Amelioration of Diabetes-Induced Nephropathy by Loranthus regularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Diabetes
2.3. Collection and Preparation of Plant Extract
2.4. Experimental Design
2.5. Blood Biochemistry
2.6. Tissue Analysis
2.7. Histopathological Analysis
2.8. Statistical Analysis
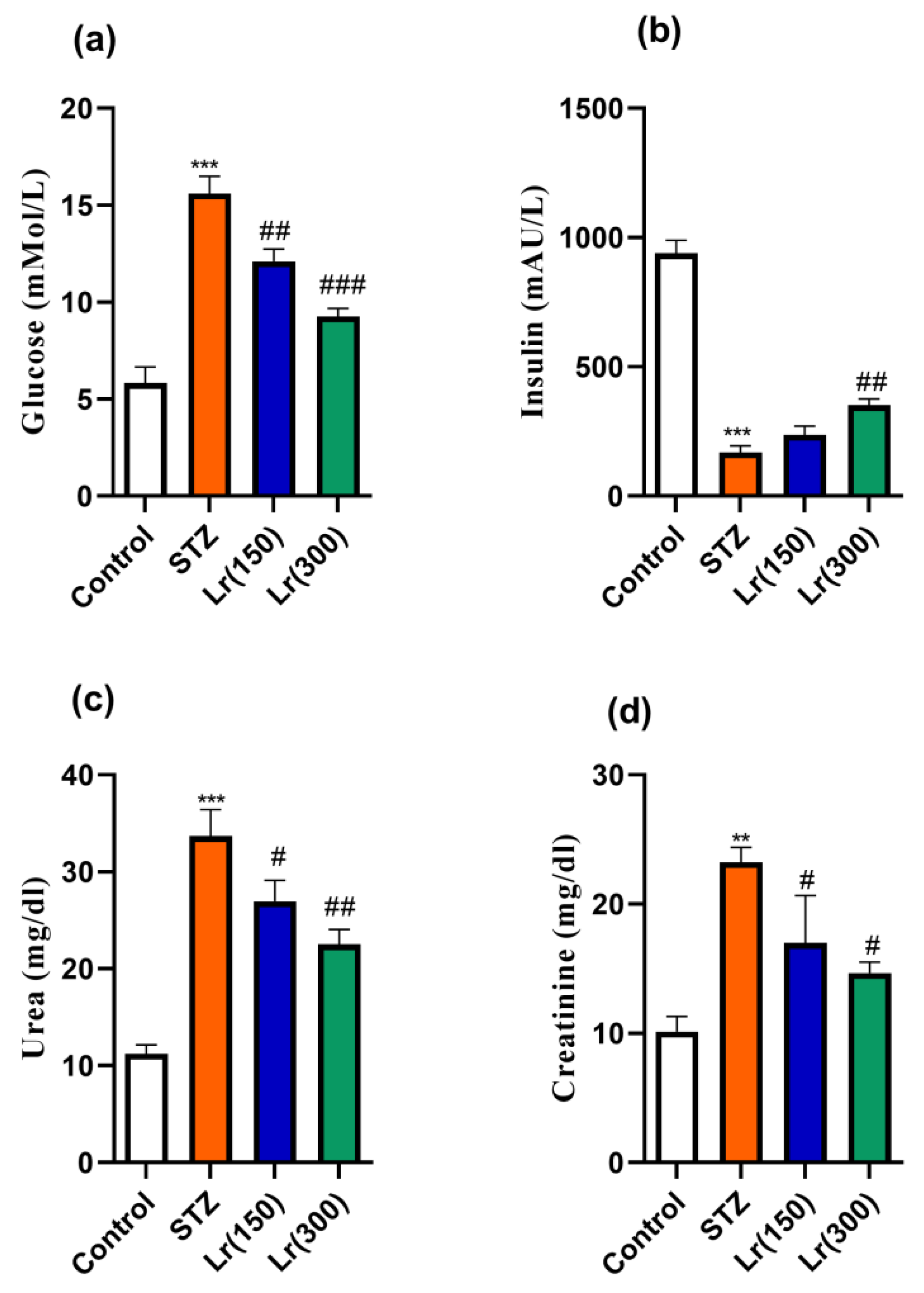
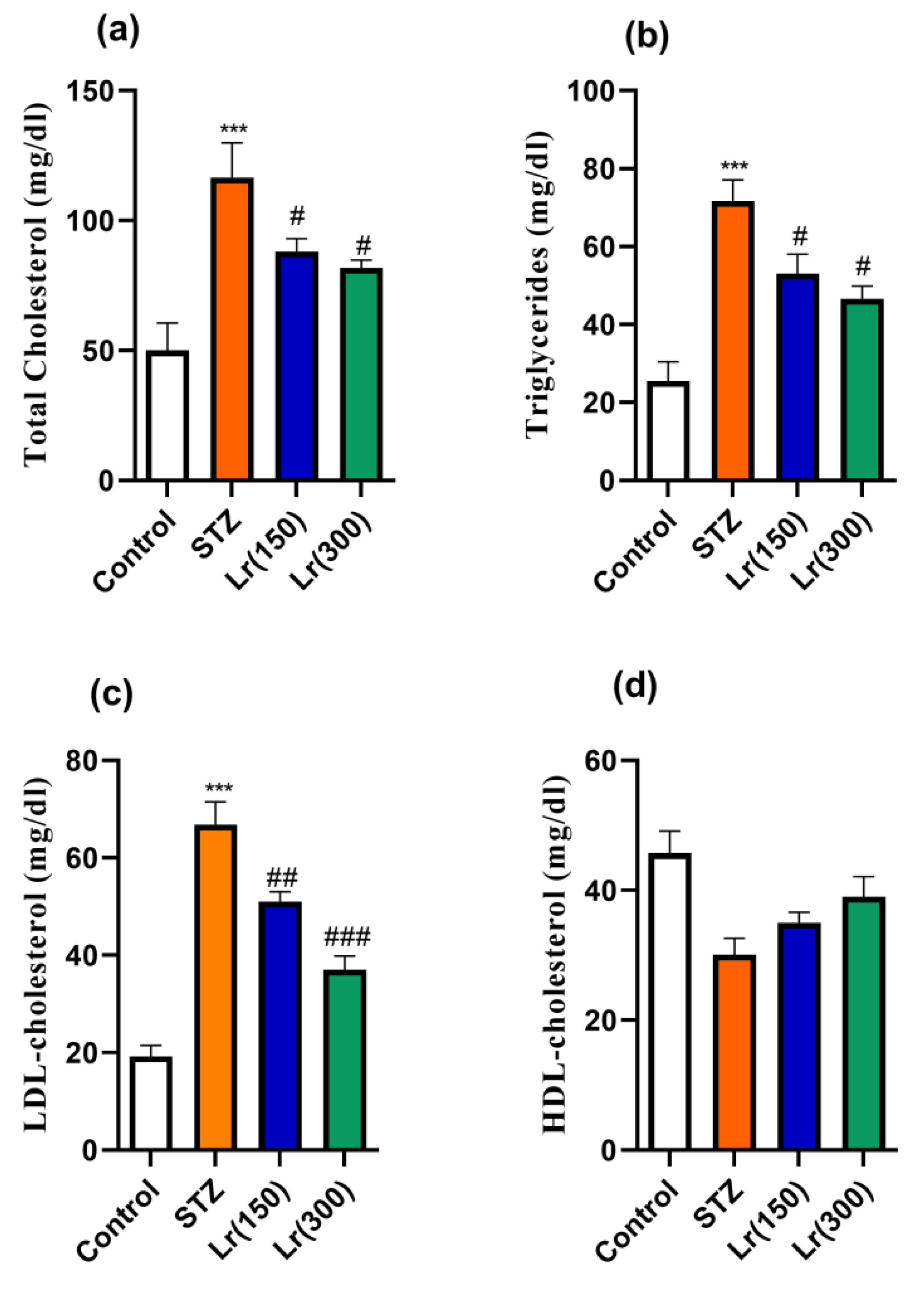
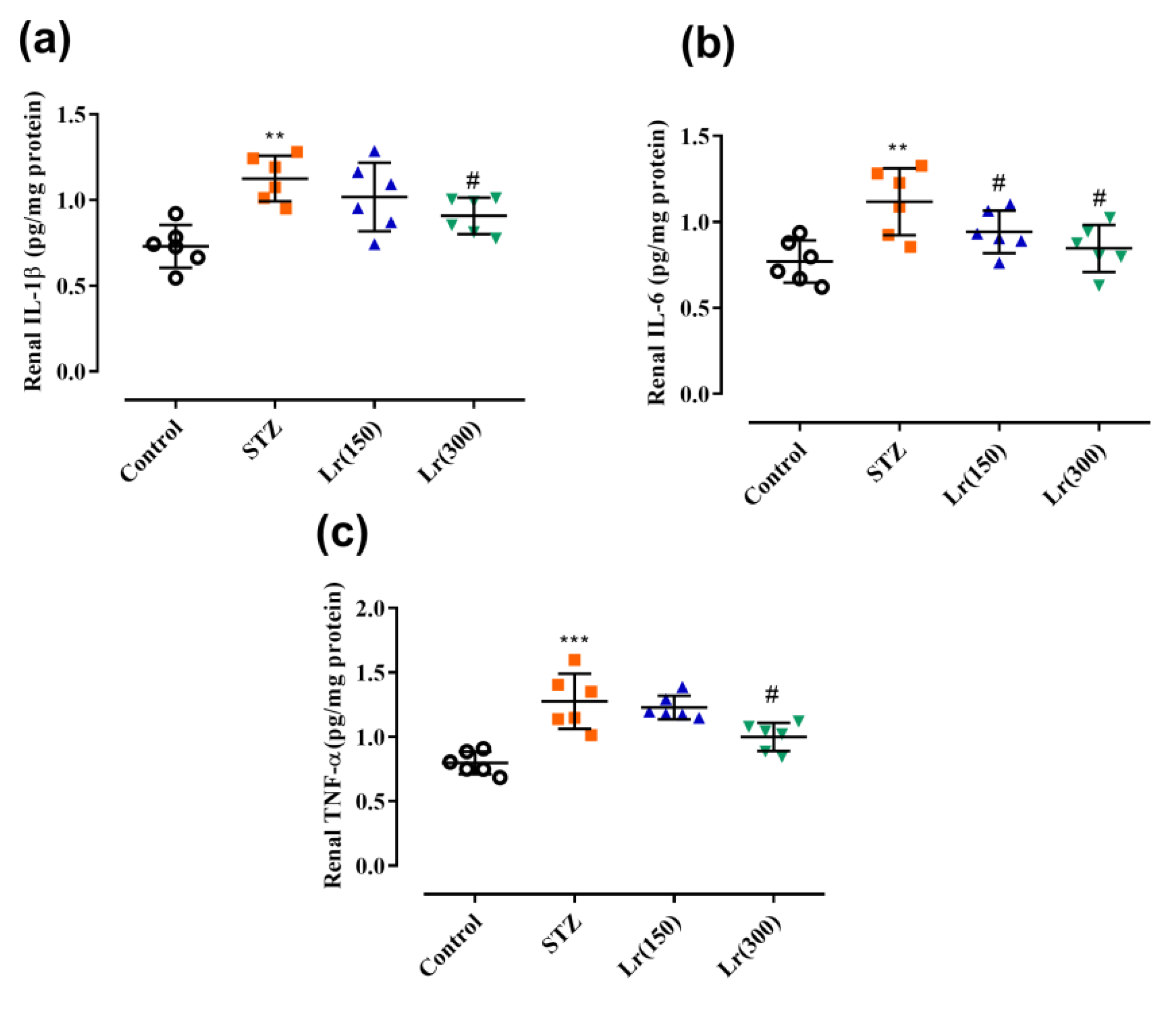
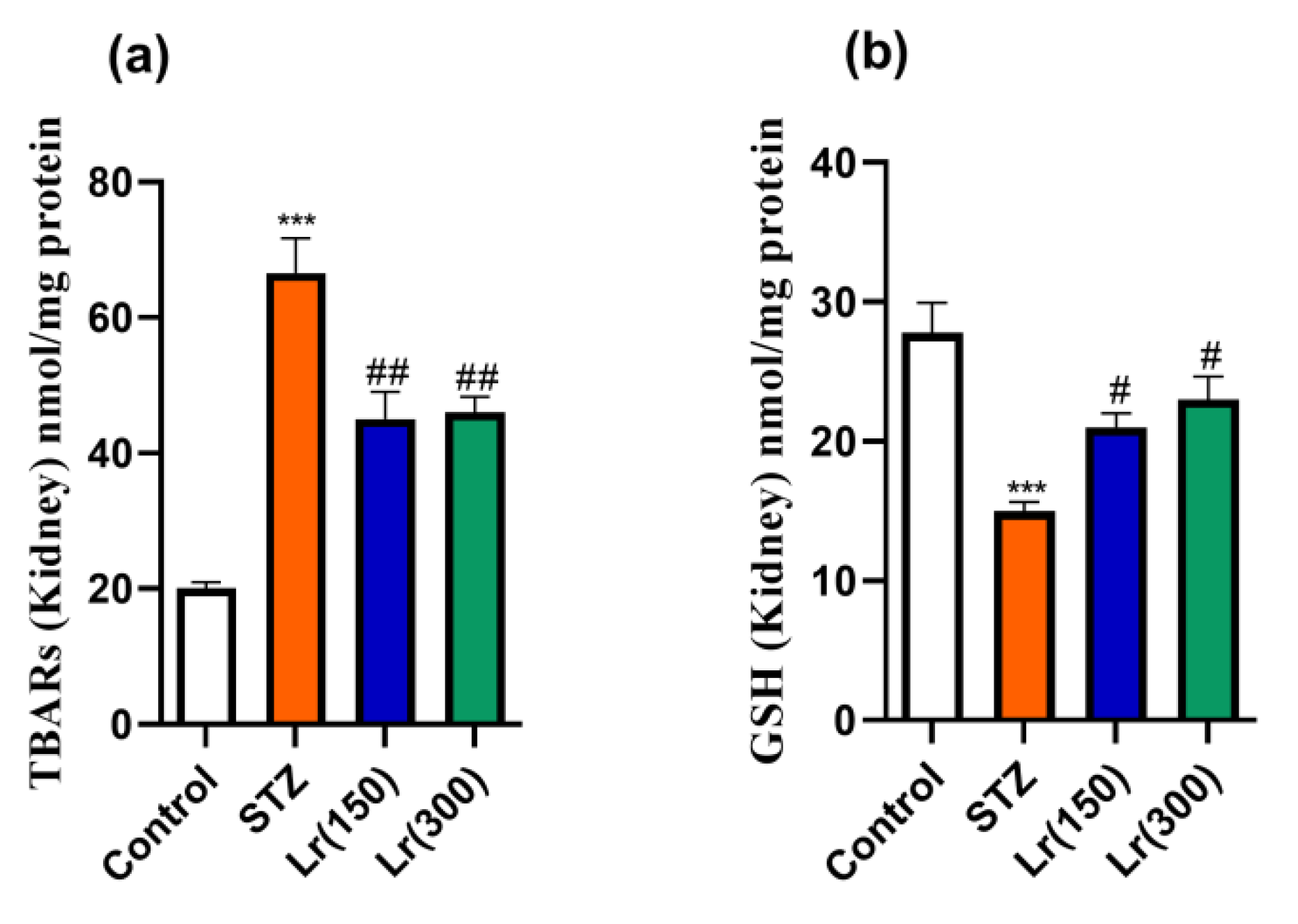
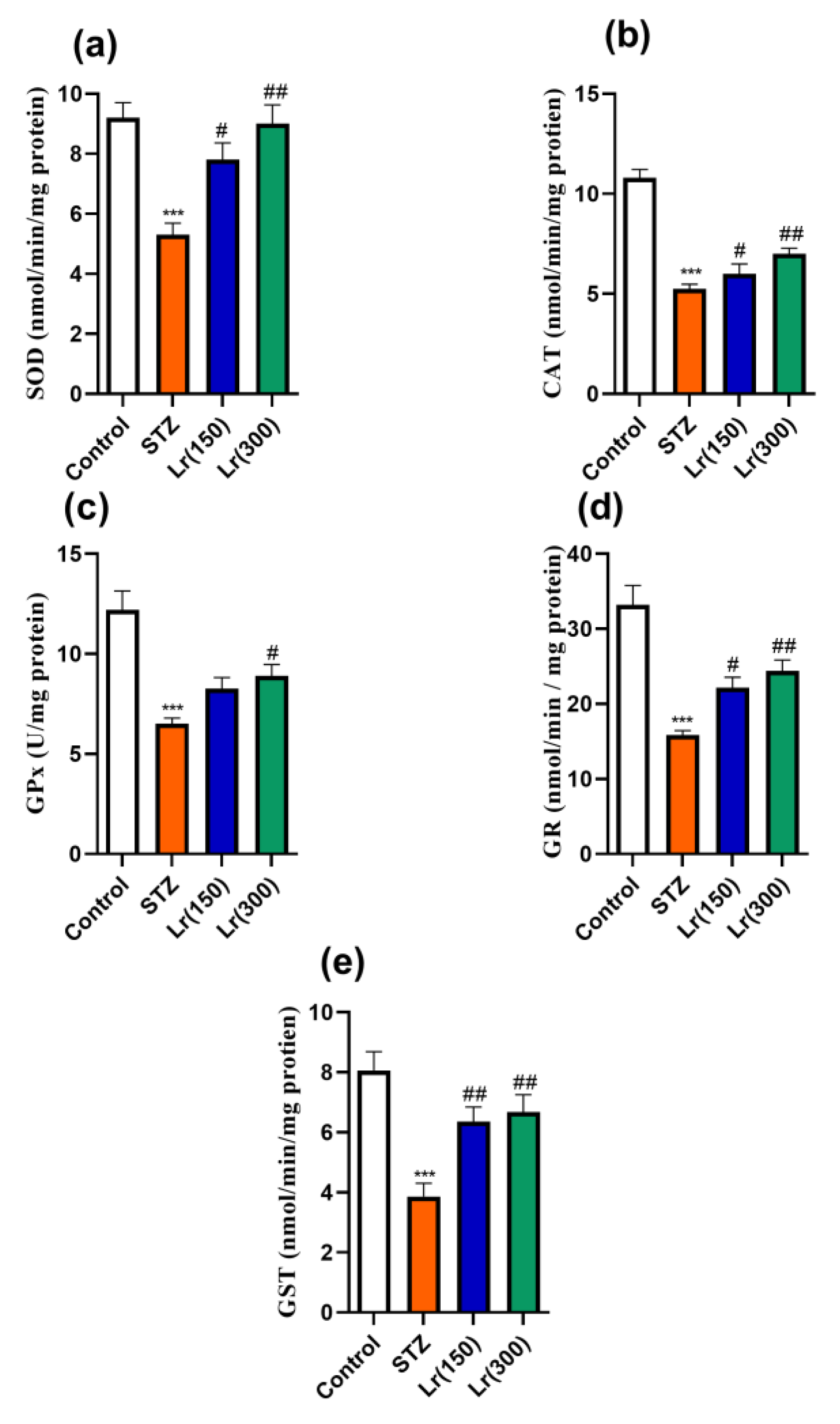
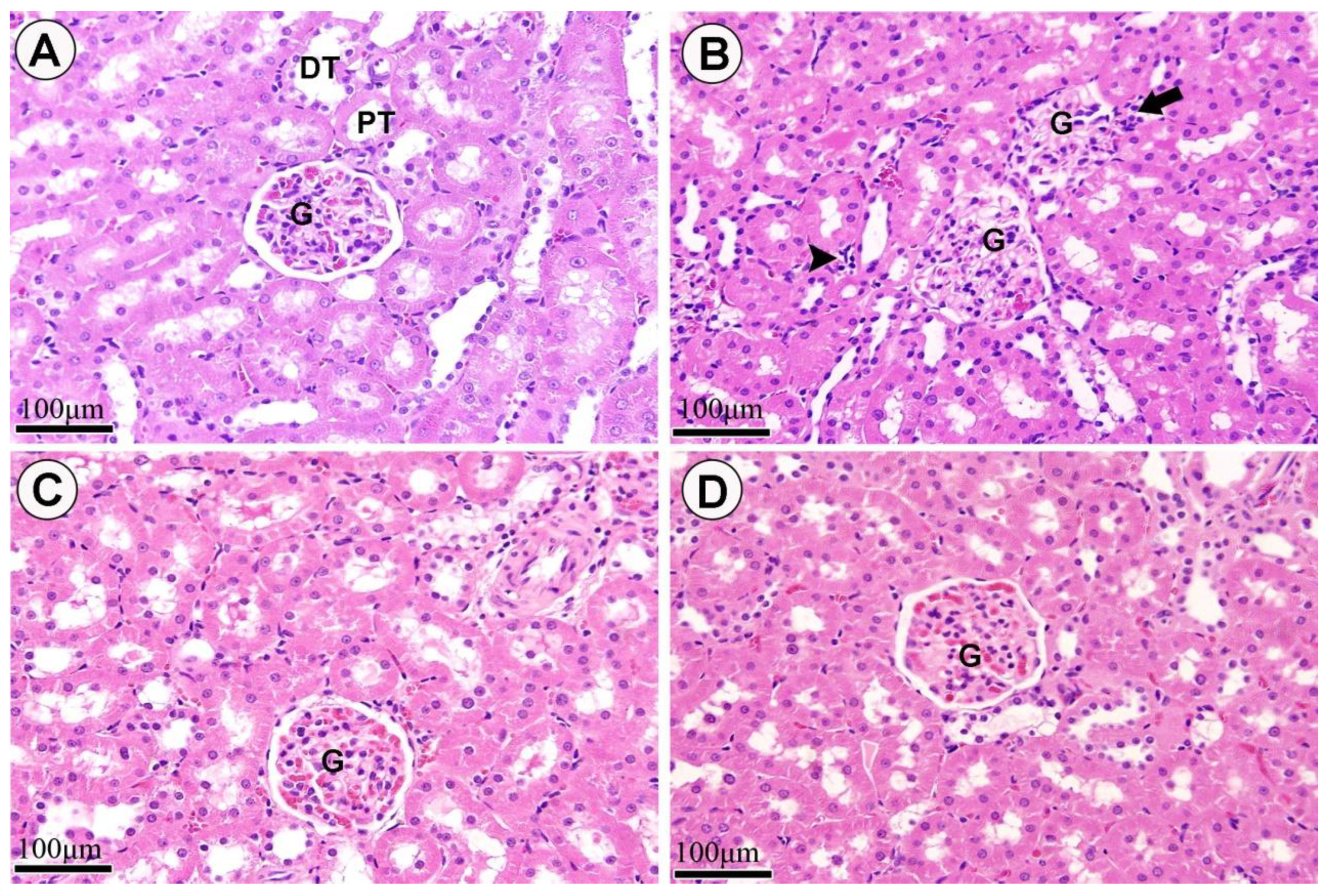
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Dey, P.; Sarkar, P.; Karmakar, S.; Tae, I.H.; Kyeong, S.K.; Park, J.H.; Lee, S.H.; Lee, B.M.; Renthlei, L.; et al. Protective effects of Croton hookeri on streptozotocin-induced diabetic nephropathy. Food Chem. Toxicol. 2020, 135, 110873. [Google Scholar] [CrossRef]
- Aleisa, A.M.; Aleisa, A.M.; Al-Rejaie, S.S.; Abuohashish, H.M.; Ahmed, M.M.; Parmar, M.Y. Nephro-protective role of morin against experimentally induced diabetic nephropathy. Digest J. Nanomater. Biostruct. 2013, 8, 395–401. [Google Scholar]
- Ola, M.S.; Ahmed, M.M.; Ahmad, R.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Neuroprotective effects of rutin in streptozotocin-induced diabetic rat retina. Journal of Molecular Neuroscience. J. Mol. Sci. 2015, 56, 440–448. [Google Scholar]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 2012, 6. [Google Scholar] [CrossRef] [PubMed]
