A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage
Abstract
1. Introduction
2. Feasibility Analysis of Steel Slag as Catalyst
3. Modification Method of Steel Slag
3.1. Acid Modification
3.2. Alkali Modification
3.3. High Temperature Activation Modification
3.4. Compound Modification
3.5. Physical Modification
4. Modified Steel Slag as Catalysts
4.1. Catalytic Pyrolysis
4.2. Organic Matter Degradation
4.3. Electrocatalysis
4.4. Photocalysis
4.5. Transesterification
4.6. Carbon Capture and Storage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.L.; Chen, J.X.; Jiang, J.J.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. The potential utilization of slag generated from iron- and steelmaking industries: A review. Environ. Geochem. Health 2020, 42, 1321–1334. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, X.Y.; Yan, X.; Tu, C.; Yu, Z.G. Environmental risks for application of iron and steel slags in soils in China: A review. Pedosphere 2021, 31, 28–42. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhang, Z.H.; Ju, J.T.; Zhao, F.Z. Comparative Analysis of Steel Slag Characteristics and Treatment Process. Adv. Mater. Res. 2014, 2817, 378–384. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, G.P.; Cheng, H.G.; Wang, J.S.; Wan, Y.F.; Chen, H. An overview of utilization of steel slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar]
- Huo, B.B.; Li, B.L.; Huang, S.Y.; Chen, Y.; Zhang, Y.; Banthia, N. Hydration and soundness properties of phosphoric acid modified steel slag powder. Constr. Build. Mater. 2020, 254, 119319. [Google Scholar] [CrossRef]
- Gao, J.T.; Li, S.Q.; Zhang, Y.T.; Zhang, Y.L.; Chen, P.Y.; Shen, P. Process of Re-Resourcing of Converter Slag. J. Iron Steel Res. 2011, 18, 32–39. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Dan, Z.K.; Wang, Q.Q.; He, S.P. Contact angle and adhesion of CaO-SiO2-and CaO-Al2O3-based mold slags on solid steel of various compositions. J. Mater. Res. Technol. 2020, 9, 7828–7837. [Google Scholar] [CrossRef]
- Lun, Y.; Zhou, M.; Cai, X.; Xu, F. Methods for improving volume stability of steel slag as fine aggregate. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 737–742. [Google Scholar] [CrossRef]
- Wu, W.; Meng, H.D.; Liu, L. Melting Characteristics of recycling slag in decarburization converter and its application effects. J. Iron Steel Res. 2013, 20, 7–12. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Wang, Q. Application of steel slag in cement treated aggregate base course. J. Clean. Prod. 2020, 269, 121733. [Google Scholar] [CrossRef]
