Heart Failure and Diabetes Mellitus: Biomarkers in Risk Stratification and Prognostication
Abstract
1. Introduction
2. Basic Underlying Mechanisms of HF Development in Diabetics
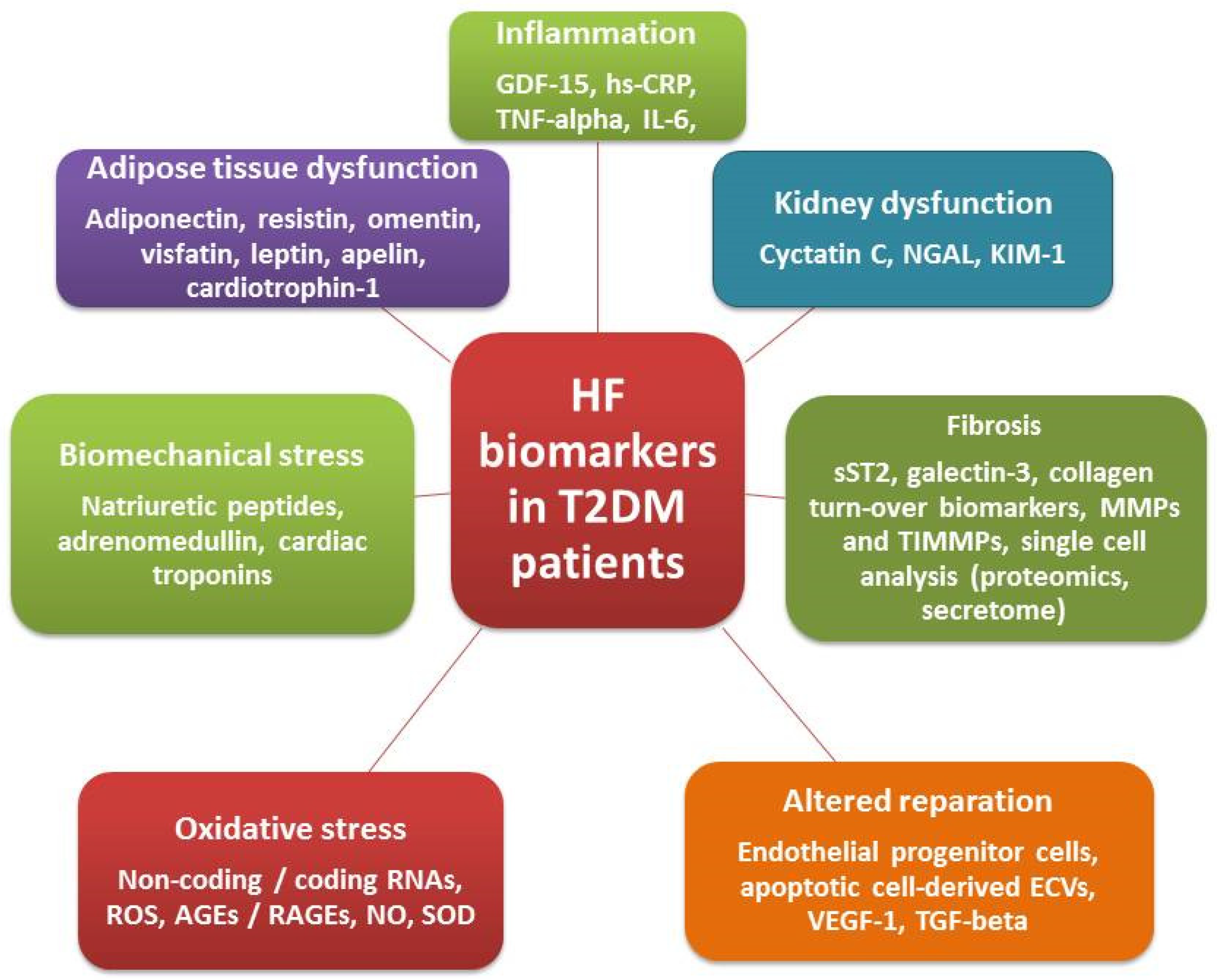
3. Biomarkers in Diabetics with Known HF
3.1. Biomechanical Stress Biomarkers
3.1.1. Natriuretic Peptides
3.1.2. Cardiac Troponins
3.1.3. Adrenomedullin
3.2. Biomarkers of Inflammation and Fibrosis
3.2.1. Growth Differential Factor-15
3.2.2. High-Sensitivity C-Reactive Protein
3.2.3. Soluble Suppression of Tumorigenicity-2
3.2.4. Galectin-3
3.2.5. Interleukin-6 Family Cytokines
3.2.6. Other Biomarkers of Fibrosis
4. Multiple Biomarker Strategies
5. Point-of-Care Clinical Diagnostics in HF
5.1. Natriuretic Peptides
5.2. Cardiac Troponins
5.3. Adrenomodullin
5.4. GDF-15
5.5. hs-CRP
5.6. ST-2
5.7. Galectin-3
5.8. IL-6
5.9. MMPs, TIMP and Collagen Degradation Products
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end-products |
| Bad | Bcl-2-associated death promoter |
| CV | cardiovascular |
| EGFR | epidermal growth factor receptor |
| ECVs | extracellular vesicles |
| FoxO1 | fork-head box-containing protein O subfamily |
| GDF | growth differential factor |
| HF | heart failure |
| HFpEF | heart failure with preserved ejection fraction |
| HFrEF | heart failure with reduced ejection fraction |
| hs-CRP | high-sensitivity C-reactive protein |
| IL | interleukin |
| JNK | c-Jun N terminal kinase |
| KIM-1 | kidney injury molecule-1 |
| MMP | matrix metalloproteinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NO | nitric oxide |
| PPAR | peroxisome proliferator-activated receptors |
| PKGIα | protein kinase G type Iα |
| RAAS | renin–angiotensin–aldosterone system |
| RAGEs | receptor for advanced glycation end-products |
| RNA | ribonucleic acid |
| ROC | reactive oxygen species |
| SNS | simpatico-adrenal nervous system |
| SOD | superoxide dismutase |
| TGF | transforming growth factor |
| TIMMP | tissue inhibitor of matrix metalloproteinase |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| β-MHC | β-isoform of myosin heavy chain |
References
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, B.Z.G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef] [PubMed]
- Thrainsdottir, I.S.; Aspelund, T.; Thorgeirsson, G.; Gudnason, V.; Hardarson, T.; Malmberg, K.; Sigurdsson, G.; Rydén, L. The Association Between Glucose Abnormalities and Heart Failure in the Population-Based Reykjavik Study. Diabetes Care 2005, 28, 612–616. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Kenny, H.C.; Abel, E.D. Heart Failure in Type 2 Diabetes Mellitus. Circ. Res. 2019, 124, 121–141. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients With Prediabetes and Known Diabetes Mellitus. Front. Cardiovasc. Med. 2020, 7, 583175. [Google Scholar] [CrossRef]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef]
- Thein, D.; Christiansen, M.N.; Mogensen, U.M.; Bundgaard, J.S.; Rørth, R.; Madelaire, C.; Fosbøl, E.L.; Schou, M.; Torp-Pedersen, C.; Gislason, G.; et al. Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: A nationwide study. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef] [PubMed]
- Marsico, F.; Gargiulo, P.; Marra, A.M.; Parente, A.; Paolillo, S. Glucose Metabolism Abnormalities in Heart Failure Patients. Heart Fail. Clin. 2019, 15, 333–340. [Google Scholar] [CrossRef]
- Berezin, A.E. Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification—A Narrative Review. Diabetes Ther. 2020, 11, 1271–1291. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, J. Diabetic cardiomyopathy: Where we are and where we are going. Korean J. Intern. Med. 2017, 32, 404–421. [Google Scholar] [CrossRef]
- Udell, J.A.; Steg, P.G.; Scirica, B.M.; Eagle, K.; Ohman, E.M.; Goto, S.; Alsheikh-Ali, A.A.; Porath, A.; Corbalan, R.; Umez-Eronini, A.A.; et al. Metabolic syndrome, diabetes mellitus, or both and cardiovascular risk in outpatients with or at risk for atherothrombosis. Eur. J. Prev. Cardiol. 2014, 21, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Diabetes, heart failure, and renal dysfunction: The vicious circles. Prog. Cardiovasc. Dis. 2019, 62, 298–302. [Google Scholar] [CrossRef]
- Cui, R.; Sun, S.Q.; Zhong, N.; Xu, M.X.; Cai, H.D.; Zhang, G.; Qu, S.; Sheng, H. The relationship between atherosclerosis and bone mineral density in patients with type 2 diabetes depends on vascular calcifications and sex. Osteoporos. Int. 2020, 31, 1135–1143. [Google Scholar] [CrossRef]
- Dillmann, W.H. Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1160–1162. [Google Scholar] [CrossRef]
- Kanamori, H.; Takemura, G.; Goto, K.; Tsujimoto, A.; Mikami, A.; Ogino, A.; Watanabe, T.; Morishita, K.; Okada, H.; Kawasaki, M.; et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy 2015, 11, 1146–1160. [Google Scholar] [CrossRef]
- Li, S.; Liang, M.; Gao, D.; Su, Q.; Laher, I. Changes in Titin and Collagen Modulate Effects of Aerobic and Resistance Exercise on Diabetic Cardiac Function. J. Cardiovasc. Transl. Res. 2019, 12, 404–414. [Google Scholar] [CrossRef]
- Hamdani, N.; Franssen, C.; Lourenço, A.; Falcão-Pires, I.; Fontoura, D.; Leite, S.; Plettig, L.; López, B.; Ottenheijm, C.A.; Becher, P.M.; et al. Myocardial Titin Hypophosphorylation Importantly Contributes to Heart Failure With Preserved Ejection Fraction in a Rat Metabolic Risk Model. Circ. Heart Fail. 2013, 6, 1239–1249. [Google Scholar] [CrossRef]
- Feng, B.; Chen, S.; Chiu, J.; George, B.; Chakrabarti, S. Regulation of cardiomyocyte hypertrophy in diabetes at the transcriptional level. Am. J. Physiol. Metab. 2008, 294, E1119–E1126. [Google Scholar] [CrossRef]
- Fender, A.C.; Pavic, G.; Drummond, G.R.; Dusting, G.J.; Ritchie, R.H. Unexpected anti-hypertrophic responses to low-level stimulation of protease-activated receptors in adult rat cardiomyocytes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.C.; Hood, S.G.; Woods, R.L.; Dusting, G.J.; Ritchie, R.H. B-type natriuretic peptide prevents acute hypertrophic responses in the diabetic rat heart: Importance of cyclic GMP. Diabetes 2003, 52, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Sundgren, N.C.; Giraud, G.D.; Schultz, J.M.; Lasarev, M.R.; Stork, P.J.S.; Thornburg, K.L. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R1481–R1489. [Google Scholar] [CrossRef] [PubMed]