- Asbun, J.; Villarreal, F.J. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Oluleye, T. Diabetic retinopathy: Current developments in pathogenesis and management. Afr. J. Med. Med. Sci. 2010, 39, 199–206. [Google Scholar]
- Sadi, G.; Eryilmaz, N.; Tütüncüoğlu, E.; Cingir, Ş.; Güray, T. Changes in expression profiles of antioxidant enzymes in diabetic rat kidneys. Diabetes/Metab. Res. Rev. 2012, 28, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Das, J.; Manna, P.; Sil, P.C. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: Role of NF-κB, p38 and JNK MAPK pathway. Toxicol. Appl. Pharmacol. 2009, 240, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; De Fuentes, M.M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-induced reactive oxygen species: Mechanism of their generation and role in renal injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Kolset, S.; Reinholt, F.; Jenssen, T. Diabetic nephropathy and extracellular matrix. J. Histochem. Cytochem. 2012, 60, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.M.; Wahab, N.A. Extracellular matrix metabolism in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Khan, H.A.; Manthiri, R.A.; Al-Khlaiwi, A.A.; Al-Asmari, B.A.; Ibrahim, K.E. Protective effects of a natural herbal compound quercetin against snake venom-induced hepatic and renal toxicities in rats. Food Chem. Toxicol. 2018, 118, 105–110. [Google Scholar] [CrossRef]
