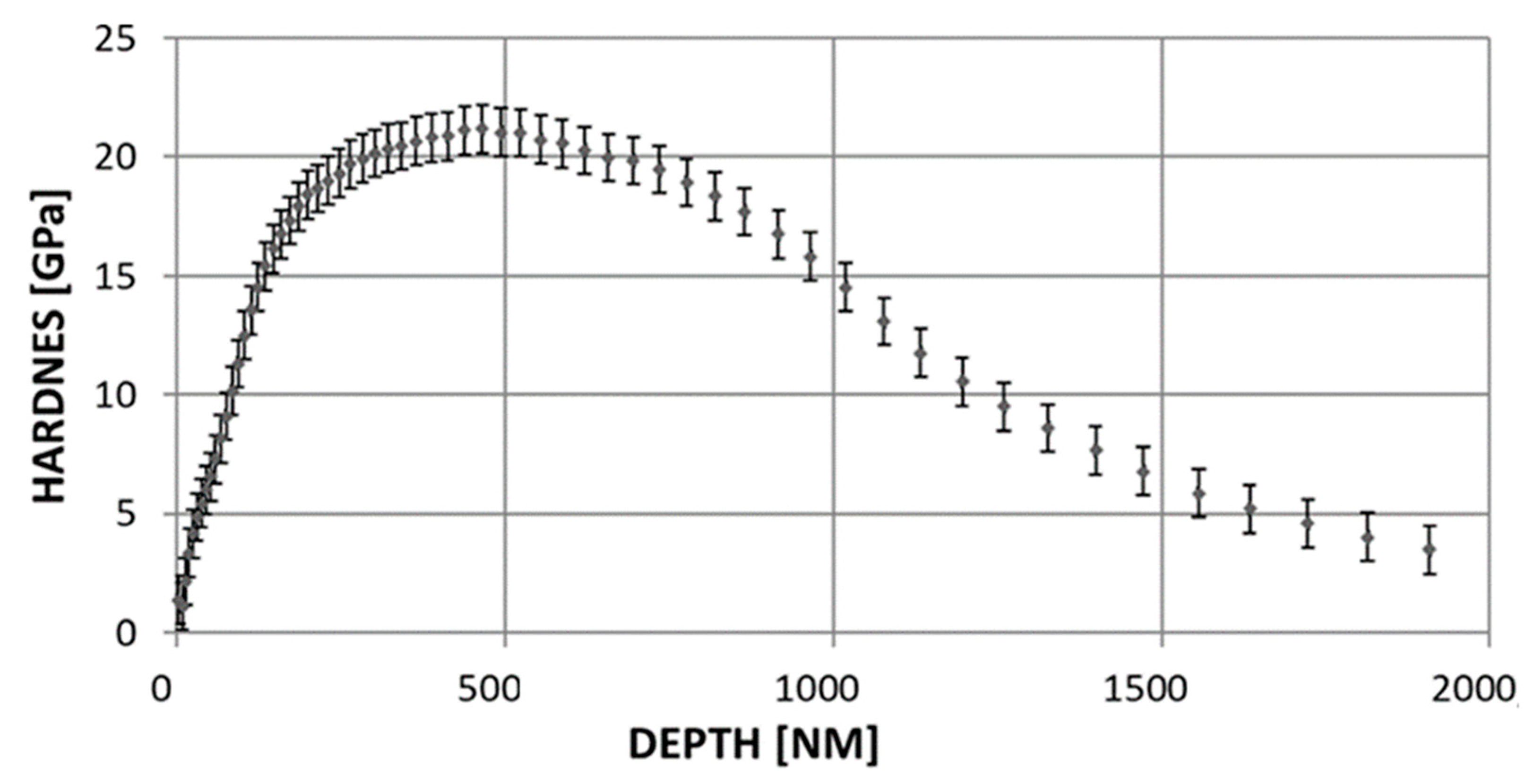
- Mechanical properties. Hardness were determined using Berkovich indenter trihedral pyramid shape with an angle equaling 65.3° and the investigation was performed according to ISO Standards [31] on a MTS NANO INSTRUMENTS G-200 nanoindeter. The results were registered and analyzed using the TestWorksPro 4 Software. The measurement was performed with the CSM (continuous stiffness measurement) [32,33] method.
- Adhesion to substrate. Examinations were carried out by the scratch test method using a sapphire cone-shaped penetrator [34]. Rounding radius of the tip was 1 μm. A 2 mm scratch was made by gradually increasing penetrator load of 0 to 200 mN.