- Mehrhof, F.B.; Müller, F.-U.; Bergmann, M.W.; Li, P.; Wang, Y.; Schmitz, W.; Dietz, R.; Von Harsdorf, R. In Cardiomyocyte Hypoxia, Insulin-Like Growth Factor-I-Induced Antiapoptotic Signaling Requires Phosphatidylinositol-3-OH-Kinase-Dependent and Mitogen-Activated Protein Kinase-Dependent Activation of the Transcription Factor cAMP Response Element-Binding Protein. Circulation 2001, 104, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.K.; Murohara, T. Diabetes-Related Heart Failure. Circ. J. 2014, 78, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.; González, A.; Kovacic, J.C. Myocardial Interstitial Fibrosis in Nonischemic Heart Disease, Part 3/4. J. Am. Coll. Cardiol. 2020, 75, 2204–2218. [Google Scholar] [CrossRef]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef]
- Passino, C.; Barison, A.; Vergaro, G.; Gabutti, A.; Borrelli, C.; Emdin, M.; Clerico, A. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin. Chim. Acta 2015, 443, 29–38. [Google Scholar] [CrossRef]
- González, A.; López, B.; Ravassa, S.; José, G.S.; Díez, J. The complex dynamics of myocardial interstitial fibrosis in heart failure. Focus on collagen cross-linking. Biochim. Biophys. Acta Bioenerg. 2019, 1866, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. Endogenous vascular repair system in cardiovascular disease: The role of endothelial progenitor cells. Australas. Med. J. 2019, 12, 42–48. [Google Scholar] [CrossRef]
- Battiprolu, P.K.; Hojayev, B.; Jiang, N.; Wang, Z.V.; Luo, X.; Iglewski, M.; Shelton, J.M.; Gerard, R.D.; Rothermel, B.A.; Gillette, T.G.; et al. Metabolic stress–induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J. Clin. Investig. 2012, 122, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhu, Q.; Zhang, K.; Thomas, C.; Wu, Y.; Kumar, R.; Baker, K.M.; Xu, Z.; Chen, S.; Guo, S. Activation of Foxo1 by Insulin Resistance Promotes Cardiac Dysfunction and -Myosin Heavy Chain Gene Expression. Circ. Heart Fail. 2015, 8, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, R.; Feng, Y.; Guo, J.; Chen, Y.; Zhang, Y.; Chen, T.; Wang, Y.; Huang, Y.; Li, C.-Y.; et al. Upregulation of MG53 Induces Diabetic Cardiomyopathy Through Transcriptional Activation of Peroxisome Proliferation-Activated Receptor α. Circulation 2015, 131, 795–804. [Google Scholar] [CrossRef]
- Wu, H.-K.; Zhang, Y.; Cao, C.-M.; Hu, X.; Fang, M.; Yao, Y.; Jin, L.; Chen, G.; Jiang, P.; Zhang, S.; et al. Glucose-Sensitive Myokine/Cardiokine MG53 Regulates Systemic Insulin Response and Metabolic Homeostasis. Circulation 2019, 139, 901–914. [Google Scholar] [CrossRef]
- Bugger, H.; Abel, E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014, 57, 660–671. [Google Scholar] [CrossRef]
- Zarich, S.W.; Arbuckle, B.E.; Cohen, L.R.; Roberts, M.; Nesto, R.W. Diastolic abnormalities in young asymptomatic diabetic patients assessed by pulsed Doppler echocardiography. J. Am. Coll. Cardiol. 1988, 12, 114–120. [Google Scholar] [CrossRef]
- Poirier, P.; Bogaty, P.; Philippon, F.; Garneau, C.; Fortin, C.; Dumesnil, J.-G. Preclinical diabetic cardiomyopathy: Relation of left ventricular diastolic dysfunction to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabolism 2003, 52, 1056–1061. [Google Scholar] [CrossRef]
- Ofstad, A.P.; Urheim, S.; Dalen, H.; Orvik, E.; Birkeland, K.I.; Gullestad, L.; Fagerland, M.W.; Johansen, O.E.; Aakhus, S. Identification of a definite diabetic cardiomyopathy in type 2 diabetes by comprehensive echocardiographic evaluation: A cross-sectional comparison with non-diabetic weight-matched controls. J. Diabetes 2015, 7, 779–790. [Google Scholar] [CrossRef]
- Kocabaş, U.; Yılmaz, Ö.; Kurtoğlu, V. Diabetic cardiomyopathy: Acute and reversible left ventricular systolic dysfunction due to cardiotoxicity of hyperglycaemic hyperosmolar state-a case report. Eur. Heart J. Case Rep. 2019, 3, ytz049. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Ceylan-Isik, A.F.; Fang, C.X.; Yang, X.; Li, S.-Y.; Sreejayan, N.; Privratsky, J.R.; Ren, J. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of Ca2+ cycling proteins, NADPH oxidase, poly (ADP-Ribose) polymerase and myosin heavy chain isozyme. Free Radic. Biol. Med. 2006, 40, 1419–1429. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Deng, L.; Wang, D.; Li, N.; Chen, X.; Cheng, X.; Yuan, J.; Gao, X.; Liao, M.; Wang, M.; et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: Initiated by hypoxia inducible factor 1α, presented by exosomes. J. Mol. Cell. Cardiol. 2012, 53, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.A.; Guo, S. Insulin receptor substrate signaling controls cardiac energy metabolism and heart failure. J. Endocrinol. 2017, 233, R131–R143. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Extracellular Endothelial Cell-Derived Vesicles: Emerging Role in Cardiac and Vascular Remodeling in Heart Failure. Front. Cardiovasc. Med. 2020, 7, 47. [Google Scholar] [CrossRef]
- Sarhene, M.; Wang, Y.; Wei, J.; Huang, Y.; Li, M.; Li, L.; Acheampong, E.; Zhengcan, Z.; Xiaoyan, Q.; Yunsheng, X.; et al. Biomarkers in heart failure: The past, current and future. Heart Fail. Rev. 2019, 24, 867–903. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L.; Kiernan, M.S.; et al. Role of Biomarkers for the Prevention, Assessment, and Management of Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Januzzi, J.L. Established and Emerging Roles of Biomarkers in Heart Failure. Circ. Res. 2018, 123, 614–629. [Google Scholar] [CrossRef]
- Butler, J.; Packer, M.; Greene, S.J.; Fiuzat, M.; Anker, S.D.; Anstrom, K.J.; Carson, P.E.; Cooper, L.B.; Fonarow, G.C.; Hernandez, A.F.; et al. Heart Failure End Points in Cardiovascular Outcome Trials of Sodium Glucose Cotransporter 2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation 2019, 140, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Suthahar, N.; Meijers, W.C.; Brouwers, F.P.; Heerspink, H.J.; Gansevoort, R.T.; Van Der Harst, P.; Bakker, S.J.; De Boer, R.A. Heart failure and inflammation-related biomarkers as predictors of new-onset diabetes in the general population. Int. J. Cardiol. 2018, 250, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, H.; Gao, C. Potential biomarkers for heart failure. J. Cell. Physiol. 2019, 234, 9467–9474. [Google Scholar] [CrossRef]
- Catalina, M.O.-S.; Redondo, P.C.; Granados, M.P.; Cantonero, C.; Sanchez-Collado, J.; Albarran, L.; Lopez, J.J. New Insights into Adipokines as Potential Biomarkers for Type-2 Diabetes Mellitus. Curr. Med. Chem. 2019, 26, 4119–4144. [Google Scholar] [CrossRef]