- Shih, P.H.; Wu, Z.Z.; Chiang, H.L. Characteristics of bricks made from waste steel slag. Waste Manag. 2004, 24, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, B.; Lijina, V.J.; Kannan, V.; Dhevasenaa, P.R. Partial replacement of fine aggregate by steel slag and coarse aggregate by walnut shell in concrete. Mater. Today 2020, 37, 1761–1766. [Google Scholar] [CrossRef]
- Jiao, W.X.; Sha, A.M.; Liu, Z.Z.; Li, W.; Jiang, W.; Qin, W.; Hu, Y.J. Study on thermal properties of steel slag asphalt concrete for snow-melting pavement. J. Clean. Prod. 2020, 277, 123574. [Google Scholar] [CrossRef]
- Xian, W.A.; Qing-Sheng, C.A. Steel Slag as an Iron Fertilizer for Corn Growth and Soil Improvement in a Pot Experiment. Pedosphere 2006, 16, 519–524. [Google Scholar]
- Diao, J.; Zhou, W.; Ke, Z.Q.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Lundkvist, K.; Brämming, M.; Larsson, M.; Samuelsson, C. System analysis of slag utilization from vanadium recovery in an integrated steel plant. J. Clean. Prod. 2013, 47, 43–51. [Google Scholar] [CrossRef]
- Altun, I.A.; Yılmaz, I. Study on steel furnace slags with high MgO as additive in Portland cement. Cem. Concr. Res. 2002, 32, 1247–1249. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.H.; Gao, Z.L. Use of steel slag as a granular material: Volume expansion prediction and usability criteria. J. Hazard. Mater. 2010, 184, 555–560. [Google Scholar] [CrossRef]
- Lee, D.J.; Jeong, K.H.; Lee, D.H.; Lee, S.H.; Jung, M.W.; Jang, Y.N.; Jo, G.G.; Kwag, J.H.; Yi, H.K.; Park, Y.K.; et al. Kwon Catalytic pyrolysis of swine manure using CO2 and steel slag. Environ. Int. 2019, 133, 105204. [Google Scholar] [CrossRef]
- Matthaiou, V.; Oulego, P.; Frontistis, Z.; Collado, S.; Hela, D.; Konstantinou, I.K.; Diaz, M.; Mantzavinos, D. Valorization of steel slag towards a Fenton-like catalyst for the degradation of paraben by activated persulfate. Chem. Eng. J. 2019, 360, 728–739. [Google Scholar] [CrossRef]
- Ekaterina, K.; Narendra, K.; Taina, O.; Juha, L.; Christian, L.; Marcus, P.; Janne, P.; Jarno, S.; Yu, M.D. Transformation of industrial steel slag with different structure-modifying agents for synthesis of catalysts. Catal. Today 2020, 355, 768–780. [Google Scholar]
- Wang, S.N.; Yao, S.J.; Du, K.; Yuan, R.F.; Chen, H.L.; Wang, F.; Zhou, B.H. The mechanisms of conventional pollutants adsorption by modified granular steel slag. Environ. Eng. Res. 2021, 26, 86–98. [Google Scholar]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Synthesis of MIL-53(Fe)/SiO2 composite from LD slag as a novel photo-catalyst for methylene blue degradation. Chem. Eng. J. 2019, 377, 119621. [Google Scholar] [CrossRef]