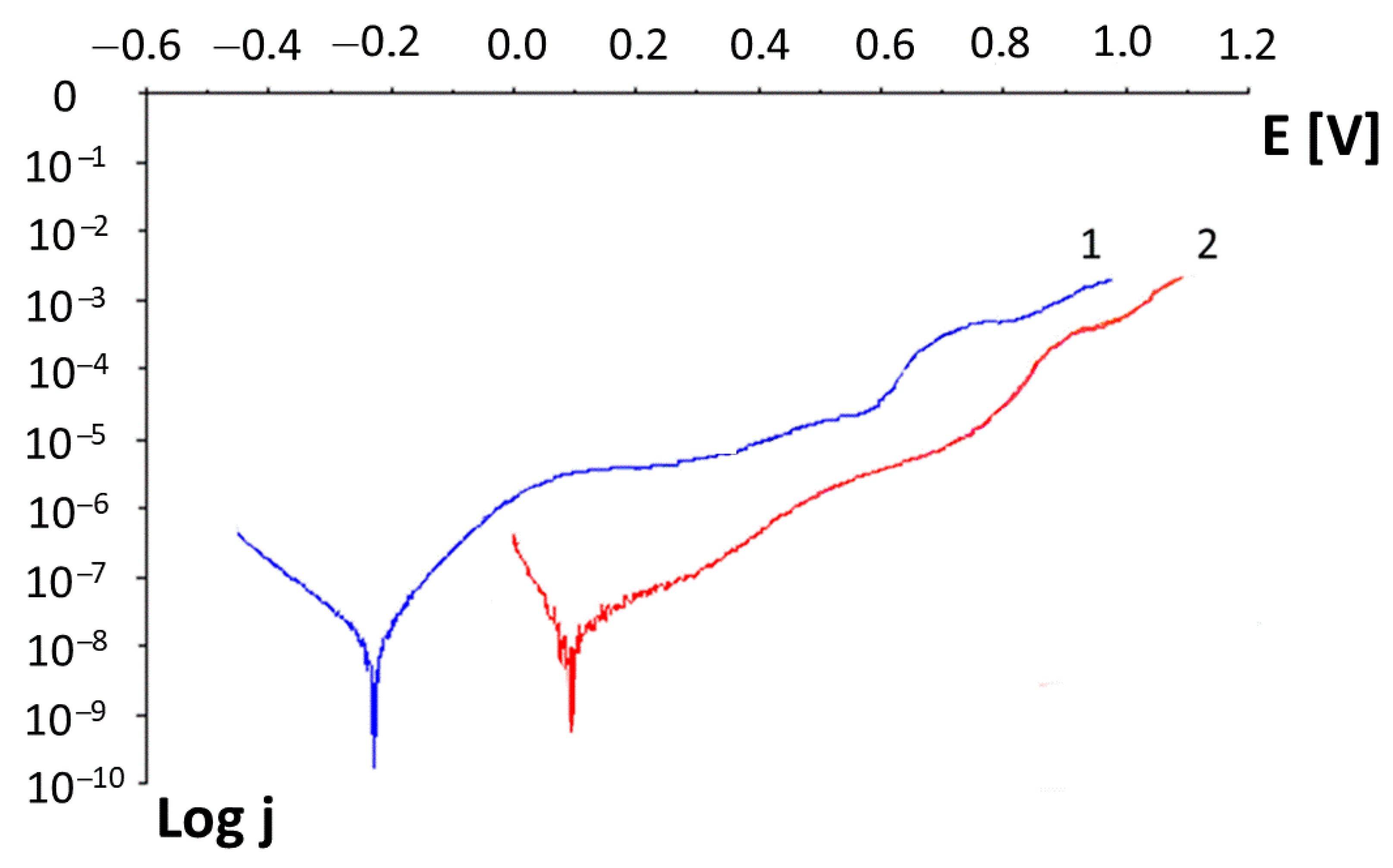
- Corrosion tests—conducted in a chemical environment of deoxidized artificial saliva in the form of a Fusayama–Meyer solution with the composition shown in Table 2 at 37 °C in the ST1 thermostatic chamber (POL-EKO), the measurements were carried out on the PGSTAT 30 galvanostat (Autolab) with GPES and FRA control software v. 4.9.
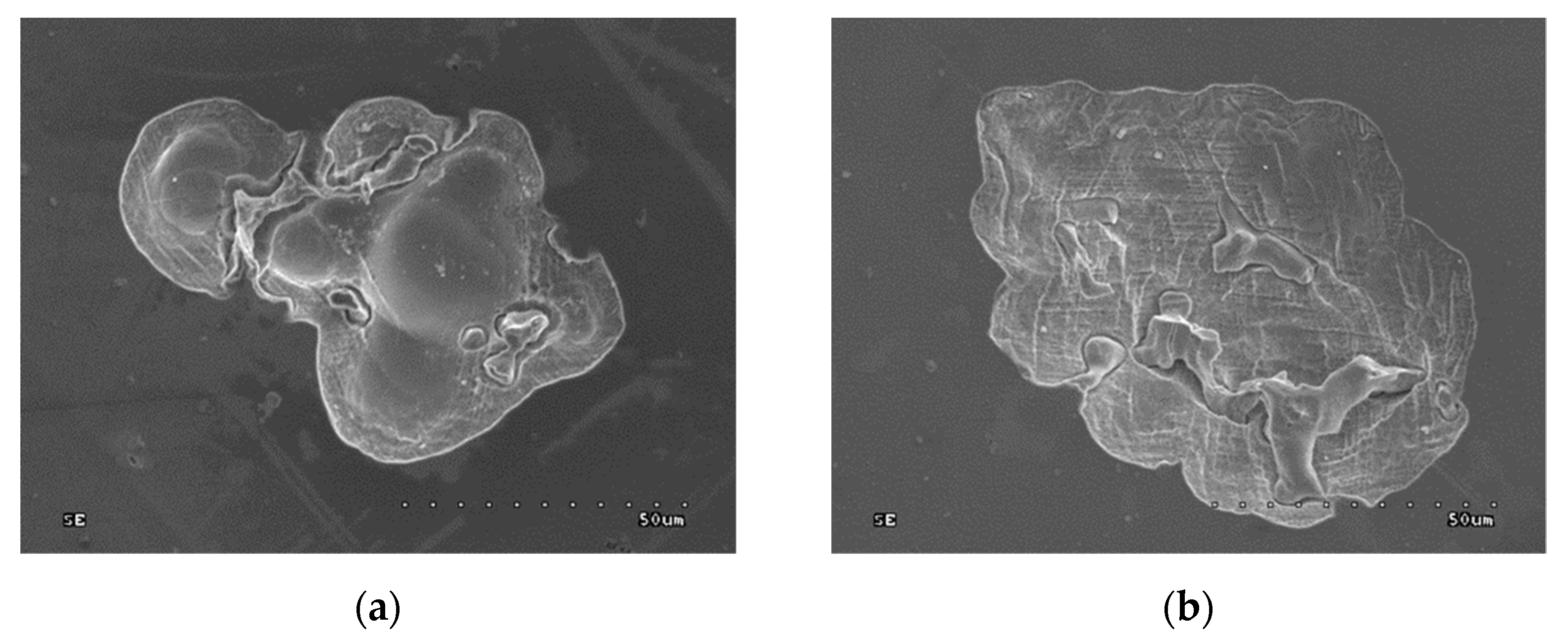
- Corrosion tests in artificial saliva with microscopic examinations of the corrosion areas. The tests were carried out on five samples from each group.