- Berezin, A.E. Circulating Biomarkers in Heart Failure. Adv. Exp. Med. Biol. 2018, 1067, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; On behalf of Master Program Students on Drug Development for Heart Failure; Monaco, I.; Brunetti, N.D.; Di Biase, M.; Metra, M.; Nodari, S.; Butler, J.; Gheorghiade, M. Redefining biomarkers in heart failure. Heart Fail. Rev. 2018, 23, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In Handbook of Experimental Pharmacology; Schmidt, H.H.H.W., Hofmann, F., Stasch, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 191, pp. 341–366. ISBN 9783540689607. [Google Scholar]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; De Bold, M.K.; De Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure with Reduced Ejection Fraction. JAMA 2020, 324, 488. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Claggett, B.L.; Prescott, M.F.; McMurray, J.J.; Packer, M.; Rouleau, J.L.; Swedberg, K.; Desai, A.S.; Gong, J.; Shi, V.C.; et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2425–2436. [Google Scholar] [CrossRef]
- Nguyen, K.; Fan, W.; Bertoni, A.; Budoff, M.J.; Defilippi, C.; Lombardo, D.; Maisel, A.; Szklo, M.; Wong, N.D. N-terminal Pro B-type Natriuretic Peptide and High-sensitivity Cardiac Troponin as Markers for Heart Failure and Cardiovascular Disease Risks According to Glucose Status (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2020, 125, 1194–1201. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-W.; Fox, S.; Mojaver, S.; Maisel, A.S. Using biomarkers to guide heart failure management. Expert Rev. Cardiovasc. Ther. 2017, 15, 729–741. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure With Reduced Ejection Fraction. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Jorsal, A.; Tougaard, R.S.; Rasmussen, J.J.; Schou, M.; Videbaek, L.; Gustafsson, I.; Faber, J.; Flyvbjerg, A.; Wiggers, H.; et al. The impact of the glucagon-like peptide-1 receptor agonist liraglutide on natriuretic peptides in heart failure patients with reduced ejection fraction with and without type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 2141–2150. [Google Scholar] [CrossRef] [PubMed]
- Moro, C. Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity. Expert Opin. Ther. Targets 2016, 20, 1445–1452. [Google Scholar] [CrossRef]
- Wolsk, E.; Claggett, B.; Pfeffer, M.A.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Lawson, F.C.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.V.; et al. Role of B-Type Natriuretic Peptide and N-Terminal Prohormone BNP as Predictors of Cardiovascular Morbidity and Mortality in Patients With a Recent Coronary Event and Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2017, 6, e004743. [Google Scholar] [CrossRef]
- Scheen, A.J. Cardiovascular Effects of New Oral Glucose-Lowering Agents. Circ. Res. 2018, 122, 1439–1459. [Google Scholar] [CrossRef]
- Jarolim, P.; White, W.B.; Cannon, C.P.; Gao, Q.; Morrow, D.A. Serial Measurement of Natriuretic Peptides and Cardiovascular Outcomes in Patients With Type 2 Diabetes in the EXAMINE Trial. Diabetes Care 2018, 41, 1510–1515. [Google Scholar] [CrossRef]
- Shyangdan, D.S.; Uthman, O.; Waugh, N. SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. BMJ Open 2016, 6, e009417. [Google Scholar] [CrossRef]
- Buckley, L.F.; Canada, J.M.; Del Buono, M.G.; Carbone, S.; Trankle, C.R.; Billingsley, H.; Kadariya, D.; Arena, R.; Van Tassell, B.W.; Abbate, A. Low NT-proBNP levels in overweight and obese patients do not rule out a diagnosis of heart failure with preserved ejection fraction. ESC Heart Fail. 2018, 5, 372–378. [Google Scholar] [CrossRef]
- Martinsson, A.; Oest, P.; Wiborg, M.-B.; Reitan, Ö.; Smith, J.G. Longitudinal evaluation of ventricular ejection fraction and NT-proBNP across heart failure subgroups. Scand. Cardiovasc. J. 2018, 52, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Lazo, M.; Chen, Y.; Shen, L.; Rubin, J.; McEvoy, J.W.; Hoogeveen, R.C.; Sharrett, A.R.; Ballantyne, C.M.; Coresh, J. Diabetes Mellitus, Prediabetes, and Incidence of Subclinical Myocardial Damage. Circulation 2014, 130, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Van Bockel, E.A.; Tulleken, J.E.; Ligtenberg, J.J.; van der Werf, T.S.; Aarts, L.P.; Zijlstra, J.G. De betekenis van een verhoogde troponinewaarde zonder acute ischemische hartziekte [The significance of elevated troponin levels in the absence of acute cardiac ischaemia]. Ned. Tijdschr. Geneeskd. 2005, 149, 1879–1883. [Google Scholar]
- Rørth, R.; Jhund, P.S.; Kristensen, S.L.; Desai, A.S.; Køber, L.; Rouleau, J.L.; Solomon, S.D.; Swedberg, K.; Zile, M.R.; Packer, M.; et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur. J. Heart Fail. 2019, 21, 40–49. [Google Scholar] [CrossRef]
- Ali, D.; Callan, N.; Ennis, S.; Powell, R.; McGuire, S.; McGregor, G.; OWeickert, M.; AMiller, M.; Cappuccio, F.P.; Banerjee, P. Heart failure with preserved ejection fraction (HFpEF) pathophysiology study (IDENTIFY-HF): Does increased arterial stiffness associate with HFpEF, in addition to ageing and vascular effects of comorbidities? Rationale and design. BMJ Open 2019, 9, e027984. [Google Scholar]
- Sharma, A.; Vaduganathan, M.; Ferreira, J.P.; Liu, Y.; Bakris, G.L.; Cannon, C.P.; White, W.B.; Zannad, F. Clinical and Biomarker Predictors of Expanded Heart Failure Outcomes in Patients With Type 2 Diabetes Mellitus After a Recent Acute Coronary Syndrome: Insights From the EXAMINE Trial. J. Am. Heart Assoc. 2020, 9, e012797. [Google Scholar] [CrossRef]
- Rezende, P.C.; Everett, B.M.; Brooks, M.M.; Vlachos, H.; Orchard, T.J.; Frye, R.L.; Bhatt, D.L.; Hlatky, M.A. Hypoglycemia and Elevated Troponin in Patients With Diabetes and Coronary Artery Disease. J. Am. Coll. Cardiol. 2018, 72, 1778–1786. [Google Scholar] [CrossRef]
- Willeit, P.; Welsh, P.; Evans, J.D.; Tschiderer, L.; Boachie, C.; Jukema, J.W.; Ford, I.; Trompet, S.; Stott, D.J.; Kearney, P.M.; et al. High-Sensitivity Cardiac Troponin Concentration and Risk of First-Ever Cardiovascular Outcomes in 154,052 Participants. J. Am. Coll. Cardiol. 2017, 70, 558–568. [Google Scholar] [CrossRef]
- Jia, X.; Sun, W.; Hoogeveen, R.C.; Nambi, V.; Matsushita, K.; Folsom, A.R.; Heiss, G.; Couper, D.J.; Solomon, S.D.; Boerwinkle, E.; et al. High-Sensitivity Troponin I and Incident Coronary Events, Stroke, Heart Failure Hospitalization, and Mortality in the ARIC Study. Circulation 2019, 139, 2642–2653. [Google Scholar] [CrossRef]
- Jougasaki, M.; Burnett, J.C. Adrenomedullin: Potential in physiology and pathophysiology. Life Sci. 2000, 66, 855–872. [Google Scholar] [CrossRef]
- Peacock, W.F. Novel biomarkers in acute heart failure: MR-pro-adrenomedullin. Clin. Chem. Lab. Med. 2014, 52, 1433–1435. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Nowak, R.M.; Peacock, W.F.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.; Richards, M.; Clopton, P.; et al. Midregion Prohormone Adrenomedullin and Prognosis in Patients Presenting with Acute Dyspnea. J. Am. Coll. Cardiol. 2011, 58, 1057–1067. [Google Scholar] [CrossRef]