- Kholkina, E.; Kumar, N.; Ohra-aho, T.; Lehtonen, J.; Lindfors, C.; Perula, M.; Peltonen, J.; Salonen, J.; Murzin, D.Y. Synthesis and Characterization of Novel Catalytic Materials Using Industrial Slag: Influence of Alkaline Pretreatment, Synthesis Time and Temperature. Top. Catal. 2019, 62, 738–751. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Liu, Y.; Zhang, C.; Wan, J.; Hu, L.; Zhou, C.Y.; Xiong, W.P. Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS/H2O2 Fenton-like system. Water Res. 2018, 138, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Sahara, R.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Hydrothermal synthesis of zeolite A using blast furnace slag. ISIJ Int. 2005, 45, 937–945. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Stabilized ladle furnace steel slag for glycerol carbonate synthesis via glycerol transesterification reaction with dimethyl carbonate. Energy Convers. Manag. 2017, 133, 477–485. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li Cai Liu, L.C.; Xu, Y.; Wang, Y.C.; Xu, D.L. A new alkali-activated steel slag-based cementitious material for photocatalytic degradation of organic pollutant from waste water. J. Hazard. Mater. 2012, 209-210, 146–150. [Google Scholar] [CrossRef]
- Cao, L.; Shen, W.; Huang, J.Q.; Yang, Y.; Zhang, D.; Huang, X.Q.; Lv, Z.J.; Ji, X.L. Process to utilize crushed steel slag in cement industry directly: Multi-phased clinker sintering technology. J. Clean. Prod. 2019, 217, 520–529. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Song, B.; Li, J.F.; Teng, X.L. Degradation of norfloxacin wastewater using kaolin/steel slag particle electrodes: Performance, mechanism and pathway. Chemosphere 2020, 270, 128652. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Feng, Y.; Liu, N.; Zhao, Y.H.; Wang, X.W.; Yang, S.M.; Yingying Long, Y.Y.; Qiu, L.P. Preparation of Sn/Mn loaded steel slag zeolite particle electrode and its removal effect on rhodamine B(RhB). J. Water Process Eng. 2020, 37, 101417. [Google Scholar] [CrossRef]
- Lian, F.; Ma, L.J.; Chou, K. Industrial research on the high-temperature modification of Basic Oxygen Furnace slag with solid waste compound. J. Clean. Prod. 2017, 143, 549–556. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, L.; Chen, K.; Xie, X.; Zhao, B.; Si, H.; Meng, G. Comparison of catalytic upgrading of biomass fast pyrolysis vapors over CaO and Fe (III)/CaO catalysts. J. Anal. Appl. Pyrolysis 2014, 108, 35–40. [Google Scholar] [CrossRef]