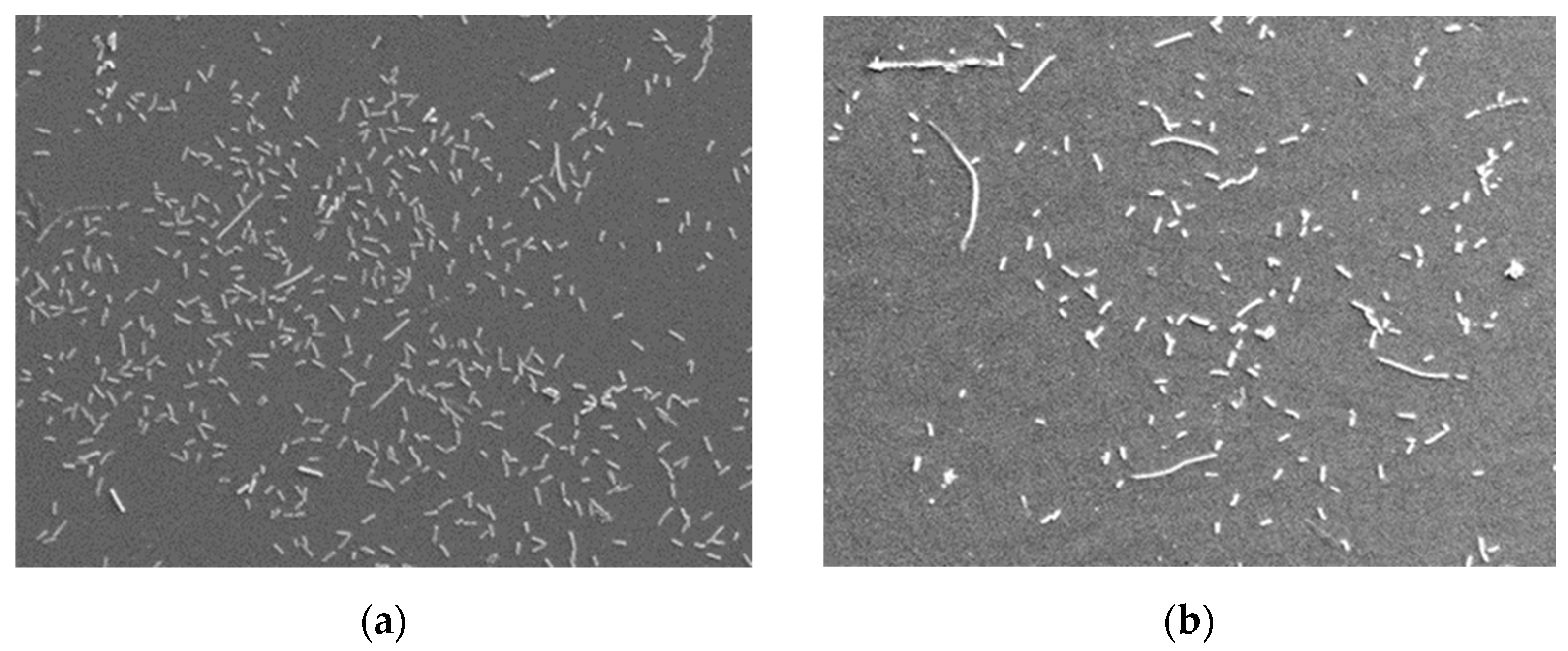
- Bacterial adhesion on the surface of the samples. To that end, the samples were sterilized in a steam autoclave at the temperature of 135 °C. The samples prepared in this way were then placed in a liquid culture of Escherichia coli bacteria (strain K12) and incubated at the temperature of 37 °C. Each sample was placed in a separate container with a solution, in which about 2 × 103 bacteria were present. After 24 h of being kept in the incubator, they were removed from the bacteria cultures and subjected to preparation procedures aiming at strengthening the bacteria which had colonized the sample surfaces [35]. Next, the samples were sprayed with a thin layer of gold by means of a sputter JEE-4X Jeol. The number of bacteria was estimated under an electron scanning microscope HITACHI S-3000N on six samples covered with a DLC coating and six samples without a coating. Every sample underwent five measurements in randomly selected areas (150 × 100 μm), which gave a total of 30 measurements.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-J.; Song, K.-Y.; Ahn, S.-G.; Choi, J.-Y.; Seo, J.-M.; Park, J.-M. Evaluation of effect of galvanic corrosion between nickel-chromium metal and titanium on ion release and cell toxicit. J. Adv. Prosthodont. 2015, 7, 172–177. [Google Scholar] [CrossRef]
- Holm, C.; Morisbak, E.; Kalfoss, T.; Dahl, J.E. In vitro element release and biological aspects of base–metal alloys for metal-ceramic applications. Acta Biomater. Odontol. Scand. 2015, 1, 2–4. [Google Scholar] [CrossRef]
- Milheiro, A.; Nozaki, K.; Kleverlaan, C.J.; Muris, J.; Miura, H.; Feilzer, A.J. In vitro cytotoxicity of metallic ions released from dental alloys. Odontology 2016, 104, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Chandar, S.; Kotian, R.; Madhyastha, P.; Kabekkodu, S.P.; Rao, P. In vitro evaluation of cytotoxicity and corrosion behavior of commercially pure titanium and Ti-6Al-4V alloy for dental implants. J. Indian Prosthodont. Soc. 2017, 17, 35–40. [Google Scholar]