- Shah, R.V.; Truong, Q.A.; Gaggin, H.K.; Pfannkuche, J.; Hartmann, O.; Januzzi, J.L. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur. Heart J. 2012, 33, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Montes, M.D.L.A.; Debray, T.P.A.; Taylor, K.S.; Speich, B.; Jones, N.; Collins, G.S.; Hobbs, F.D.R.R.; Magriplis, E.; Maruri-Aguilar, H.; Moons, K.G.M.; et al. UMBRELLA protocol: Systematic reviews of multivariable biomarker prognostic models developed to predict clinical outcomes in patients with heart failure. Diagn. Progn. Res. 2020, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhong, J.; Ling, Y.; Zhang, Y.; Lin, W.; Tang, L.; Liu, J.; Li, S. Diagnostic value of novel biomarkers for heart failure. Herz 2018, 45, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Holmager, P.; Schou, M.; Egstrup, M.; Gustafsson, I.; Goetze, J.P.; Gustafsson, F.; Klausen, T.W.; Faber, J.; Kistorp, C. The Influence of Diabetes Mellitus on Midregional Proadrenomedullin Concentrations and Prognostic Value in Heart Failure Outpatients. J. Card. Fail. 2015, 21, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, F.S.; Kistorp, C.; Kümler, T.; Hassager, C.; Tønder, N.; Iversen, K.; Kamstrup, P.R.; Faber, J.; Køber, L.; Schou, M. Early Stages of Obesity-related Heart Failure Are Associated with Natriuretic Peptide Deficiency and an Overall Lack of Neurohormonal Activation: The Copenhagen Heart Failure Risk Study. Glob. Heart 2020, 15, 25. [Google Scholar] [CrossRef]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef]
- Kempf, T.; Guba-Quint, A.; Torgerson, J.; Magnone, M.C.; Haefliger, C.; Bobadilla, M.; Wollert, K.C. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: Results from the XENDOS trial. Eur. J. Endocrinol. 2012, 167, 671–678. [Google Scholar] [CrossRef]
- Berezin, A.E. Diabetes mellitus related biomarker: The predictive role of growth-differentiation factor-15. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S154–S157. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Daya, N.; Matsushita, K.; Wang, D.; Ndumele, C.E.; Al Rifai, M.; Hoogeveen, R.C.; Ballantyne, C.M.; Selvin, E. Growth Differentiation Factor (GDF)-15 and Cardiometabolic Outcomes among Older Adults: The Atherosclerosis Risk in Communities Study. Clin. Chem. 2021, 67, 653–661. [Google Scholar] [CrossRef]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic Utility of Growth Differentiation Factor-15 in Patients With Chronic Heart Failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF-15, Galectin 3, Soluble ST2, and Risk of Mortality and Cardiovascular Events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef]
- Moutachakkir, M.; Hanchi, A.L.; Baraou, A.; Boukhira, A.; Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin. 2017, 75, 225–229. [Google Scholar] [CrossRef]
- Liu, P.; Li, G.; Chen, Y.; Luo, H.; Huang, D.; Wang, Y.; Ge, S.; Zhang, J.; Xia, N. Chemiluminescent immunoassay for high-sensitivity C-reactive protein. Sheng Wu Gong Cheng Xue Bao 2010, 26, 1150–1156. [Google Scholar] [PubMed]
- Bagherniya, M.; Khayyatzadeh, S.S.; Bakavoli, A.R.H.; Ferns, G.A.; Ebrahimi, M.; Safarian, M.; Nematy, M.; Ghayour-Mobarhan, M. Serum high-sensitive C-reactive protein is associated with dietary intakes in diabetic patients with and without hypertension: A cross-sectional study. Ann. Clin. Biochem. Int. J. Lab. Med. 2017, 55, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Y.; Yuan, J.-M.; Koh, W.-P. High-sensitive C-reactive protein and risk of incident type 2 diabetes: A case–control study nested within the Singapore Chinese Health Study. BMC Endocr. Disord. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, A.; Standl, E.; Strotmann, H.-J.; Schulze, J.; Hohberg, C.; Lübben, G.; Pahler, S.; Schöndorf, T.; Forst, T. Association of high-sensitive C-reactive protein with advanced stage? Cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus. Clin. Chem. Lab. Med. 2006, 44, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Noordam, R.; Oudt, C.; Bos, M.; Smit, R.; van Heemst, D. High-sensitivity C-reactive protein, low-grade systemic inflammation and type 2 diabetes mellitus: A two-sample Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 795–802. [Google Scholar] [CrossRef]
- Aryan, Z.; Ghajar, A.; Faghihi-Kashani, S.; Afarideh, M.; Nakhjavani, M.; Esteghamati, A. Baseline High-Sensitivity C-Reactive Protein Predicts Macrovascular and Microvascular Complications of Type 2 Diabetes: A Population-Based Study. Ann. Nutr. Metab. 2018, 72, 287–295. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Wang, W.; Gao, C.; Yu, C.; Liu, S.; Wu, J. An Elevated High-Sensitivity C-Reactive Protein Level Is Associated with Unfavorable Functional Outcomes of Small-Artery Occlusion in Patients without Diabetes. Eur. Neurol. 2017, 78, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Gao, Y.; Hou, D.; Wang, Y.; Yu, C.; Wang, W.; Liu, S.; Gao, C.; Tong, X.; Wu, J. Association between hs-CRP Levels and the Outcomes of Patients with Small-Artery Occlusion. Front. Aging Neurosci. 2016, 8, 191. [Google Scholar] [CrossRef][Green Version]
- Yang, Q.-Q.; Shao, D.; Li, J.; Yang, C.-L.; Fan, M.-H.; Cao, F.-L. Positive Association between Serum Levels of High-Sensitivity C-Reactive Protein and Depression/Anxiety in Female, but Not Male, Patients With Type 2 Diabetes Mellitus. Biol. Res. Nurs. 2019, 22, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. High-sensitivity C-reactive protein and atherosclerotic disease: From improved risk prediction to risk-guided therapy. Int. J. Cardiol. 2013, 168, 5126–5134. [Google Scholar] [CrossRef]
- Dubrock, H.M.; AbouEzzeddine, O.F.; Redfield, M.M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS ONE 2018, 13, e0201836. [Google Scholar] [CrossRef] [PubMed]
- Al Aseri, Z.; Habib, S.S.; Marzouk, A. Predictive value of high sensitivity C-reactive protein on progression to heart failure occurring after the first myocardial infarction. Vasc. Health. Risk Manag. 2019, 15, 221–227. [Google Scholar] [CrossRef]
- Kang, S.; Fan, L.-Y.; Chen, M.; Li, J.; Liu, Z.-M. Relationship of high-sensitivity C-reactive protein concentrations and systolic heart failure. Curr. Vasc. Pharmacol. 2017, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.; Pereira, J.; Ribeiro, A.; Ferreira-Coimbra, J.; Barroso, I.; Guimarães, J.-T.; Leite-Moreira, A.; Bettencourt, P. C-reactive protein decrease associates with mortality reduction only in heart failure with preserved ejection fraction. J. Cardiovasc. Med. 2019, 20, 23–29. [Google Scholar] [CrossRef]
- Araújo, J.P.; Lourenço, P.; Azevedo, A.; Friões, F.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Prognostic Value of High-Sensitivity C-Reactive Protein in Heart Failure: A Systematic Review. J. Card. Fail. 2009, 15, 256–266. [Google Scholar] [CrossRef]
