Characteristic Study of Briquette Cyanobacteria as Fuel in Chemical Looping Combustion with Hematite as Oxygen Carrier
Abstract
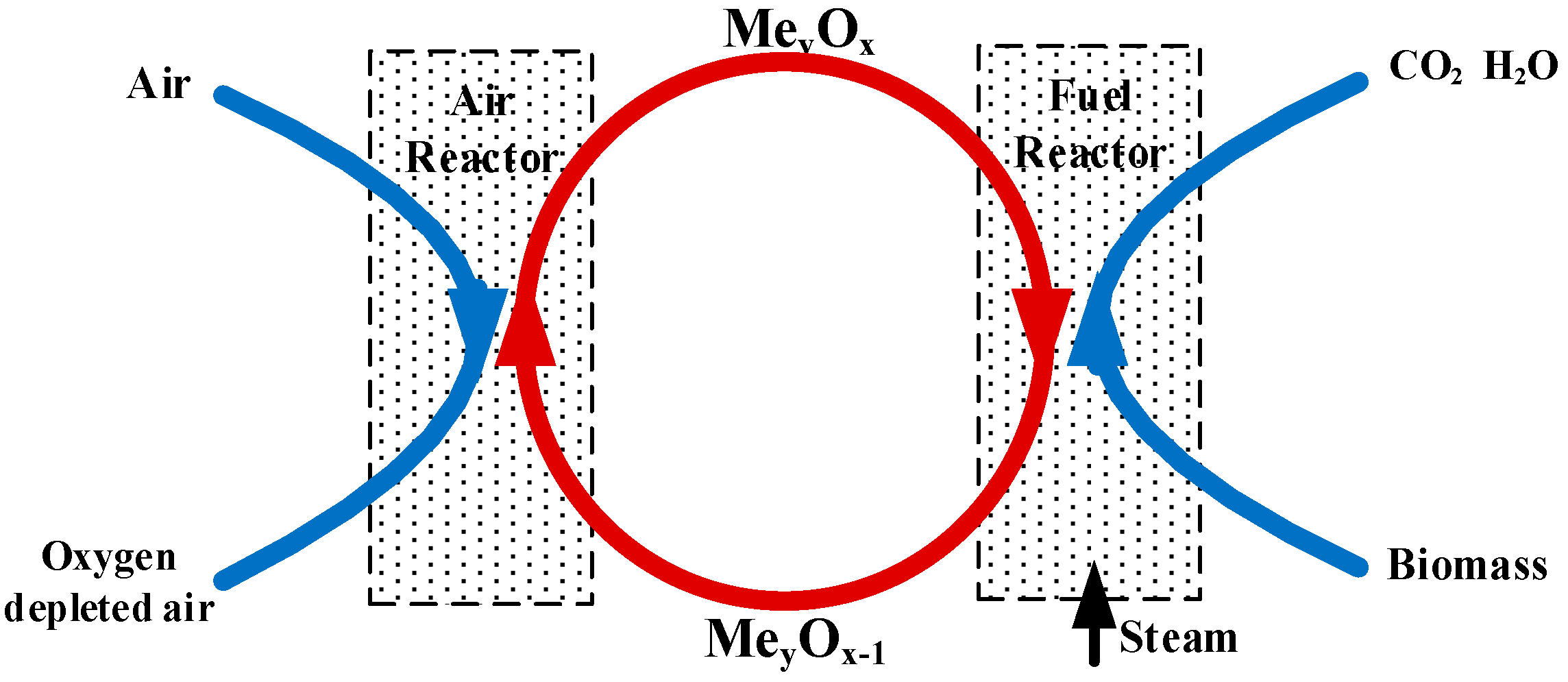
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus and Experimental Steps
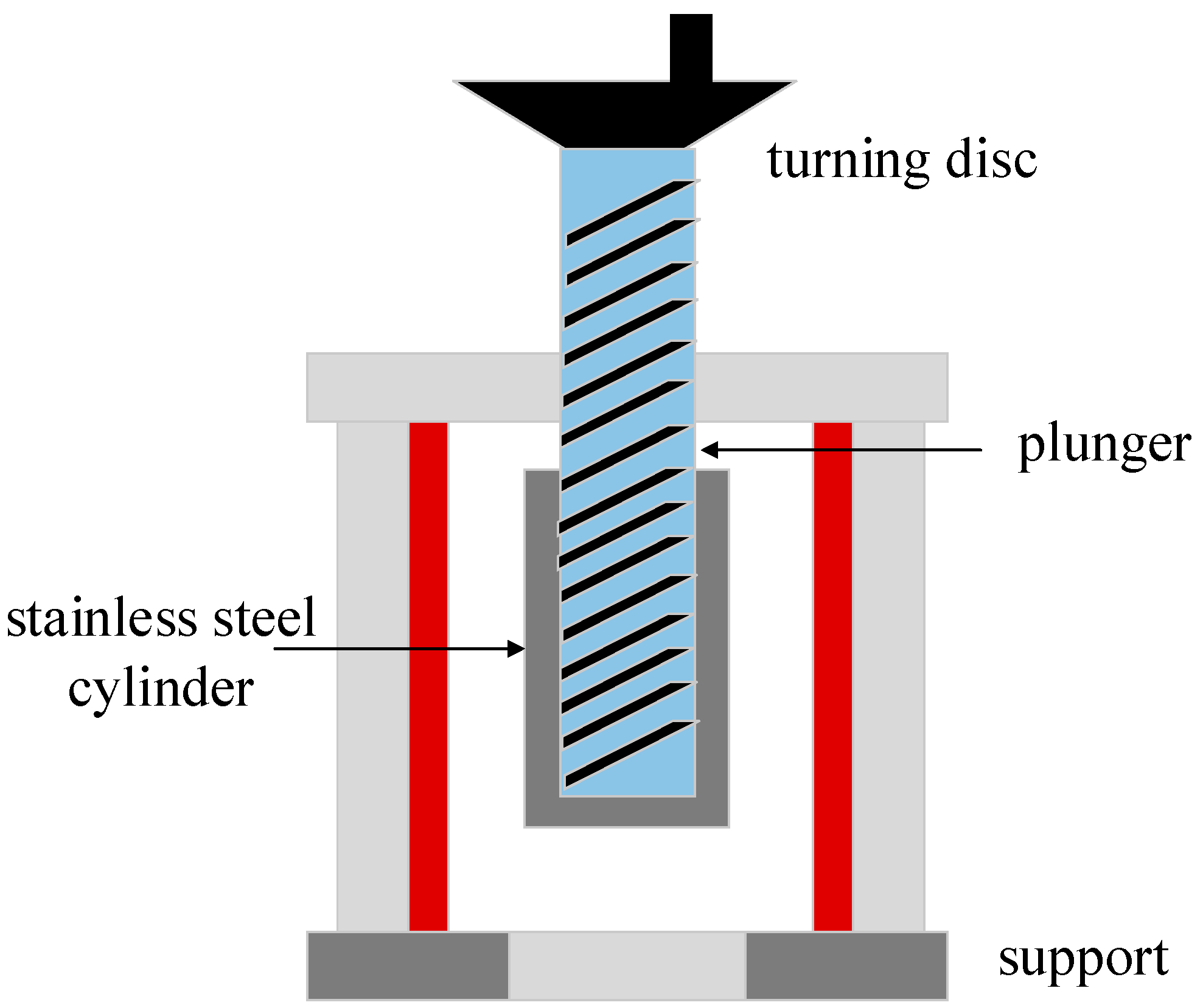
2.2.1. Single Briquetting Unit
2.2.2. Pressure Crushing Detector
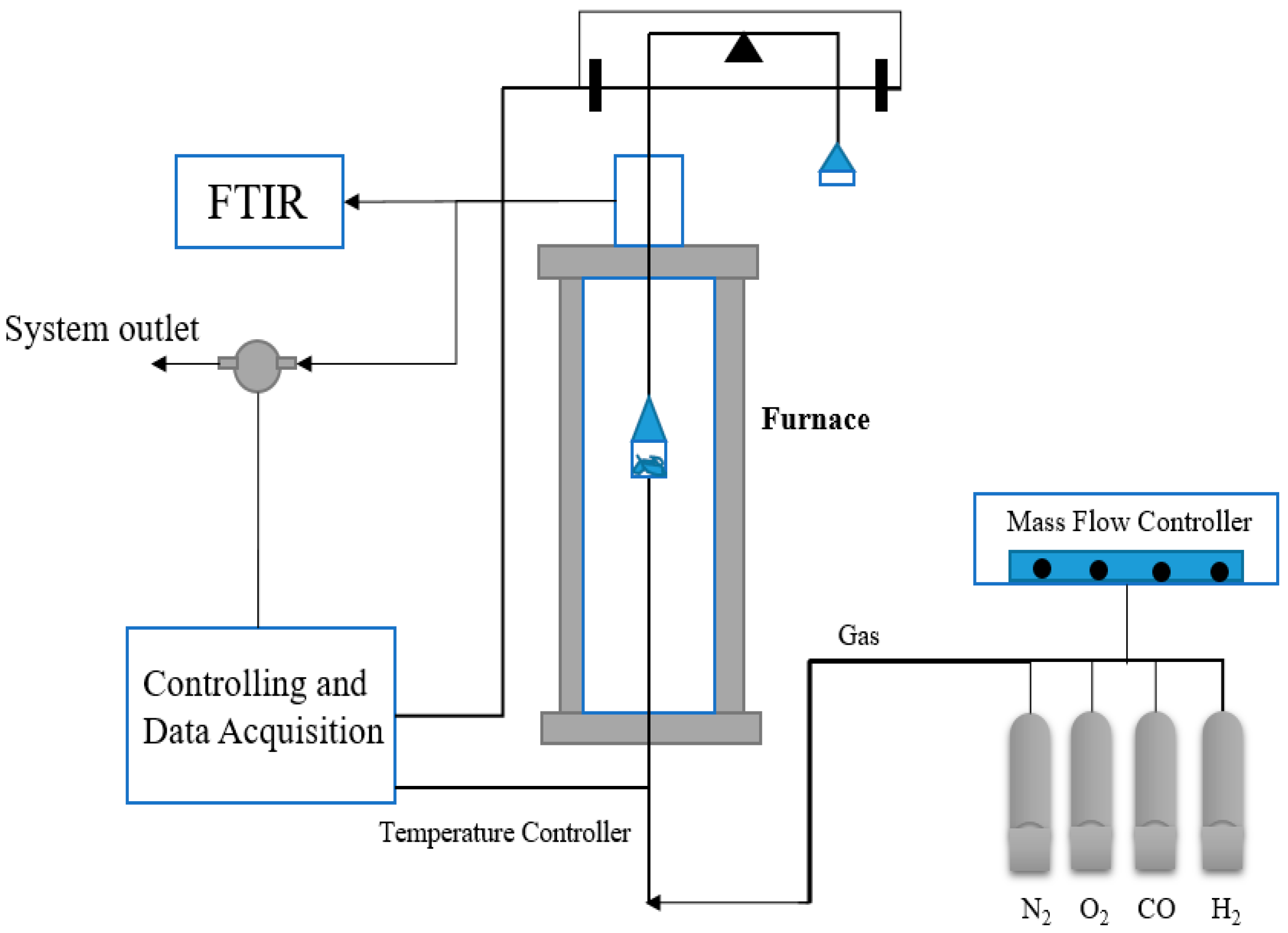