- Mc Ginley, E.L.; Fleming, G.J.P.; Moran, G.P. Development of a discriminatory biocompatibility testing model for non-precious dental casting alloys. Dent. Mater. 2011, 27, 1295–1306. [Google Scholar] [CrossRef]
- Ramírez-Ledesma, A.L.; Roncagliolo, P.; Álvarez-Pérez, M.A.; Lopez, H.F.; Juárez-Islas, J.A. Corrosion Assessment of an Implantable Dental Co-Cr Alloy in Artificial Saliva and Biocompatibility Behavior. J. Mater. Eng. Perform. 2020, 29, 1657–1670. [Google Scholar] [CrossRef]
- Pochrząst, M.; Marciniak, J.; Wróbel, K.; Bączkowski, B. Electrochemical Properties of Ni-Cr and Co-Cr Alloys Used in Prosthodontics. Solid State Phenom. 2011, 183, 143–148. [Google Scholar] [CrossRef]
- Schmutz, P.; Quach-Vu, N.-C.; Gerber, I. Metallic Medical Implants: Electrochemical Characterization of Corrosion Processes. Electrochem. Soc. Interface 2020, 17, 35–40. [Google Scholar] [CrossRef]
- Nierlich, J.; Papageorgiou, S.N.; Bourauel, C.; Hültenschmidt, R.; Bayer, S.; Stark, H.; Keilig, L. Corrosion behavior of dental alloys used for retention elements in prosthodontics. Eur. J. Oral Sci. 2016, 124, 287–294. [Google Scholar] [CrossRef]
- Mercieca, S.; Conti, C.M.; Buhagiar, J.; Camilleri, J. Assessment of corrosion resistance of cast cobalt- and nickel-chromium dental alloys in acidic environments. J. Appl. Biomater. Funct. Mater. 2018, 16, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Ferreira, J.A.; Leszczyńska, K.; Chmielewska, S.; Dąbrowski, J.R.; Wieciński, P.; Kurzydłowski, K. Biocorrosion of 316LV steel used in oral cavity due to Desulfotomaculum nigrificans bacteria. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef]
- Adya, N.; Alam, M.; Ravindranath, T.; Mubeen, A.; Saluja, B. Corrosion in titanium dental implants: Literature review. J. Indian Prosthodont. Soc. 2005, 53, 126–131. [Google Scholar] [CrossRef]
- Wu, F.; Chen, T.; Wang, H.; Liu, D. Effect of Mo on Microstructures and Wear Properties of In Situ Synthesized Ti(C, N)/Ni-Based Composite Coatings by Laser Cladding. Materials 2017, 10, 1047. [Google Scholar] [CrossRef]