- Gómez-Sierra, T.; Eugenio-Pérez, D.; Sánchez-Chinchillas, A.; Pedraza-Chaverri, J. Role of food-derived antioxidants against cisplatin induced-nephrotoxicity. Food Chem. Toxicol. 2018, 120, 230–242. [Google Scholar] [CrossRef]
- Álvarez-Cilleros, D.; Ramos, S.; Goya, L.; Martín, M.Á. Colonic metabolites from flavanols stimulate nitric oxide production in human endothelial cells and protect against oxidative stress-induced toxicity and endothelial dysfunction. Food Chem. Toxicol. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Adesina, S.K.; Illoh, H.C.; Johnny, I.I.; Jacobs, I.E. African mistletoes (Loranthaceae); ethnopharmacology, chemistry and medicinal values: An update. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 161–170. [Google Scholar] [CrossRef]
- Ameer, O.; Salman, I.M.; Siddiqui, M.J.A.; Yam, M.F.; Sriramaneni, R.N.; Sadikun, A.; Ismail, Z.; Shah, A.M.; Asmawi, M.Z. Cardiovascular activity of the n-butanol fraction of the methanol extract of Loranthus ferrugineus Roxb. Braz. J. Med. Biol. Res. 2010, 43, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Alam, A.K.; Hossain, M.A.; Mosaddik, M.A.; Sadik, G. Biological screening of Bangladeshi mango mistletoe bark extracts. Fitoterapia 2004, 75, 405–408. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kim, Y.S.; Choi, S.U.; Ryu, S.Y. Isolation of flavonol rhamnosides from Loranthus tanakae and cytotoxic effect of them on human tumor cell lines. Arch. Pharm. Res. 2004, 27, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Obatomi, D.K.; Bikomo, E.O.; Temple, V.J. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 1994, 43, 13–17. [Google Scholar] [CrossRef]
- Schopen, A. Traditionelle Heilmittel in Jemen; Steiner Wiesbaden: Stuttgart, Germany, 1983. [Google Scholar]
- Mothana, R.A.; Kriegisch, S.; Harms, M.; Wende, K.; Lindequist, U. Assessment of selected Yemeni medicinal plants for their in vitro antimicrobial, anticancer, and antioxidant activities. Pharm. Biol. 2011, 49, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.; Al-Said, M.S.; Al-Rehaily, A.J.; Thabet, T.M.; Awad, N.A.; Lalk, M.; Lindequist, U. Anti-inflammatory, antinociceptive, antipyretic and antioxidant activities and phenolic constituents from Loranthus regularis Steud. ex Sprague. Food Chem. 2012, 130, 344–349. [Google Scholar] [CrossRef]
- Đorđević, M.; Mihailović, M.; Jovanović, J.A.; Grdović, N.; Uskoković, A.; Tolić, A.; Sinadinović, M.; Rajić, J.; Mišić, D.; Šiler, B.; et al. Centaurium erythraea methanol extract protects red blood cells from oxidative damage in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2017, 202, 172–183. [Google Scholar] [CrossRef]