- Lin, X.N.; Zhang, Z.F.; Zhang, Z.J.; Sun, J.P.; Wang, Q.W.; Pittman, C.U. Catalytic fast pyrolysis of a wood-plastic composite with metal oxides as catalysts. J. Waste Manag. 2018, 79, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Lappas, A.A.; Pilavachi, P.A. In-situ upgrading of biomass pyrolysis vapors: Catalyst screening on a fifixed bed reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Y.Q.; Yang, H.P.; Wang, X.H.; Che, Q.F.; Wei Chen Chen, H.P. Catalytic fast pyrolysis of biomass: Selective deoxygenation to balance the quality and yield of bio-oil. Bioresour. Technol. 2019, 273, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.Q.; Liang, S.; Zhao, X.M.; Jia, X.P.; Peng, K.Y.; Jiang, X.C.; Qian, L. Catalytic reforming of biomass pyrolysis tar using the low-cost steel slag as catalyst. Energy 2019, 189, 116161. [Google Scholar] [CrossRef]
- Guo, F.Q.; Zhao, X.M.; Peng, K.Y.; Liang, S.; Jia, X.P.; Lin Qian, L. Catalytic reforming of biomass primary tar from pyrolysis over waste steel slag based catalysts. Int. J. Hydrogen Energy 2019, 44, 16224–16233. [Google Scholar] [CrossRef]
- Kabir, G.; Mohd Din, A.T.; Hameed, B.H. Pyrolysis of oil palm mesocarp fiber catalyzed with steel slag-derived zeolite for bio-oil production. Bioresour. Technol. 2018, 249, 42–48. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Qi, J.Y.; Feng, Y.; Li, K.; Li, X. Preparation of catalytic particle electrodes from steel slag and its performance in a three-dimensional electrochemical oxidation system. J. Ind. Eng. Chem. 2014, 20, 3672–3677. [Google Scholar] [CrossRef]
- Wang, Z.Y.; He, X.L.; Li, J.F.; Qi, J.Y.; Zhao, C.; Yang, G. Preparation of magnetic steel-slag particle electrode and its performance in a novel electrochemical reactor for oilfield wastewater advanced treatment. J. Ind. Eng. Chem. 2018, 58, 18–23. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.M.; Huang, D.L.; Lai, C.; Liu, Y.; Xu, P.; Zhang, C.; Wan, J.; Hu, L.; Xiong, W.P.; et al. Salicylic acid–methanol modified steel converter slag as heterogeneous Fenton-like catalyst for enhanced degradation of alachlor. Chem. Eng. J. 2017, 327, 686–693. [Google Scholar] [CrossRef]
- Shen, W.G.; Zhou, M.K.; Ma, W.; Hu, J.Q.; Cai, Z. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, S. Development of magnetically separable Cu catalyst supported by pre-treated steel slag. Korean J. Chem. Eng. 2019, 36, 1814–1825. [Google Scholar] [CrossRef]
- Jaehyeong, P.; Sungjun, B. Formation of Fe nanoparticles on water-washed coal fly ash for enhanced reduction of p-nitrophenol. Chemosphere 2018, 202, 733–741. [Google Scholar]
- Wei, J.Z.; Feng, Y.J.; Sun, X.J.; Liu, J.F.; Zhu, L.M. Effectiveness and pathways of electrochemical degradation of pretilachlor herbicides. J. Hazard. Mater. 2011, 189, 84–91. [Google Scholar] [CrossRef]