- Nematia, A.; Saghafia, M.; Khamseh, S.; Alibakhshic, E.; Zarrintajd, P.; Saebe, M.R. Magnetron-sputtered TixNy thin films applied on titanium-based alloys for biomedical applications: Composition-microstructure-property relationships. Surf. Coat. Technol. 2018, 349, 251–259. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Li, P.; Bilek, M.M.; Boxman, R.L.; McKenzie, D.R.; Chu, P.K. Evaluation of corrosion resistance and cytocompatibility of graded metal carbon film on Ti and NiTi prepared by hybrid cathodic arc/glow discharge plasma-assisted chemical vapor deposition. Corros. Sci. 2015, 97, 126–138. [Google Scholar] [CrossRef]
- Wang, G.; Zreiqat, H. Functional Coatings or Films for Hard-Tissue Applications. Materials 2010, 3, 3994–4050. [Google Scholar] [CrossRef]
- Banaszek, K.; Wiktorowska-Owczarek, A.; Kowalczyk, E.; Klimek, L. Possibilities of applying Ti (C, N) coatings on prosthetic elements—Research with the use of human endothelial cells. Acta Bioeng. Biomater. 2016, 18, 119–126. [Google Scholar]
- Banaszek, K.; Klimek, L.; Zgorzynska, E.; Swarzynska, A.; Walczewska, A. Cytotoxicity of titanium carbonitride coatings for prostodontic alloys with different amounts of carbon and nitro gen. Biomed. Mater. 2018, 13, 045003. [Google Scholar] [CrossRef]
- Calderon, S.; Alves, C.F.A.; Manninen, N.K.; Cavaleiro, A.; Carvalho, S. Electrochemical Corrosion of Nano-Structured Magnetron-Sputtered Coatings. Coatings 2019, 9, 682. [Google Scholar] [CrossRef]
- Banaszek, K.; Klimek, L. Ti(C, N) as Barrier Coatings. Coatings 2019, 9, 432. [Google Scholar] [CrossRef]
- Li, J.; Wang, K.; Li, Z.; Tu, J.P.; Jin, G.; Su, J.; Zhai, B. Mechanical tests, wear simulation and wear particle analysis of carbon-based nanomultilayer coatings on Ti6Al4V alloys as hip prostheses. RSC Adv. 2018, 8, 6849–6857. [Google Scholar] [CrossRef]