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Achi, N.; Ohaeri, O.C.; Ijeh, I.I.; Eleazu, C. Modulation of the lipid profile and insulin levels of streptozotocin induced diabetic rats by ethanol extract of Cnidoscolus aconitifolius leaves and some fractions: Effect on the oral glucose tolerance of normoglycemic rats. Biomed. Pharmacother. 2017, 86, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, T.; Gong, Y.; Dong, X.; Zhang, W.; Sun, S.; Wang, H.; Gu, Y.; Lu, X.; Yan, M.; et al. Attenuation of diabetic nephropathy by Chaihuang-Yishen granule through anti-inflammatory mechanism in streptozotocin-induced rat model of diabetics. J. Ethnopharmacol. 2014, 151, 556–564. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Al-Said, M.S.; Al-Rehaily, A.J.; Thabet, T.M.; Awad, N.A.; Lalk, M.; Lindequist, U. Anti-inflammatory, antinociceptive, antipyretic and antioxidant activities and phenolic constituents from Loranthus regularis growing in Saudi Arabia. Planta Med. 2013, 79, P66. [Google Scholar] [CrossRef]
- Eid, M.H.; Haddad, P.S. The antidiabetic potential of quercetin: Underlying mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar]
- Zhu, L.; Han, J.; Yuan, R.; Xue, L.; Huang, S.; Tan, M.; Guo, F.; Dong, L.; Liu, Z.; Yuan, R.; et al. Berberine ameliorates diabetic nephropathy by inhibiting TLR4/NF-κB pathway. Biol. Res. 2018, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tan, M.; Guo, F.; Dong, L.; Liu, Z.; Yuan, R.; Dongzhi, Z.; Lee, D.-S.; Wang, Y.; Li, B. Nepeta angustifolia CY Wu improves renal injury in HFD/STZ-induced diabetic nephropathy and inhibits oxidative stress-induced apoptosis of mesangial cells. J. Ethnopharmacol. 2020, 255, 112771. [Google Scholar] [CrossRef]
- Hartz, J.C.; de Ferranti, S.; Gidding, S. Hypertriglyceridemia in diabetes mellitus: Implications for pediatric care. J. Endocr. Soc. 2018, 2, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Siddiqui, W.A.; Arif, J.M.; Kuddus, M.; Shahid, S.M.A.; adnan Kausar, M. Evaluation of vegetables and fish oils for the attenuation of diabetes complications. Cell. Mol. Biol. 2019, 65, 38–45. [Google Scholar] [CrossRef]
- Pirmoghani, A.; Salehi, I.; Moradkhani, S.; Karimi, S.A.; Salehi, S. Effect of Crataegus extract supplementation on diabetes induced memory deficits and serum biochemical parameters in male rats. IBRO Rep. 2019, 7, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Park, E.; Lee, H.J.; Kim, M.O.; Cha, Y.J.; Kim, J.M.; Shin, M.J. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr. Res. Pract. 