- Shi, C.J.; Qian, J.S. High performance cementing materials from industrial slags—A review, Resources. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Yuan, M.; Yan, F.R.; Chen, Y.G.; Luo, J.J.; Li, Z.Y. A three-dimensional electrochemical oxidation system with alpha-Fe2O3/PAC as the particle electrode for ammonium nitrogen wastewater treatment. Resources. Conserv. Recycl. 2020, 29, 195–207. [Google Scholar]
- Wang, Z.Y.; Qi, J.Y.; Feng, Y.; Li, K.; Li, X. Fabrication and electrocatalytic performance of a novel particle electrode. J. Catal. Commun. 2014, 46, 165–168. [Google Scholar] [CrossRef]
- Zhang, B.G.; Hou, Y.P.; Yu, Z.B.; Liu, Y.X.; Huang, J.; Qian, L.; Xiong, J.H. Three-dimensional electro-Fenton degradation of Rhodamine B with efficient Fe-Cu/kaolin particle electrodes: Electrode’s optimization, kinetics, influencing factors and mechanism. Sep. Purif. Technol. 2019, 210, 60–68. [Google Scholar] [CrossRef]
- Ramezani, H.; Naser, S.; Reza, S. NaY zeolite as a platform for preparation of Ag nanoparticles arrays in order to construction of H2O2 sensor. Sens. Actuators B Chem. 2017, 248, 571–579. [Google Scholar] [CrossRef]
- Kuang, P.J.; Chen, N.; Feng, C.F.; Li, M.; Dong, S.S.; Lv, L.; Zhang, J.; Hu, Z.X.; Deng, Y. Construction and optimization of an iron particle–zeolite packing electrochemical–adsorption system for the simultaneous removal of nitrate and by-products. J. Taiwan Inst. Chem. Eng. 2018, 86, 101–112. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-zeolite composites: A review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Wei, Y.; He, P.; Lv, B.; Ma, H.; Lei, Z. MIL-53(Fe)-graphene nanocomposites: Efficient visible-light photocatalysts for the selective oxidation of alcohols. Appl. Catal. B Environ. 2016, 198, 112–123. [Google Scholar] [CrossRef]
- Chen, X.E.; Cui, X.M.; Huang, X.M.; Huang, Z.C. Photocatalytic degradation of phenol with steel slag supported TiO2. New Chem. Mater. 2014, 42, 162–163+172. [Google Scholar]
- Shao, N.N.; Li, S.; Yan, F.; Su, Y.P.; Liu, F.; Zhang, Z.T. An all-in-one strategy for the adsorption of heavy metal ions and photodegradation of organic pollutants using steel slag-derived calcium silicate hydrate. J. Hazard. Mater. 2020, 382, 121120. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2008, 27, 30–39. [Google Scholar] [CrossRef]