- Lotierzo, M.; Dupuy, A.M.; Kalmanovich, E.; Roubille, F.; Cristol, J.P. sST2 as a value-added biomarker in heart failure. Clin. Chim. Acta 2020, 501, 120–130. [Google Scholar] [CrossRef]
- Figal, D.A.P.; Lax, A.; Perez-Martinez, M.T.; Asensio-Lopez, M.D.C.; Sánchez-Más, J.; Network, O.B.O.G. Clinical relevance of sST2 in cardiac diseases. Clin. Chem. Lab. Med. 2016, 54, 29–35. [Google Scholar] [CrossRef]
- Aimo, A.; Januzzi, J.L.; Bayes-Genis, A.; Vergaro, G.; Sciarrone, P.; Passino, C.; Emdin, M. Clinical and Prognostic Significance of sST2 in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Vergaro, G.; Bayes-Genis, A.; Lupón, J.; Latini, R.; Meessen, J.; Anand, I.S.; Cohn, J.N.; Gravning, J.; et al. sST2 Predicts Outcome in Chronic Heart Failure Beyond NT−proBNP and High-Sensitivity Troponin T. J. Am. Coll. Cardiol. 2018, 72, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Barutaut, M.; Fournier, P.; Peacock, W.F.; Evaristi, M.-F.; Dambrin, C.; Caubère, C.; Koukoui, F.; Galinier, M.; Smih, F.; Rouet, P. sST2 adds to the prognostic value of Gal-3 and BNP in chronic heart failure. Acta Cardiol. 2020, 75, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Plawecki, M.; Morena, M.; Kuster, N.; Chenine, L.; Leray-Moragues, H.; Jover, B.; Fesler, P.; Lotierzo, M.; Dupuy, A.-M.; Klouche, K.; et al. sST2 as a New Biomarker of Chronic Kidney Disease-Induced Cardiac Remodeling: Impact on Risk Prediction. Mediat. Inflamm. 2018, 2018, 3952526. [Google Scholar] [CrossRef]
- Huang, A.; Qi, X.; Hou, W.; Qi, Y.; Zhao, N.; Liu, K. Prognostic value of sST2 and NT-proBNP at admission in heart failure with preserved, mid-ranged and reduced ejection fraction. Acta Cardiol. 2017, 73, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Lichtenauer, M.; Jirak, P.; Wernly, B.; Paar, V.; Rohm, I.; Jung, C.; Schernthaner, C.; Kraus, J.; Motloch, L.J.; Yilmaz, A.; et al. A comparative analysis of novel cardiovascular biomarkers in patients with chronic heart failure. Eur. J. Intern. Med. 2017, 44, 31–38. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy. Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.; Ho, J.E.; Liu, F.-T.; De Boer, R.A. Galectin-3 Activation and Inhibition in Heart Failure and Cardiovascular Disease: An Update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef]
- Funasaka, T.; Raz, A.; Nangia-Makker, P. Galectin-3 in angiogenesis and metastasis. Glycobiology 2014, 24, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Brugnolaro, C.; Ibarrola, J.; Ravassa, S.; Buonafine, M.; López, B.; Fernández-Celis, A.; Querejeta, R.; Santamaria, E.; Fernández-Irigoyen, J.; et al. CT-1 (Cardiotrophin-1)-Gal-3 (Galectin-3) Axis in Cardiac Fibrosis and Inflammation. Hypertension 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Vora, A.; De Lemos, J.A.; Ayers, C.; Grodin, J.L.; Lingvay, I. Association of Galectin-3 With Diabetes Mellitus in the Dallas Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 4449–4458. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Cheung, C.-L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia 2018, 61, 1212–1219. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Cheung, C.-L.; Lee, A.C.; Lam, J.K.; Wong, Y.; Shiu, S.W. Galectin-3 and risk of cardiovascular events and all-cause mortality in type 2 diabetes. Diabetes Metab. Res. Rev. 2018, 35, e3093. [Google Scholar] [CrossRef]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in Heart Failure. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Gabius, H.-J. Galectin-3: Is this member of a large family of multifunctional lectins (already) a therapeutic target? Expert Opin. Ther. Targets 2019, 23, 819–828. [Google Scholar] [CrossRef]
- Pugliese, G.; Iacobini, C.; Ricci, C.; Fantauzzi, C.B.; Menini, S. Galectin-3 in diabetic patients. Clin. Chem. Lab. Med. 2014, 52, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Qian, X.; Chen, G.; Song, X. The role of galectin-3 in heart failure and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2019, 46, 197–203. [Google Scholar] [CrossRef]
- Oikonomou, E.; Karlis, D.; Tsalamadris, S.; Siasos, G.; Chrysohoou, C.; Vogiatzi, G.; Dimitropoulos, S.; Charalambous, G.; Kouskouni, E.; Tousoulis, D. Galectin-3 and Arterial Stiffness in Patients with Heart Failure: A Pilot Study. Curr. Vasc. Pharmacol. 2019, 17, 396–400. [Google Scholar] [CrossRef]
- Gaggin, H.K.; Januzzi, J.L. Biomarkers and diagnostics in heart failure. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Edelmann, F.; Cohen-Solal, A.; Mamas, M.A.; Maisel, A.; Pieske, B. Galectin-3 in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2013, 15, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Trippel, T.D.; Mende, M.; Düngen, H.; Hashemi, D.; Petutschnigg, J.; Nolte, K.; Herrmann-Lingen, C.; Binder, L.; Hasenfuss, G.; Pieske, B.; et al. The diagnostic and prognostic value of galectin-3 in patients at risk for heart failure with preserved ejection fraction: Results from the DIAST-CHF study. ESC Heart Fail. 2021, 8, 829–841. [Google Scholar] [CrossRef]
- Gocer, H.; Günday, M.; Ünal, M. Plasma galectin-3 as a biomarker for clinical staging of heart failure: A cross-sectional evaluation of 100 cases. Clin. Ther 2019, 170, e267–e271. [Google Scholar]
- Chen, Y.-S.; Gi, W.-T.; Liao, T.-Y.; Lee, M.-T.G.; Lee, S.-H.; Hsu, W.-T.; Chang, S.-S.; Lee, C.-C. Using the galectin-3 test to predict mortality in heart failure patients: A systematic review and meta-analysis. Biomarkers Med. 2016, 10, 329–342. [Google Scholar] [CrossRef]
- Srivatsan, V.; George, M.; Shanmugam, E. Utility of galectin-3 as a prognostic biomarker in heart failure: Where do we stand? Eur. J. Prev. Cardiol. 2015, 22, 1096–1110. [Google Scholar] [CrossRef]