2.2.3. Thermogravimetric Analyzer
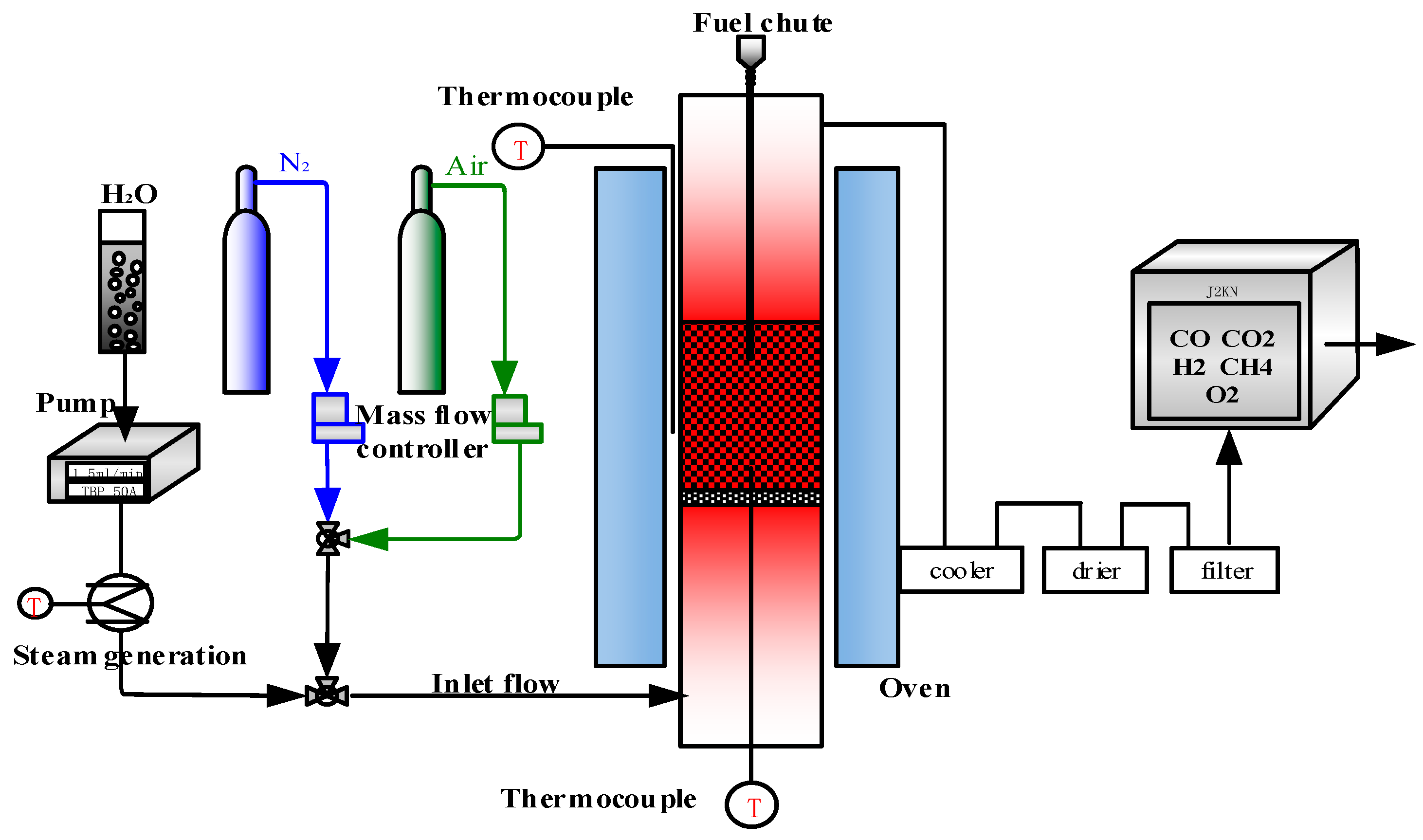
2.2.4. Single Fluidized Bed Reactor
- (1)
- Thirty grams of hematite particles are placed on the porous plate of the single fluidized bed, and air flow of 1.5 L/min is introduced to ensure that the hematite oxygen carrier is in the oxidation state;
- (2)
- When the single fluidized bed reactor is heated to the experimental temperature, steam as gasification agent is introduced and air flow is switched to nitrogen (1.5 L/min);
- (3)
- When the temperature of the reactor is stable, a batch of 0.5 g briquette cyanobacteria fuel is put into the reactor through the storage bin, and the reaction starts;
- (4)
- The total reaction time is set at 30 min, and the gas composition and concentration in the flue gas are monitored online;
- (5)
- When there is no carbonaceous gas detected, steam flow is cut off and nitrogen flow is switched to air again (1.5 L/min), reducted hematite starts to be oxidized and completes regeneration. Then, the air flow is switched to nitrogen (1.5 L/min), and briquette cyanobacteria of 0.5 g are put into the reactor again.