- Okoye, P.U.; Hameed, B.H. Review on recent progress in catalytic carboxylation and acetylation of glycerol as a byproduct of biodiesel production. Renew. Sustain. Energy Rev. 2016, 74, 387–401. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Xie, W.L.; Zhao, L.L. Production of biodiesel by transesterification of soybean oil using calcium supported tin oxides as heterogeneous catalysts. Energy Convers. Manag. 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Yi, Z.; Li, S.; Tian, S.; Wang, B. Study on preparation of Ca/Al/Fe3O4 magnetic composite solid catalyst and its application in biodiesel transesterification. Fuel Process. Technol. 2012, 95, 84–89. [Google Scholar] [CrossRef]
- Ezzah-Mahmudah, S.; Lokman, I.M.; Saiman, M.I.; Yun, T.Y. Synthesis and characterization of Fe2O3/CaO derived from Anadara Granosa for methyl ester production. Energy Convers. Manag. 2016, 126, 124–131. [Google Scholar] [CrossRef]
- Saba, T.; Estephane, J.; Khoury, B.E.; Khoury, M.E.; Khazma, M.; Zakhem, H.E.; Aouad, S. Biodiesel production from refined sunflower vegetable oil over KOH/ZSM5 catalysts. Renew. Energy 2016, 90, 301–306. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, Y.B.; Cong, W.J.; Zhao, X.B.; Jidon, J.; Wei, T.; Fang, Z. Soybean biodiesel production using synergistic CaO/Ag nano catalyst: Process optimization, kinetic study, and economic evaluation. Ind. Crop. Prod. 2021, 166, 113479. [Google Scholar] [CrossRef]
- Li, H.; Liu, F.S.; Ma, X.L.; Cui, P.; Guo, M.; Li, Y.; Gao, Y.; Zhou, S.J.; Yu, M.Z. An efficient basic heterogeneous catalyst synthesis of magnetic mesoporous Fe@C support SrO for transesterification. Renew. Energy 2020, 149, 816–827. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterification of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Zheng, L.P.; Xia, S.Z.; Hou, Z.T.; Zhang, M.Y.; Hou, Z.Y. Transesterification of glycerol with dimethyl carbonate over Mg-Al hydrotalcites. Chin. J. Catal. 2014, 35, 310–318. [Google Scholar] [CrossRef]
- Zheng, L.P.; Xia, S.X.; Lu, X.Y.; Hou, Z.Y. Transesterification of glycerol with dimethyl carbonate over calcined Ca-Al hydrocalumite. Chin. J. Catal. 2015, 36, 1759–1765. [Google Scholar] [CrossRef]
- Simanjuntak, F.S.H.; Kim, T.K.; Lee, S.D.; Ahn, B.S.; Kim, H.S.; Lee, H. CaO-catalyzed synthesis of glycerol carbonate from glycerol and dimethyl carbonate: Isolation and characterization of an active Ca species. Appl. Catal. A Gen. 2011, 401, 220–225. [Google Scholar] [CrossRef]
- Liu, G.H.; Yang, J.Y.; Xu, X.R. Synthesis of hydrotalcite-type mixed oxide catalysts from waste steel slag for transesterification of glycerol and dimethyl carbonate. Sci. Rep. 2020, 10, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Skillen, N.C.; Robertson, P.K.J.; Rooney, D.W.; Morgan, K.e.v.i.n. Exploring the photocatalytic hydrogen production potential of titania doped with alumina derived from foil waste. Int. J. Hydrogen Energy 2020, 45, 34494–34502. [Google Scholar] [CrossRef]