- Mueller, T.; Gegenhuber, A.; Leitner, I.; Poelz, W.; Haltmayer, M.; Dieplinger, B. Diagnostic and prognostic accuracy of galectin-3 and soluble ST2 for acute heart failure. Clin. Chim. Acta 2016, 463, 158–164. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Stejskal, D.; Ruzicka, V. Cardiotrophin-1 Review. Biomed. Pap. 2008, 152, 9–19. [Google Scholar] [CrossRef]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, A.; Fülöp, G.Á.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2021, 117, 495–507. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Araki, S.; Maegawa, H. Sodium–glucose cotransporter 2 inhibitors represent a paradigm shift in the prevention of heart failure in type 2 diabetes patients. J. Diabetes Investig. 2021, 12, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Petrie, M.C.; McMurray, J.J.; Sattar, N. How Do SGLT2 (Sodium-Glucose Cotransporter 2) Inhibitors and GLP-1 (Glucagon-Like Peptide-1) Receptor Agonists Reduce Cardiovascular Outcomes? Arter. Thromb. Vasc. Biol. 2020, 40, 506–522. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Ferreira, S.M.; Ferreira, M.J.; Falcão-Pires, I. Circulating Biomarkers of Collagen Metabolism and Prognosis of Heart Failure with Reduced or Mid-Range Ejection Fraction. Curr. Pharm. Des. 2017, 23, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Jhund, P.S.; Baicu, C.F.; Claggett, B.L.; Pieske, B.; Voors, A.A.; Prescott, M.F.; Shi, V.; Lefkowitz, M.; McMurray, J.J.; et al. Plasma Biomarkers Reflecting Profibrotic Processes in Heart Failure With a Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002551. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Khammy, O.; Mariani, J.; Maeder, M.T. Relationship of circulating matrix biomarkers to myocardial matrix metabolism in advanced heart failure. Eur. J. Heart Fail. 2013, 15, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.M.; Kuster, N.; Curinier, C.; Huet, F.; Plawecki, M.; Solecki, K.; Roubille, F.; Cristol, J.P. Exploring collagen remodeling and regulation as prognosis biomarkers in stable heart failure. Clin. Chim. Acta 2019, 490, 167–171. [Google Scholar] [CrossRef]
- Sanchis, L.; Andrea, R.; Falces, C.; Llopis, J.; Morales-Ruiz, M.; López-Sobrino, T.; Pérez-Villa, F.; Sitges, M.; Sabate, M.; Brugada, J. Prognosis of new-onset heart failure outpatients and collagen biomarkers. Eur. J. Clin. Investig. 2015, 45, 842–849. [Google Scholar] [CrossRef]
- Duprez, D.A.; Gross, M.D.; Kizer, J.R.; Ix, J.H.; Hundley, W.G.; Jacobs, D.R. Predictive Value of Collagen Biomarkers for Heart Failure With and Without Preserved Ejection Fraction: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Heart Assoc. 2018, 7, e007885. [Google Scholar] [CrossRef]
- Yang, J.; Savvatis, K.; Kang, J.S.; Fan, P.; Zhong, H.; Schwartz, K.; Barry, V.; Mikels-Vigdal, A.; Karpinski, S.; Kornyeyev, D.; et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat. Commun. 2016, 7, 13710. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Verdonschot, J.; Collier, T.; Wang, P.; Pizard, A.; Bär, C.; Björkman, J.; Boccanelli, A.; Butler, J.; Clark, A.; et al. Proteomic Bioprofiles and Mechanistic Pathways of Progression to Heart Failure. Circ. Heart Fail. 2019, 12, e005897. [Google Scholar] [CrossRef]
- Ravassa, S.; Ballesteros, G.; López, B.; Ramos, P.; Bragard, J.; González, A.; Moreno, M.U.; Querejeta, R.; Vives, E.; García-Bolao, I.; et al. Combination of Circulating Type I Collagen-Related Biomarkers Is Associated with Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 73, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Michalski, B.; Trzciński, P.; Kupczyńska, K.; Miśkowiec, D.; Pęczek, L.; Nawrot, B.; Lipiec, P.; Kasprzak, J.D. The differences in the relationship between diastolic dysfunction, selected biomarkers and collagen turn-over in heart failure patients with preserved and reduced ejection fraction. Cardiol. J. 2017, 24, 35–42. [Google Scholar] [CrossRef]
- Löfsjögård, J.; Kahan, T.; Díez, J.; López, B.; González, A.; Edner, M.; Henriksson, P.; Mejhert, M.; Persson, H. Biomarkers of collagen type I metabolism are related to B-type natriuretic peptide, left ventricular size, and diastolic function in heart failure. J. Cardiovasc. Med. 2014, 15, 463–469. [Google Scholar] [CrossRef]
- Topf, A.; Mirna, M.; Ohnewein, B.; Jirak, P.; Kopp, K.; Fejzic, D.; Haslinger, M.; Motloch, L.J.; Hoppe, U.C.; Berezin, A.; et al. The Diagnostic and Therapeutic Value of Multimarker Analysis in Heart Failure. An Approach to Biomarker-Targeted Therapy. Front. Cardiovasc. Med. 2020, 7, 579567. [Google Scholar] [CrossRef] [PubMed]
- Düngen, H.-D.; Tscholl, V.; Obradovic, D.; Radenovic, S.; Matic, D.; Bright, L.M.; Tahirovic, E.; Marx, A.; Inkrot, S.; Hashemi, D.; et al. Prognostic performance of serial in-hospital measurements of copeptin and multiple novel biomarkers among patients with worsening heart failure: Results from the MOLITOR study. ESC Heart Fail. 2018, 5, 288–296. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, Q.; Qi, X.; Han, Q.; Qi, X.; Wang, F.; Du, B. Comparison of multiple biomarkers for mortality prediction in patients with acute heart failure of ischemic and nonischemic etiology. Biomarkers Med. 2018, 12, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vaduganathan, M.; Patel, K.V.; Ayers, C.; Ballantyne, C.M.; Kosiborod, M.N.; Carnethon, M.; DeFilippi, C.; McGuire, D.K.; Khan, S.S.; et al. Biomarker-Based Risk Prediction of Incident Heart Failure in Pre-Diabetes and Diabetes. JACC Heart Fail. 2021, 9, 215–223. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A.; Samura, T.; Berezina, T. Altered signature of apoptotic endothelial cell-derived microvesicles predicts chronic heart failure phenotypes. Biomarkers Med. 2019, 13, 737–750. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; Mccord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.; et al. Rapid Measurement of B-Type Natriuretic Peptide in the Emergency Diagnosis of Heart Failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef]
- Wieczorek, S.J.; Wu, A.H.; Christenson, R.; Krishnaswamy, P.; Gottlieb, S.; Rosano, T.; Hager, D.; Gardetto, N.; Chiu, A.; Bailly, K.R.; et al. A rapid B-type natriuretic peptide assay accurately diagnoses left ventricular dysfunction and heart failure: A multicenter evaluation. Am. Heart J. 2002, 144, 834–839. [Google Scholar] [CrossRef]
- Rawlins, M.L.; Owen, W.E.; Roberts, W.L. Performance Characteristics of Four Automated Natriuretic Peptide Assays. Am. J. Clin. Pathol. 2005, 123, 439–445. [Google Scholar] [CrossRef]
- Wu, A.H.; Packer, M.; Smith, A.; Bijou, R.; Fink, D.; Mair, J.; Wallentin, L.; Johnston, N.; Feldcamp, C.S.; Haverstick, D.M.; et al. Analytical and Clinical Evaluation of the Bayer ADVIA Centaur Automated B-Type Natriuretic Peptide Assay in Patients with Heart Failure: A Multisite Study. Clin. Chem. 2004, 50, 867–873. [Google Scholar] [CrossRef]
- Mueller, T.; Gegenhuber, A.; Poelz, W.; Haltmayer, M. Preliminary Evaluation of the AxSYM B-Type Natriuretic Peptide (BNP) Assay and Comparison with the ADVIA Centaur BNP Assay. Clin. Chem. 2004, 50, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Barak, M.; Weinberger, R.; Marcusohn, J.; Froom, P. Harmonization of the Bayer ADVIA Centaur and Abbott AxSYM automated B-type natriuretic peptide assay in patients on hemodialysis. Clin. Chem. Lab. Med. 2005, 43, 554–556. [Google Scholar] [CrossRef] [PubMed]
- Lingervelder, D.; Koffijberg, H.; Kusters, R.; Ijzerman, M.J. Point-of-care testing in primary care: A systematic review on implementation aspects addressed in test evaluations. Int. J. Clin. Pract. 2019, 73, e13392. [Google Scholar] [CrossRef]