2.3. Procedures
2.3.1. Densification Process from Single Briquetting Unit
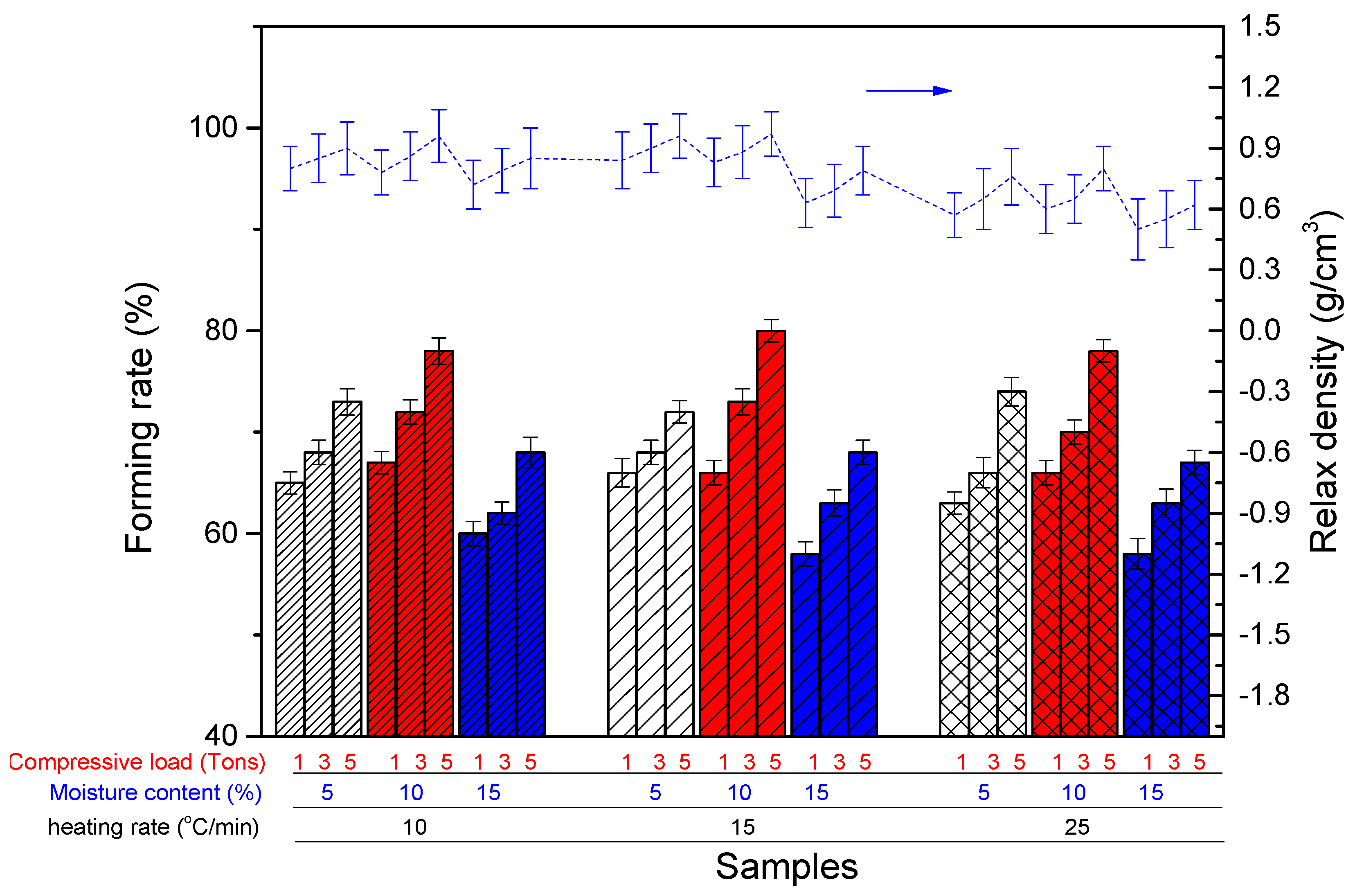
2.3.2. Drying Process from Thermogravimetric