- Martín-Gómez, J.; Hidalgo-Carrillo, J.; Montes, V.; Estévez-Toledano, R.C.; Escamilla, J.C.; Marinas, A.; Urbano, F.J. EPR and CV studies cast further light on the origin of the enhanced hydrogen production through glycerol photoreforming on CuO:TiO2 physical mixtures. J. Environ. Chem. Eng. 2021, 9, 105336. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Italiano, C.; Pino, L.; Sebastian, V.S.; Vita, A.; Goula, M.A. Hydrogen production via steam reforming of glycerol over Rh/γ-Al2O3 catalysts modified with CeO2, MgO or La2O3. Renew. Energy 2020, 162, 908–925. [Google Scholar] [CrossRef]
- Aissaoui, M.; Sahraei, O.A.Z.; Yancheshmeh, M.S.; Iliut, M.C. Development of a Fe/Mg-bearing metallurgical waste stabilized-CaO/NiO hybrid sorbent-catalyst for high purity H2 production through sorption-enhanced glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 18452–18465. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilization technologies: A review. Environ. Chem. Lett. 2020, 19, 797–849. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Walther-Darío, B.P.; Brenda, A.V.; Rosa-María, R.Z. Synthesis and evaluation in the CO2 capture process of potassium-modified lithium silicates produced from steel metallurgical slags. Mater. Res. Bull. 2021, 141, 111353. [Google Scholar]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Doucet, F.J. Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behavior in view of industrial mineral carbonation. Miner. Eng. 2009, 23, 262–269. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, K.; Sun, L.F.; Liu, C.J.; Jiang, M.; Saxén, H.; Zevenhoven, R. Towards carbon sequestration using stainless steel slag via phase modification and co-extraction of calcium and magnesium. Process Saf. Environ. Prot. 2020, 133, 73–81. [Google Scholar] [CrossRef]
- Li, H.W.; Tang, Z.G.; Li, N.; Cui, L.P.; Mao, X.Z. Mechanism and process study on steel slag enhancement for CO2 capture by seawater. Appl. Energy 2020, 276, 115515. [Google Scholar] [CrossRef]



| Type | Chemical Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CaO | MgO | Fe2O3/FeO | SiO2 | Al2O3 | MnO | SO3 | P2O5 | |
| Steel slag | 40.65 | 10.88 | 20.57 | 10.10 | 1.70 | 0.06 | 0.24 | 1.04 |
| Catalysis Field | Raw Material | Modification Method | Reference |
|---|---|---|---|
| Catalytic pyrolysis | 1. Steel slag | 1. Physical modification 2. High-temperature activation modification | [39] |
| 1. Pine sawdust 2. Steel slag | Loaded with Ni by wet impregnation and high temperature calcined | [40] | |