- Müller-Bardorff, M.; Freitag, H.; Scheffold, T.; Remppis, A.; Kübler, W.; Katus, H.A. Development and Characterization of a Rapid Assay for Bedside Determinations of Cardiac Troponin T. Circulation 1995, 92, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Alp, N.; Bell, J.; Shahi, M. A rapid troponin-I-based protocol for assessing acute chest pain. QJM Int. J. Med. 2001, 94, 687–694. [Google Scholar] [CrossRef][Green Version]
- Ramparany, L.; Ramirez, J.; Nizou, J.-Y.; Le Saux, D.; Richard, V.; Talarmin, A. Evaluation of Four Rapid Immunochromatographic Tests for the Detection of Cardiac Troponin I. Clin. Vaccine Immunol. 2011, 18, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Wettersten, N.; Maisel, A.; University of California San Diego; System, L.J.V.A.S.D.H. Role of Cardiac Troponin Levels in Acute Heart Failure. Card. Fail. Rev. 2015, 1, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Ishimitsu, T.; Kojima, M.; Kangawa, K.; Hino, J.; Matsuoka, H.; Kitamura, K.; Eto, T.; Matsuo, H. Genomic Structure of Human Adrenomedullin Gene. Biochem. Biophys. Res. Commun. 1994, 203, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Jougasaki, M.; Wei, C.-M.; McKinley, L.J.; Burnett, J.C. Elevation of Circulating and Ventricular Adrenomedullin in Human Congestive Heart Failure. Circulation 1995, 92, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Gegenhuber, A.; Struck, J.; Dieplinger, B.; Poelz, W.; Pacher, R.; Morgenthaler, N.G.; Bergmann, A.; Haltmayer, M.; Mueller, T. Comparative Evaluation of B-Type Natriuretic Peptide, Mid-Regional Pro-A-type Natriuretic Peptide, Mid-Regional Pro-Adrenomedullin, and Copeptin to Predict 1-Year Mortality in Patients With Acute Destabilized Heart Failure. J. Card. Fail. 2007, 13, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.K.; Smith, M.W.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G. Adrenomedullin (1–52) measured in human plasma by radioimmunoassay: Plasma concentration, adsorption, and storage. Clin. Chem. 1998, 44, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Struck, J.; Tao, C.; Morgenthaler, N.G.; Bergmann, A. Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 2004, 25, 1369–1372. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Measurement of Midregional Proadrenomedullin in Plasma with an Immunoluminometric Assay. Clin. Chem. 2005, 51, 1823–1829. [Google Scholar] [CrossRef]
- Izumiya, Y.; Hanatani, S.; Kimura, Y.; Takashio, S.; Yamamoto, E.; Kusaka, H.; Tokitsu, T.; Rokutanda, T.; Araki, S.; Tsujita, K.; et al. Growth Differentiation Factor-15 Is a Useful Prognostic Marker in Patients with Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2014, 30, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Black, S.; Kushner, I.; Samols, D. C-reactive Protein. J. Biol. Chem. 2004, 279, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Perin, D.P.; Gehle, A.; Nsiah-Kumi, P.A. Feasibility of using C-reactive protein for point-of-care testing. Technol. Health Care 2013, 21, 233–240. [Google Scholar] [CrossRef]
- Lindström, J.; Nordeman, L.; Hagstrom, B. What a difference a CRP makes. A prospective observational study on how point-of-care C-reactive protein testing influences antibiotic prescription for respiratory tract infections in Swedish primary health care. Scand. J. Prim. Health Care 2015, 33, 275–282. [Google Scholar] [CrossRef]
- Prajapati, A.; Verma, N.; Pandya, A. Highly sensitive vertical flow based point-of-care immunokit for rapid and early detection of human CRP as a cardiovascular risk factor. Biomed. Microdevices 2020, 22, 28. [Google Scholar] [CrossRef]
- Patti, G.; Mangiacapra, F.; Ricottini, E.; Cannatà, A.; Cavallari, I.; Vizzi, V.; D’Ambrosio, A.; Dicuonzo, G.; Di Sciascio, G. Correlation of Platelet Reactivity and C-Reactive Protein Levels to Occurrence of Peri-Procedural Myocardial Infarction in Patients Undergoing Percutaneous Coronary Intervention (from the ARMYDA-CRP Study). Am. J. Cardiol. 2013, 111, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-W.; Lee, S.-W.; Yun, S.-C.; Song, H.-G.; Ahn, J.-M.; Lee, J.-Y.; Kim, W.-J.; Kang, S.-J.; Kim, Y.-H.; Lee, C.W.; et al. A Point-of-Care Platelet Function Assay and C-Reactive Protein for Prediction of Major Cardiovascular Events After Drug-Eluting Stent Implantation. J. Am. Coll. Cardiol. 2011, 58, 2630–2639. [Google Scholar] [CrossRef]
- O’Meara, E.; Prescott, M.F.; Claggett, B.; Rouleau, J.L.; Chiang, L.-M.; Solomon, S.D.; Packer, M.; McMurray, J.J.; Zile, M.R. Independent Prognostic Value of Serum Soluble ST2 Measurements in Patients With Heart Failure and a Reduced Ejection Fraction in the PARADIGM-HF Trial (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure). Circ. Heart Fail. 2018, 11, e004446. [Google Scholar] [CrossRef] [PubMed]
- Krum, H. Prospective Comparison of ARNi with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF): Paragon of a study or further investigation paramount? Circulation 2015, 131, 11–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gruson, D.; Ferracin, B.; Ahn, S.; Rousseau, M.F. Testing for Soluble ST2 in Heart Failure Patients: Reliability of a Point of Care Method. Clin. Lab. 2017, 63, 141–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dieplinger, B.; Egger, M.; Gegenhuber, A.; Haltmayer, M.; Mueller, T. Analytical and clinical evaluation of a rapid quantitative lateral flow immunoassay for measurement of soluble ST2 in human plasma. Clin. Chim. Acta 2015, 451, 310–315. [Google Scholar] [CrossRef][Green Version]
- Anand, I.S.; Rector, T.S.; Kuskowski, M.; Adourian, A.; Muntendam, P.; Cohn, J.N. Baseline and serial measurements of galectin-3 in patients with heart failure: Relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur. J. Heart Fail. 2013, 15, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Van Der Velde, A.R.; Gullestad, L.; Ueland, T.; Aukrust, P.; Guo, Y.; Adourian, A.; Muntendam, P.; Van Veldhuisen, D.J.; De Boer, R.A. Prognostic Value of Changes in Galectin-3 Levels Over Time in Patients With Heart Failure. Circ. Heart Fail. 2013, 6, 219–226. [Google Scholar] [CrossRef]
- Gaze, D.C.; Prante, C.; Dreier, J.; Knabbe, C.; Collet, C.; Launay, J.-M.; Franekova, J.; Jabor, A.; Lennartz, L.; Shih, J.; et al. Analytical evaluation of the automated galectin-3 assay on the Abbott ARCHITECT immunoassay instruments. Clin. Chem. Lab. Med. 2014, 52, 919–926. [Google Scholar] [CrossRef]