- Jachowicz, M.; Kaczmarek, Ł.; Rylski, A.; Przybylski, K.; Danielewski, M.; Wendler, B. New type AlMo-, AlTi- or Si-based magnetron sputtered protective coatings on metallic substrates. J. Mater. Process. Technol. 2006, 175, 427–432. [Google Scholar]
- Kaczmarek, Ł.; Wendler, B.; Siniarski, D.; Rylski, A.; Bieliński, D.; Dobrowolski, O.; Lipiński, P. Oxidation resistance of refractory γ-TiAlW coatings. Surf. Coat. Technol. 2007, 201, 167–6170. [Google Scholar] [CrossRef]
- Viswanathan, S.; Mohan, L.; Chakraborty, M.; Mandal, C.; Bera, P.; Aruna, S.T.; Anandan, C. Carbon plasma immersion ion implantation and DLC deposition on Ni−Ti alloy. Mater. Manuf. Process. 2018, 33, 1121–1127. [Google Scholar] [CrossRef]
- Rajib, P. Diamond-Like-Carbon Coatings for Advanced Biomedical Applications. Glob. J. Nanomed. 2017, 5, 1–5. [Google Scholar]
- Grabarczyk, J.; Batory, D.; Louda, P.; Couvrat, P.; Kotela, I.; Bąkowicz-Mitura, K. Carbon coatings for medical implants. J. Achiev. Mater. Manuf. Eng. 2007, 20, 107–110. [Google Scholar]
- Mitura, E.; Niedzielska, A.; Niedzielski, P.; Klimek, L.; Rylski, A.; Mitura, S.; Moll, J. The properities of carbon layers deposited onto titanium substrates. Diam. Relat. Mater. 1996, 5, 998–1001. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Boxman, R.L.; Bilek, M.M.; Kocer, C.; Hu, T.; Zhang, X.; McKenzie, D.R.; Chu, P.K. Effects of pulse voltage and deposition time on the adhesion strength of graded metal/carbon films deposited on bendable stainless-steel foils by hybrid cathodic arc—Glow discharge plasma assisted chemical vapor deposition. Appl. Surf. Sci. 2016, 366, 535–544. [Google Scholar] [CrossRef]
- Batory, D.; Blaszczyk, T.; Clapa, M.; Mitura, S. Investigation of anti-corrosion properties of Ti:C gradient layers manufactured in hybrid deposition system. Mater. Sci. 2008, 43, 3385–3391. [Google Scholar] [CrossRef]