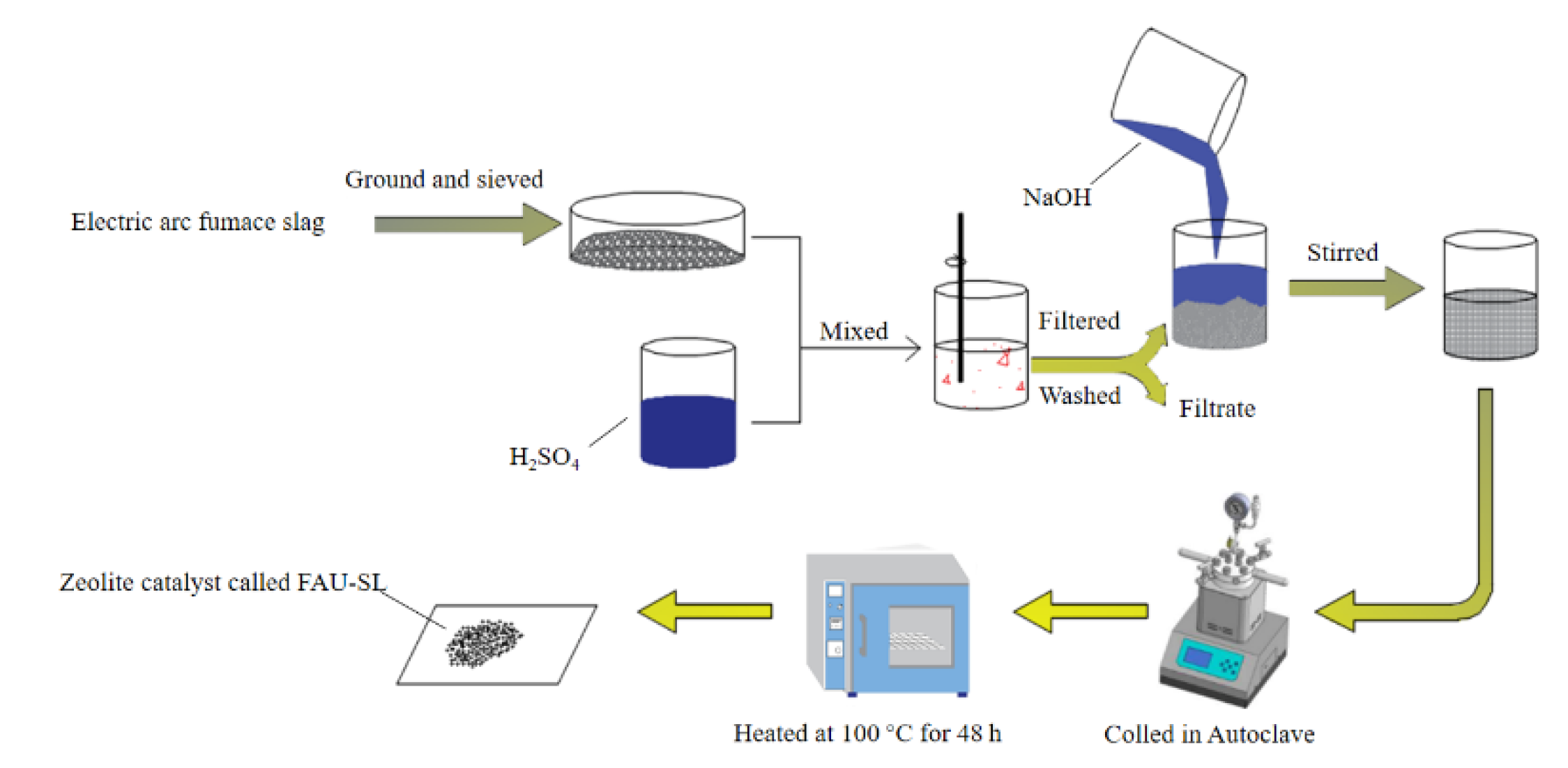
| 1. Electric arc furnace slag 2. H2SO4 and NaOH | Compound modification | [41] | |
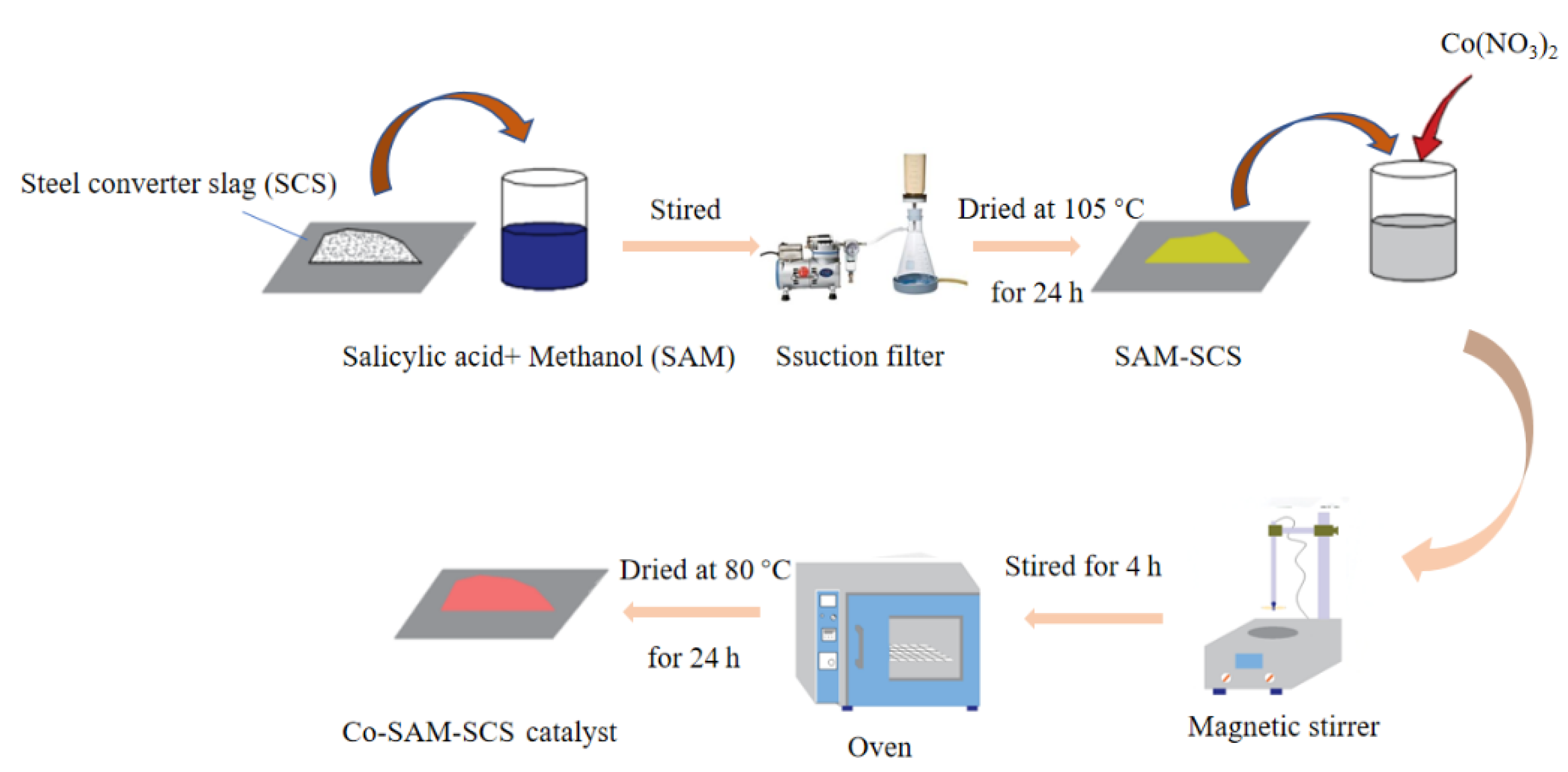
| Organic matter degradation | 1. Steel converter slag (SCS) 2. Methanol and salicylic acid (SAM) | 1. Physical modification 2. Acid modification | [44] |
| 1. Steel converter slag (SCS) 2. Methanol and salicylic acid (SAM) 3. Co(NO3)2 | 1. Physical modification 2. Acid modification | [26] | |
| 1. Steel slag 2. Cu | Compound modification | [46] | |
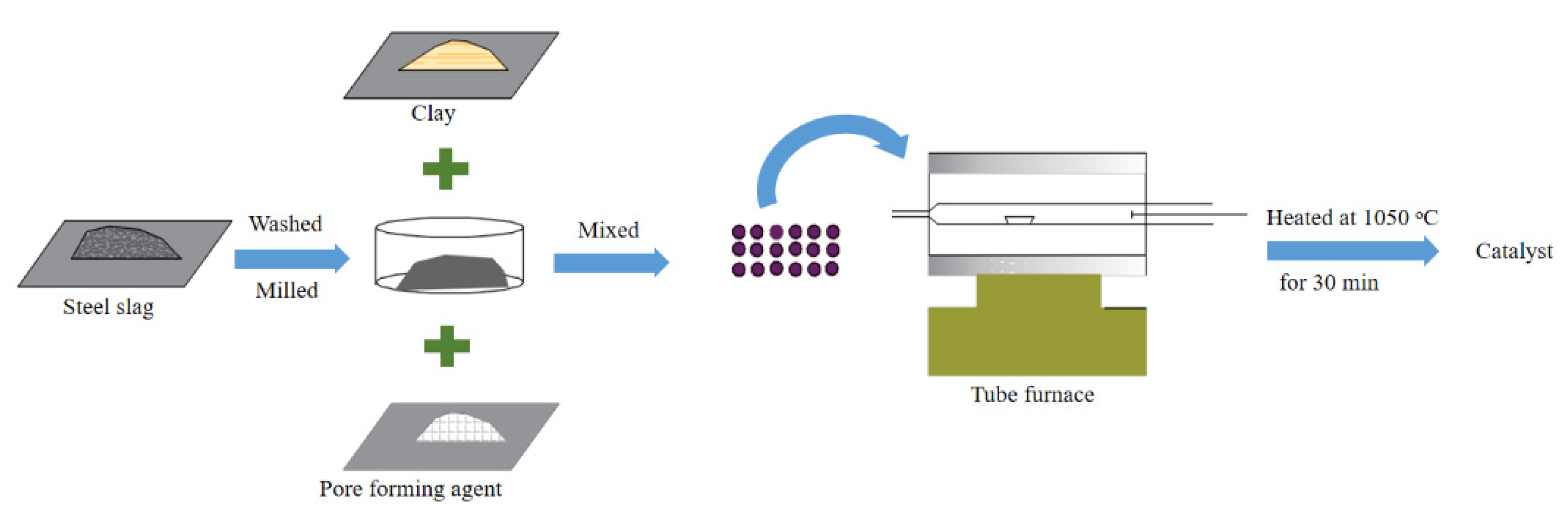
| Electrocatalysis | 1. Steel slag 2. Clay 3. Pore | 1. Physical modification 2. High-temperature activation modification | [43,51] |
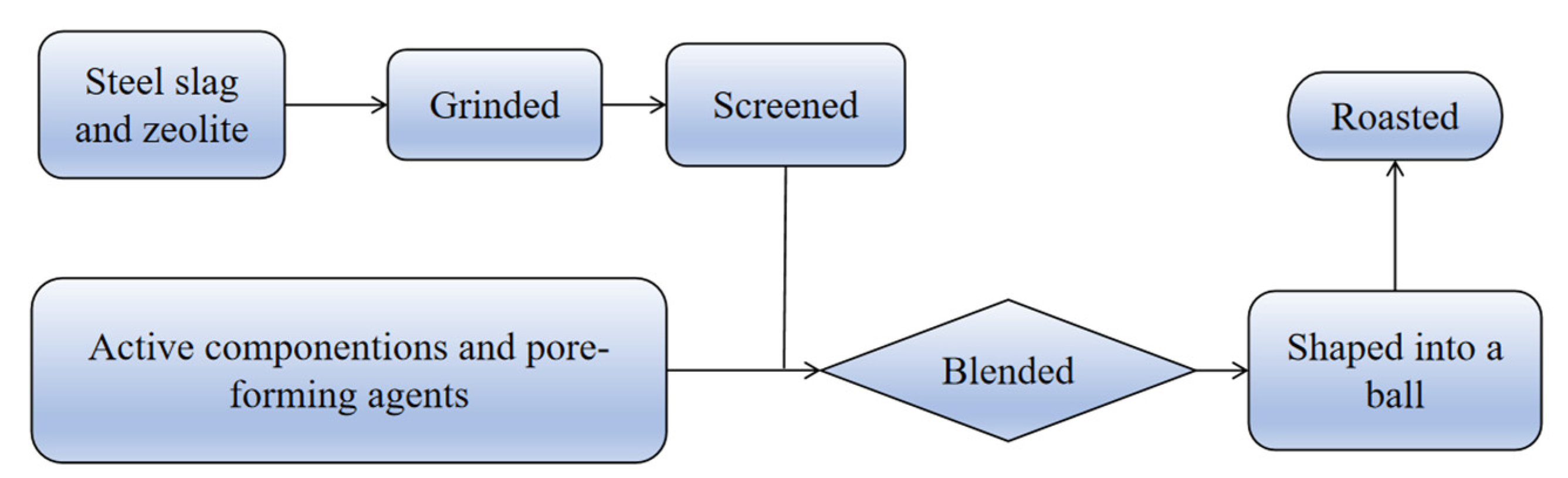
| 1. Steel slag 2. Zeolite 3. Pore-forming | Compound modification | [32] | |
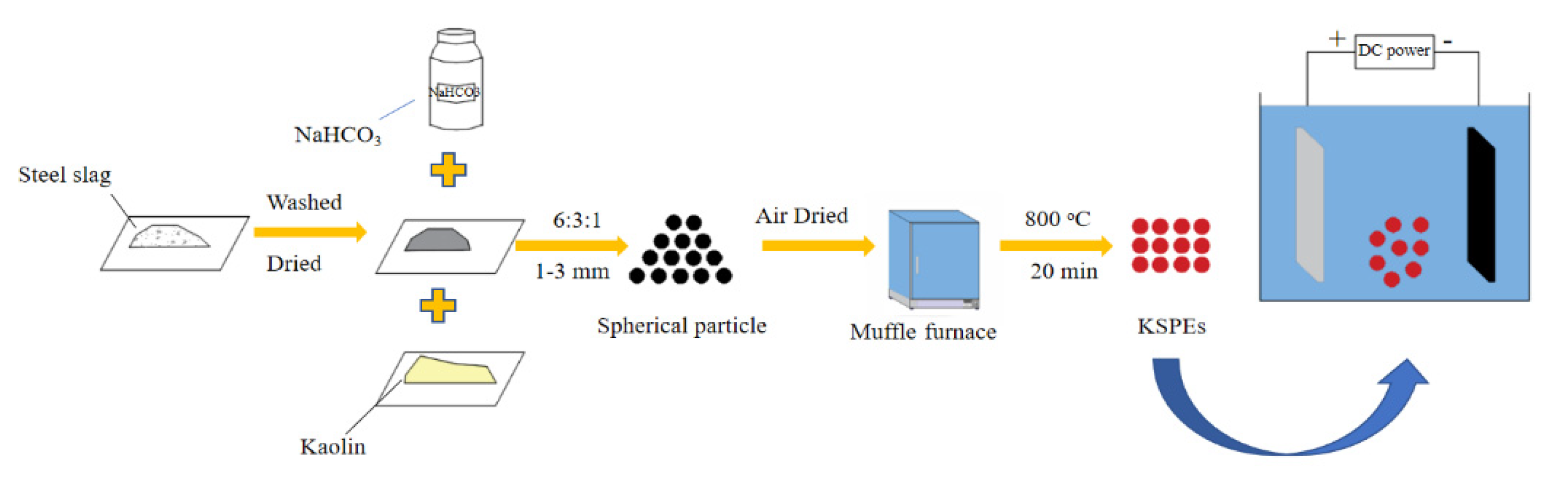
| 1. Kaolin 2. Steel slag 3. NaHCO3 | Compound modification | [31] | |
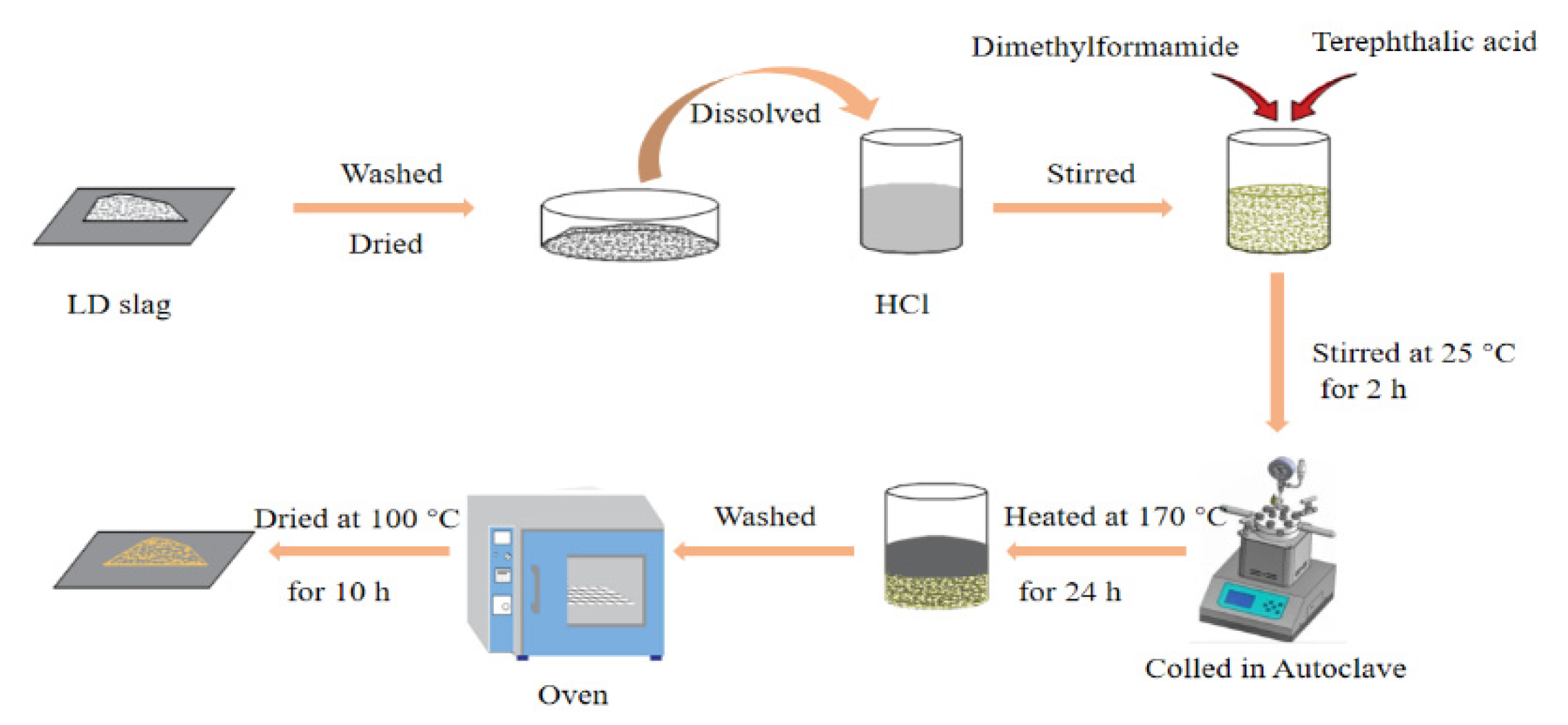
| light catalytic | 1. LD slag 2. HCl 3. Dimethylformamide 4. Terephthalic acid | Acid modification | [24] |
| 1. Steel slag 2. Industrial Titanium Liquid 3. Ammonia and H2O2 | Compound modification | [57] | |
| 1. Steel slag 2. NaOH | Alkali modification | [29] | |
| Transesterification | 1. Steel slag 2. HCl3. NaOH and Na2CO3 | 1. Acid modification 2. Alkali modification 3. High-temperature activation modification | [62] |
| 1. ladle furnace steel slag 2. NaOH | 1. Alkali modification 2. High-temperature activation modification | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.-P.; Liu, T.-J.; Cai, S.; Gao, D.; Yu, Q.; Wang, X.-M.; Wang, Y.-T.; Zeng, Y.-N.; Li, J.-G. A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage. Appl. Sci. 2021, 11, 4539. https://doi.org/10.3390/app11104539
Wang F-P, Liu T-J, Cai S, Gao D, Yu Q, Wang X-M, Wang Y-T, Zeng Y-N, Li J-G. A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage. Applied Sciences. 2021; 11(10):4539. https://doi.org/10.3390/app11104539
Chicago/Turabian StyleWang, Fu-Ping, Tian-Ji Liu, Shuang Cai, Di Gao, Qing Yu, Xiao-Man Wang, Yi-Tong Wang, Ya-Nan Zeng, and Jun-Guo Li. 2021. "A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage" Applied Sciences 11, no. 10: 4539. https://doi.org/10.3390/app11104539
APA StyleWang, F.-P., Liu, T.-J., Cai, S., Gao, D., Yu, Q., Wang, X.-M., Wang, Y.-T., Zeng, Y.-N., & Li, J.-G. (2021). A Review of Modified Steel Slag Application in Catalytic Pyrolysis, Organic Degradation, Electrocatalysis, Photocatalysis, Transesterification and Carbon Capture and Storage. Applied Sciences, 11(10), 4539. https://doi.org/10.3390/app11104539