- La’Ulu, S.L.; Apple, F.S.; Murakami, M.M.; Ler, R.; Roberts, W.L.; Straseski, J.A. Performance characteristics of the ARCHITECT Galectin-3 assay. Clin. Biochem. 2013, 46, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Tomaniak, M.; Filipiak, K.J.; Kołtowski, Ł.; Sitkiewicz, D. Galectin-3 in Patients with Acute Heart Failure: Preliminary Report on First Polish Experience. Adv. Clin. Exp. Med. 2016, 25, 617–623. [Google Scholar] [CrossRef]
- Gruson, D.; Mancini, M.; Ahn, S.; Rousseau, M. Galectin-3 testing: Validity of a novel automated assay in heart failure patients with reduced ejection fraction. Clin. Chim. Acta 2014, 429, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; Van Der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef]
- Huang, D.; Ying, H.; Jiang, D.; Liu, F.; Tian, Y.; Du, C.; Zhang, L.; Pu, X. Rapid and sensitive detection of interleukin-6 in serum via time-resolved lateral flow immunoassay. Anal. Biochem. 2020, 588, 113468. [Google Scholar] [CrossRef]
- Chaemsaithong, P.; Romero, R.; Korzeniewski, S.J.; Dong, Z.; Yeo, L.; Hassan, S.S.; Kim, Y.M.; Yoon, B.H.; Chaiworapongsa, T. A point of care test for the determination of amniotic fluid interleukin-6 and the chemokine CXCL-10/IP-10. J. Matern. Neonatal Med. 2015, 28, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Chaemsaithong, P.; Romero, R.; Docheva, N.; Chaiyasit, N.; Bhatti, G.; Pacora, P.; Hassan, S.S.; Yeo, L.; Erez, O. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes. J. Matern. Neonatal Med. 2018, 31, 228–244. [Google Scholar] [CrossRef]
- Louzao-Martinez, L.; Vink, A.; Harakalova, M.; Asselbergs, F.W.; Verhaar, M.C.; Cheng, C. Characteristic adaptations of the extracellular matrix in dilated cardiomyopathy. Int. J. Cardiol. 2016, 220, 634–646. [Google Scholar] [CrossRef]
- Gunja-Smith, Z.; Morales, A.R.; Romanelli, R.; Woessner, J.F. Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am. J. Pathol. 1996, 148, 1639–1648. [Google Scholar]
- Kai, H.; Ikeda, H.; Yasukawa, H.; Kai, M.; Seki, Y.; Kuwahara, F.; Ueno, T.; Sugi, K.; Imaizumi, T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 1998, 32, 368–372. [Google Scholar] [CrossRef]
- Mihailovici, A.R.; Deliu, R.C.; Mărgăritescu, C.; Simionescu, C.E.; Donoiu, I.; Istrătoaie, O.; Tudoraşcu, D.R.; Târtea, E.A.; Gheonea, D.I. Collagen I and III, MMP-1 and TIMP-1 immunoexpression in dilated cardiomyopathy. Rom. J. Morphol. Embryol. 2017, 58, 777–781. [Google Scholar] [PubMed]
- Lubos, E.; Schnabel, R.; Rupprecht, H.J.; Bickel, C.; Messow, C.-M.; Prigge, S.; Cambien, F.; Tiret, L.; Münzel, T.; Blankenberg, S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: Results from the AtheroGene study. Eur. Heart J. 2005, 27, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tervahartiala, T.; Leppilahti, J.; Hernandez, M.; Gamonal, J.; Tuomainen, A.M.; Lauhio, A.; Pussinen, P.J.; Mäntylä, P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol. Res. 2011, 63, 108–113. [Google Scholar] [CrossRef]
- Leppilahti, J.; Ahonen, M.-M.; Hernández, M.; Munjal, S.; Netuschil, L.; Uitto, V.-J.; Sorsa, T.; Mäntylä, P. Oral rinse MMP-8 point-of-care immuno test identifies patients with strong periodontal inflammatory burden. Oral Dis. 2010, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Anand, I.S. Troponin T and Plasma Collagen Peptides in Heart Failure. Circ. Heart Fail. 2012, 5, 394–397. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Strategy | Biomarkers | ESC, 2016 | ACC/AHA/HFSA, 2017 | ||||
|---|---|---|---|---|---|---|---|
| COR | LOE | Phenotype of HF | COR | LOE | Phenotype of HF | ||
| Diagnosis | BNP/NT-proBNP/MR-proANP * | I | A | AHF, HFpEF, HFmrEF | I | A | AHF, CHF |
| Risk of in-hospital death | BNP/NT-proBNP | I | C | AHF | I | A | AHF, CHF |
| hs-cTr | I | C | AHF | I | A | AHF, CHF | |
| Risk of recurrent hospital admission | BNP/NT-proBNP | - | I | A | AHF, CHF | ||
| Risk of post-discharged death | BNP/NT-proBNP | I | A | AHF, CHF | I | A | AHF, CHF |
| hs-cTr | I | C | AHF, CHF | I | IIa | AHF, CHF | |
| Galectin-3 | - | IIb | B | AHF, CHF | |||
| sST2 | - | IIb | B | AHF, CHF | |||
| Prevention of HF onset | BNP/NT-proBNP | - | IIa | B | AHF, CHF | ||
| Guided therapy | BNP/NT-proBNP | - | I | A | HFrEF/HFpEF | ||
| Biomarkers | Underlying Pathophysiological Mechanisms | Possible Application for HF Phenotype | Advantages | Disadvantages |
|---|---|---|---|---|
| NPs | Biomechanical stress | HFrEF, HFpEF | Available for diagnosis, risk stratification, prognosis, and point-to-care therapy | High serum level variability, variable cut-off points in patients with AF, CKD, AO, prediction in HFrEF is higher than HFpEF |
| hs-cTn | Myocardial injury | Manly HFrEF | Available for risk stratification and prognosis | No add-on prediction to NPs |
| Mid-regional-pro-adrenomedullin | Neurohumoral activation | HFrEF, HFpEF | Better than NPs in predicting short-term mortality in acute HF | No superiority to NPs in predictive ability among chronic HFrEF/HFpEF |
| hs-CRP, IL-6 | Inflammation | HFrEF, HFpEF | Prediction of all-cause mortality, CVD, HF-related events | Not suitable for point-of-care therapy, no ability to increase predictive ability of NPs, not recommended by reputed medical societies |
| GDF-15 | Inflammation | HFrEF, HFpEF | Available for improving predictive ability of NPs, suitable for multiple biomarker strategy and point-of-care therapy | High cost, not recommended by reputed medical societies |
| sST2, galectin-3 | Fibrosis/inflammation | HFpEF | Better than NPs for predicting mortality and HF-related events in non-HF patients, low individual serum level variability | High cost |
| Collagen turn-over biomarkers | Fibrosis | HFpEF | Available for risk stratification and prognosis | High cost, not recommended by reputed medical societies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtenauer, M.; Jirak, P.; Paar, V.; Sipos, B.; Kopp, K.; Berezin, A.E. Heart Failure and Diabetes Mellitus: Biomarkers in Risk Stratification and Prognostication. Appl. Sci. 2021, 11, 4397. https://doi.org/10.3390/app11104397
Lichtenauer M, Jirak P, Paar V, Sipos B, Kopp K, Berezin AE. Heart Failure and Diabetes Mellitus: Biomarkers in Risk Stratification and Prognostication. Applied Sciences. 2021; 11(10):4397. https://doi.org/10.3390/app11104397
Chicago/Turabian StyleLichtenauer, Michael, Peter Jirak, Vera Paar, Brigitte Sipos, Kristen Kopp, and Alexander E. Berezin. 2021. "Heart Failure and Diabetes Mellitus: Biomarkers in Risk Stratification and Prognostication" Applied Sciences 11, no. 10: 4397. https://doi.org/10.3390/app11104397
APA StyleLichtenauer, M., Jirak, P., Paar, V., Sipos, B., Kopp, K., & Berezin, A. E. (2021). Heart Failure and Diabetes Mellitus: Biomarkers in Risk Stratification and Prognostication. Applied Sciences, 11(10), 4397. https://doi.org/10.3390/app11104397