- PN-EN-ISO 14577-1, -2, -3. Metals—Apparatus Testing of Hardness and Material Properties of Indenter Indentation; International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Li, X.; Bharat, B. A review of nanoindentation continuous stiffness measurement technique and its applications. Mat. Charact 2002, 48, 11–36. [Google Scholar]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Huang, L.; Lu, J.; Xu, K. Elasto-plastic deformation and fracture mechanism of a diamond-like carbon film deposited on a Ti–6Al–4V substrate in nano-scratch test. Thin Solid Film. 2004, 466, 175–182. [Google Scholar]
- Banaszek, K.; Szymanski, W.; Pietrzyk, B.; Klimek, L. Adhesion of E. coli Bacteria Cells to Prosthodontic Alloys Surfaces Modified by TiO2 Sol-Gel Coatings. Adv. Mater. Sci. Eng. 2013, 2013, 179241. [Google Scholar] [CrossRef]
- Tai, F.C.; Lee, S.C.; Chen, J.; Wei, C.; Chang, S.H. Multipeak fitting analysis of Raman spectra on DLCH film. J. Raman Spectrosc. 2009, 40, 1055–1059. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Świątek, H.; Łuczak, K.; Głuszek, M.; Cłapa, M. Impact of Plasma Pre-Treatment on the Tribological Properties of DLC Coatings on PDMS Substrates. Materials 2021, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Batory, D.; Szymański, W.; Cłapa, M. Mechanical and tribological properties of gradient a-C:H/Ti coatings. Mater. Sci. Pol. 2013, 31, 415–423. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Y.; Guo, P.; Xu, D.; Hu, L.; Wang, A. Adhesion, biological corrosion resistance and biotribological properties of carbon films deposited on MAO coated Ti substrates. J. Mech. Behav. Biomed. Mater. 2020, 101, 1–6. [Google Scholar] [CrossRef]
- Cano, D.; Lousa, A.; Esteve, J.; Ferrer-Anglada, N. Low Wear and Low Friction DLC Coating with Good Adhesion to CoCrMo Metal Substrates. Phys. Status Solidi 2018, 255, 1800255. [Google Scholar] [CrossRef]
- Jakubowski, W.; Ślósarczyk, A.; Paszkiewicz, Z.; Szymański, W.; Walkowiak, B. Bacterial colonisation of bioceramic surfaces. Adv. Appl. Ceram. 2008, 107, 217–221. [Google Scholar] [CrossRef]
- Liu, C.Q.; Zhao, Y.; Liu, S.; Wang, E.W. Abel: Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B Biointerfaces 2018, 61, 182–187. [Google Scholar] [CrossRef]