2011, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Seiva, F.R.; Chuffa, L.G.A.; Braga, C.P.; Amorim, J.P.A.; Fernandes, A.A.H. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem. Toxicol. 2012, 50, 3556–3561. [Google Scholar] [CrossRef]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2–Keap1 signaling. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2011, 1812, 719–731. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Pandey, K.B.; Abidi, A.B.; Rizvi, S.I. Markers of oxidative stress during diabetes mellitus. J. Biomark. 2013, 2013, 378790. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Liou, S.S.; Hong, T.Y.; Kao, S.T.; Liu, I.M. Gigantol has protective effects against high glucose-evoked nephrotoxicity in mouse glomerulus mesangial cells by suppressing ROS/MAPK/NF-κB signaling pathways. Molecules 2019, 24, 80. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Chen, H.; Zhou, Y. Lycium chinense leaves extract ameliorates diabetic nephropathy by suppressing hyperglycemia mediated renal oxidative stress and inflammation. Biomed. Pharmacother. 2018, 102, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, M.; Chopra, K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.M.; Mohamed, T.; Moustafa, H.; Hamdy, H.; Ahmed, R.R.; Aboud, E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed. Pharmacother. 2018, 101, 58–73. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, A.Z.; Mohany, M.; Alasmari, F.; Mothana, R.A.A.; Alshehri, A.O.A.; Alhazzani, K.; Ahmed, M.M.; Al-Rejaie, S.S. Amelioration of Diabetes-Induced Nephropathy by Loranthus regularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia. Appl. Sci. 2021, 11, 4548. https://doi.org/10.3390/app11104548
Alanazi AZ, Mohany M, Alasmari F, Mothana RAA, Alshehri AOA, Alhazzani K, Ahmed MM, Al-Rejaie SS. Amelioration of Diabetes-Induced Nephropathy by Loranthus regularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia. Applied Sciences. 2021; 11(10):4548. https://doi.org/10.3390/app11104548
Chicago/Turabian StyleAlanazi, Ahmed Z., Mohamed Mohany, Fawaz Alasmari, Ramzi A. A. Mothana, Abdulaziz O. A. Alshehri, Khalid Alhazzani, Mohammed M. Ahmed, and Salim S. Al-Rejaie. 2021. "Amelioration of Diabetes-Induced Nephropathy by Loranthus regularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia" Applied Sciences 11, no. 10: 4548. https://doi.org/10.3390/app11104548
APA StyleAlanazi, A. Z., Mohany, M., Alasmari, F., Mothana, R. A. A., Alshehri, A. O. A., Alhazzani, K., Ahmed, M. M., & Al-Rejaie, S. S. (2021). Amelioration of Diabetes-Induced Nephropathy by Loranthus regularis: Implication of Oxidative Stress, Inflammation and Hyperlipidaemia. Applied Sciences, 11(10), 4548. https://doi.org/10.3390/app11104548