- Robertson, S.N.; Gibson, D.; Mac Kay, W.G.; Reid, S.; Williams, C.; Birney, R. Investigation of the antimicrobial properties of modified multilayer diamond-like carbon coatings on 316 stainless steel. Surf. Coat. Technol. 2017, 314, 72–78. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Mangolin, J.F.; Da-Silva, N.S.; Corata, E.J.; Trava-Airoldia, V.J. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vacuum 2011, 85, 662–666. [Google Scholar] [CrossRef]
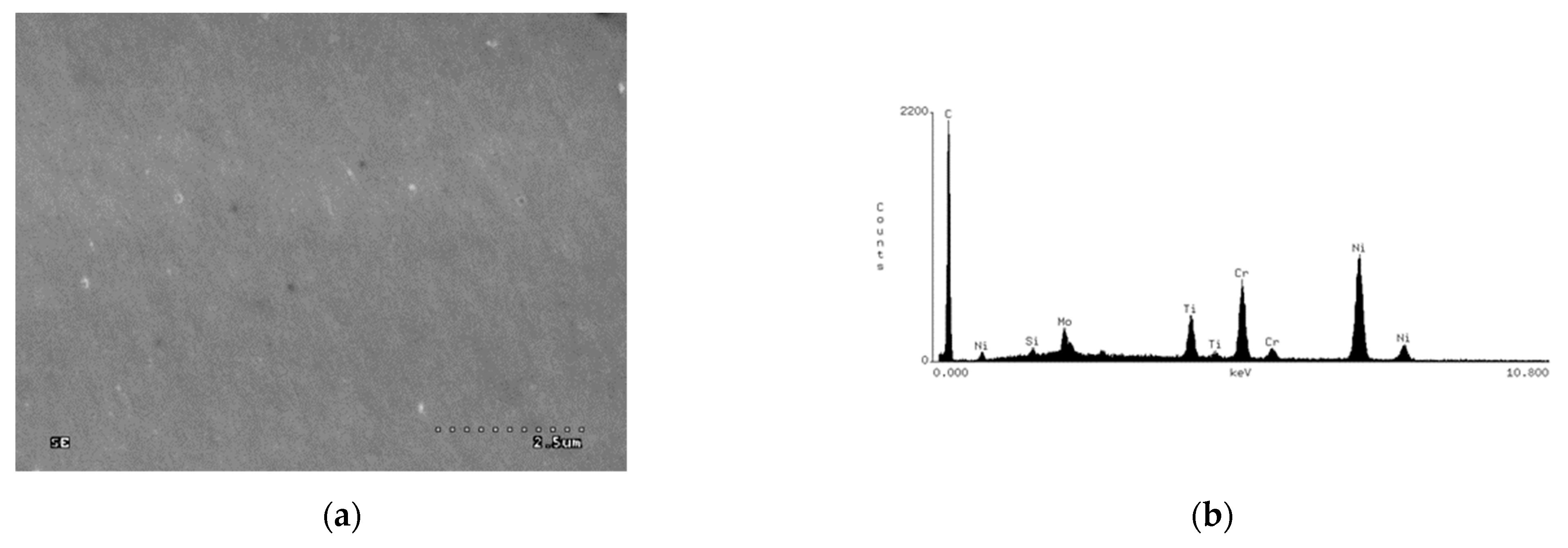
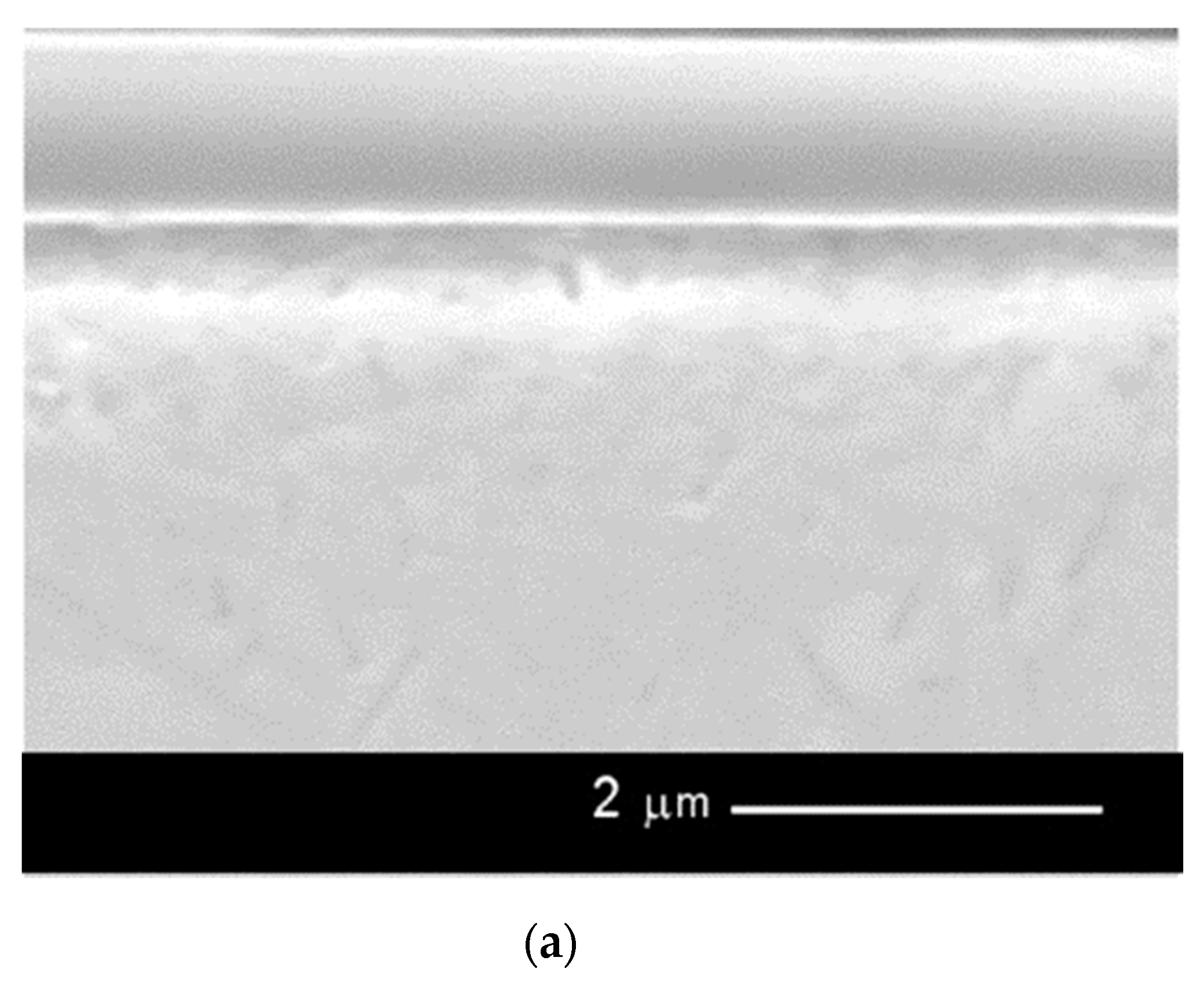
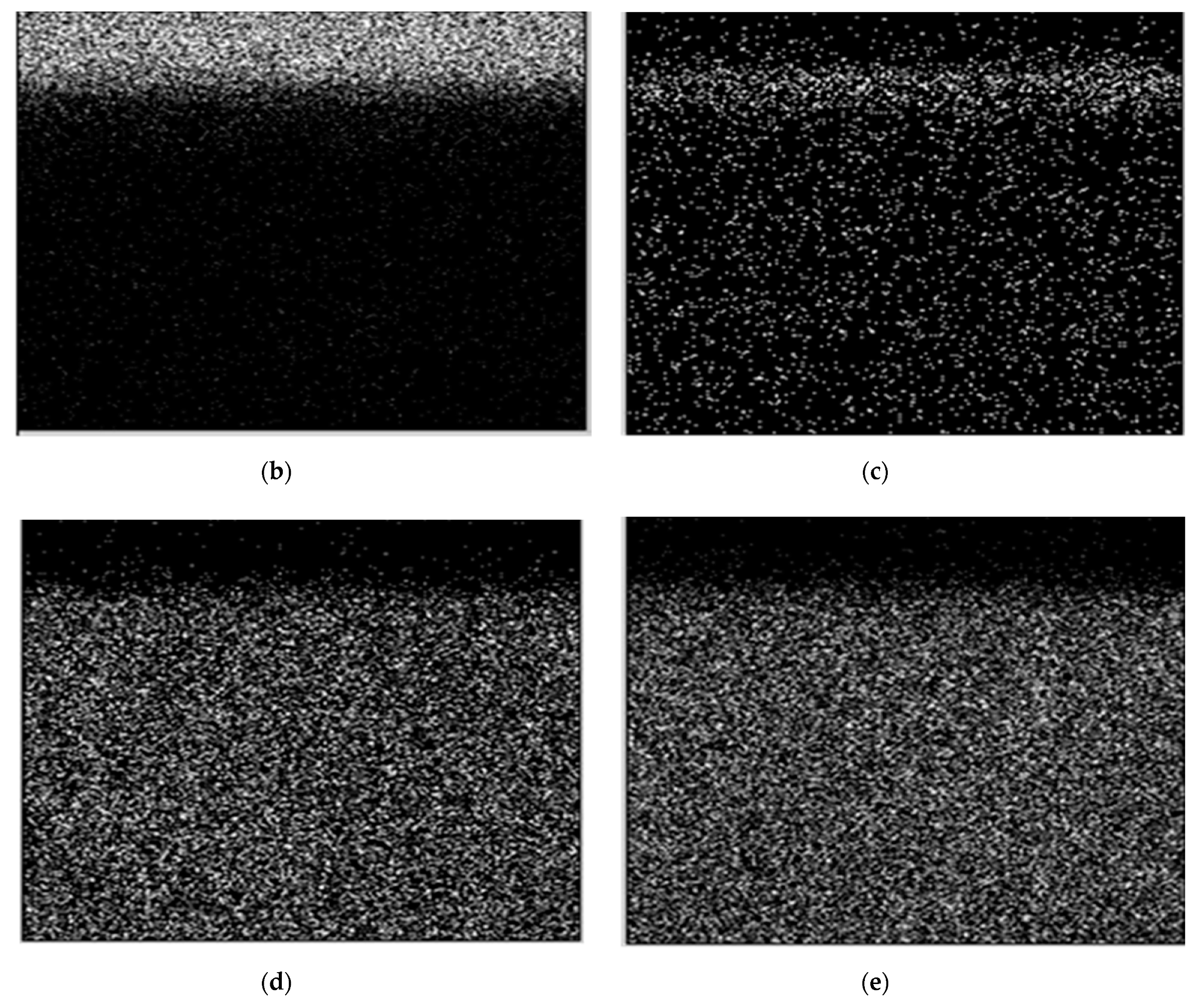
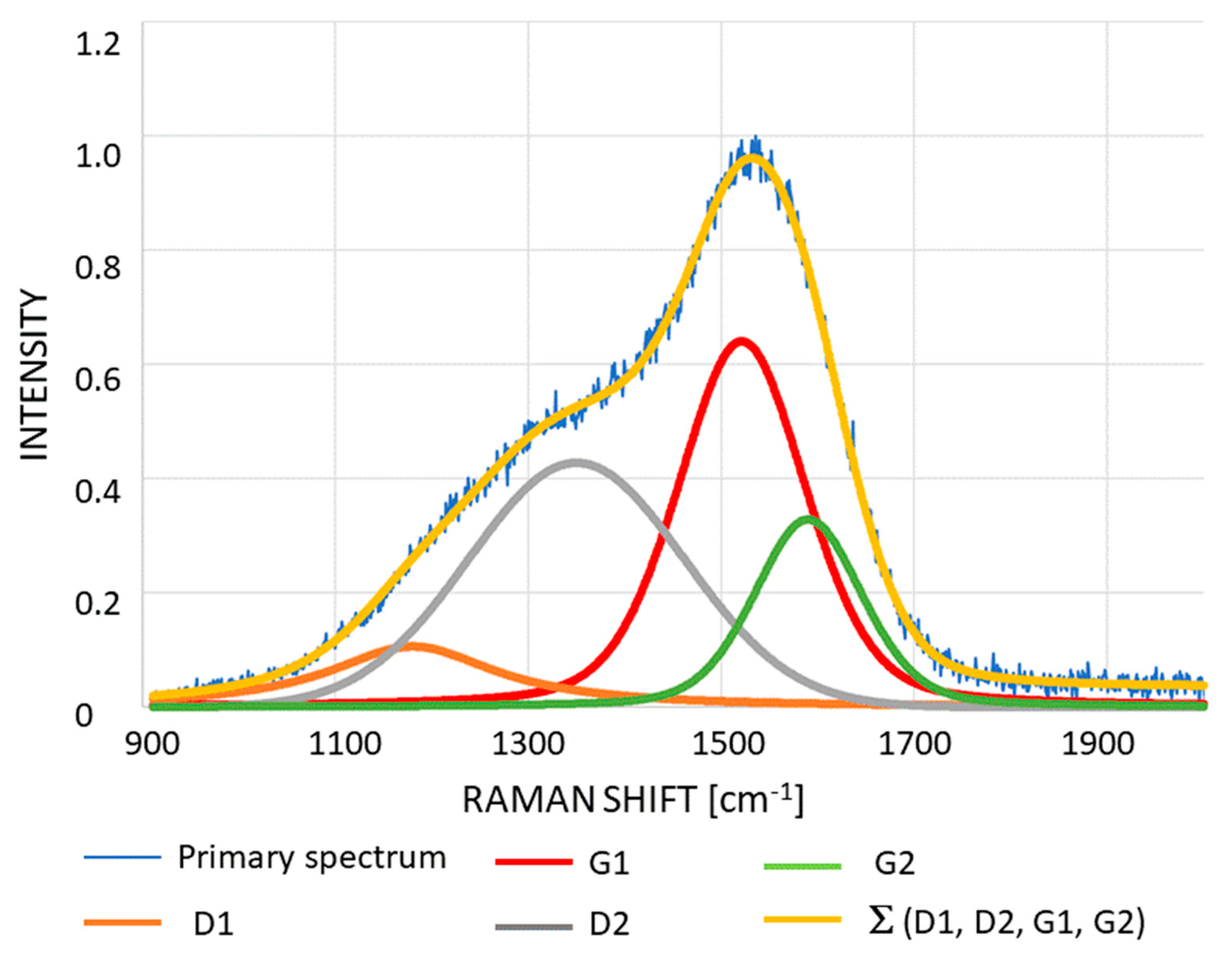
| Element Percentage by Weight (wt.%) | ||||||
|---|---|---|---|---|---|---|
| Cr | Mo | Si | Fe | Co | Mn | Ni |
| 24.79 | 8.89 | 1.57 | 1.33 | 0.17 | 0.13 | rest |
| KCl | NaCl | CaCl2·2H2O | NaH2PO4·2H2O | Na2S·9H2O | Urea | Triple Distilled Water |
|---|---|---|---|---|---|---|
| 0.4 g | 0.4 g | 0.906 g | 0.69 g | 0.005 g | 1 g | 1 dm3 |
| Sample | Mean Value | Standard Deviation |
|---|---|---|
| Ni-Cr with a coating | 0.619 V | 0.010 |
| Ni-Cr without a coating | 0.823 V | 0.003 |
| Sample | Average Value | Standard Deviation |
|---|---|---|
| Ni-Cr with a coating | 84 | 10 |
| Ni-Cr without a coating | 173 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, Z.; Semenov, M.; Klimek, L. Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy. Appl. Sci. 2021, 11, 4551. https://doi.org/10.3390/app11104551
Kula Z, Semenov M, Klimek L. Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy. Applied Sciences. 2021; 11(10):4551. https://doi.org/10.3390/app11104551
Chicago/Turabian StyleKula, Zofia, Michael Semenov, and Leszek Klimek. 2021. "Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy" Applied Sciences 11, no. 10: 4551. https://doi.org/10.3390/app11104551
APA StyleKula, Z., Semenov, M., & Klimek, L. (2021). Carbon Coatings Deposited on Prosthodontic Ni-Cr Alloy. Applied Sciences, 11(10), 4551. https://doi.org/10.3390/app11104551