Analysis Reactor
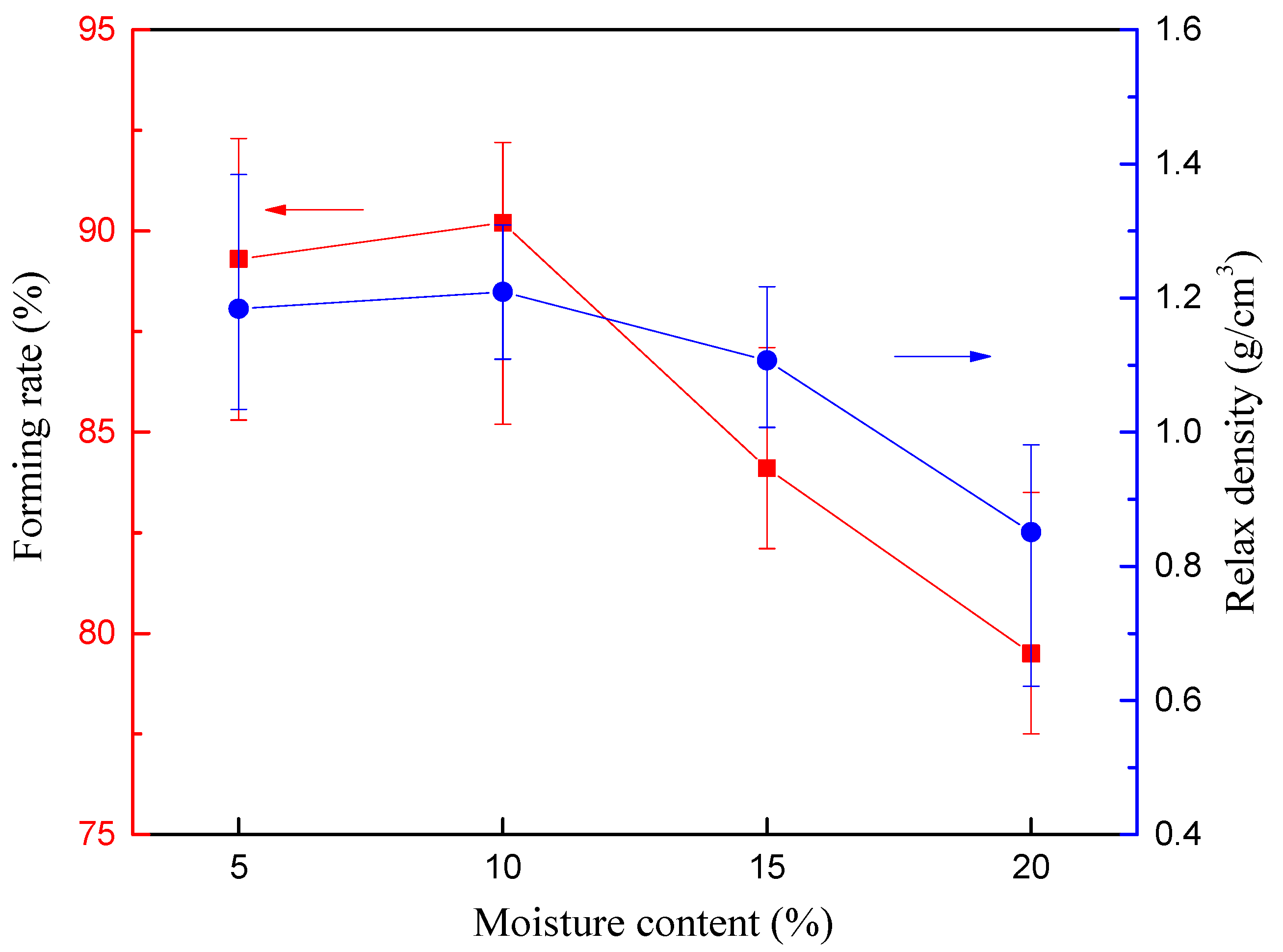
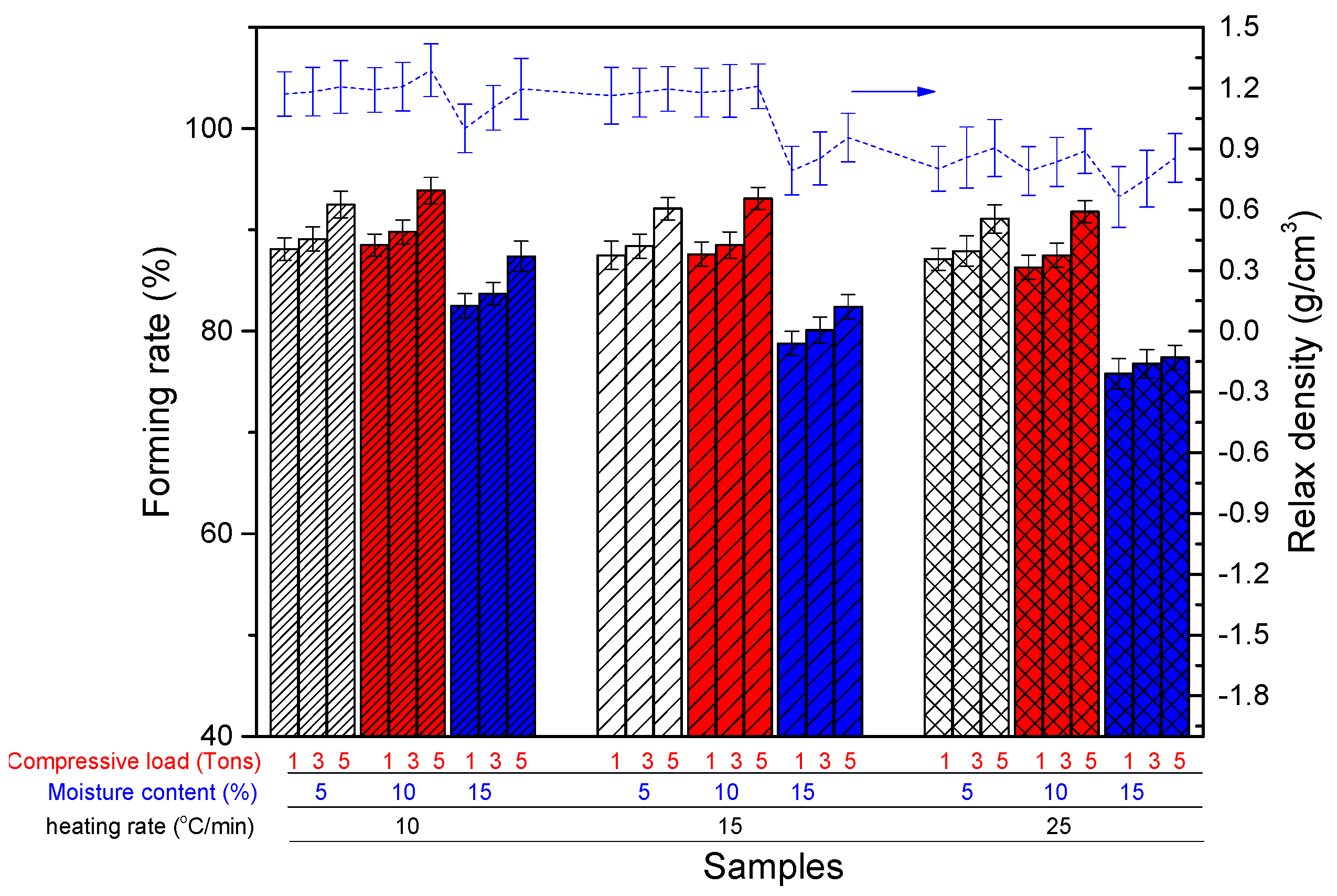
2.3.3. Devolatilization Process from Thermogravimetric Analysis Reactor
2.3.4. Drying, Devolatilization and Char Burnout Process from Single Fluidized Bed
2.4. Quality Attributes and Analytical Method
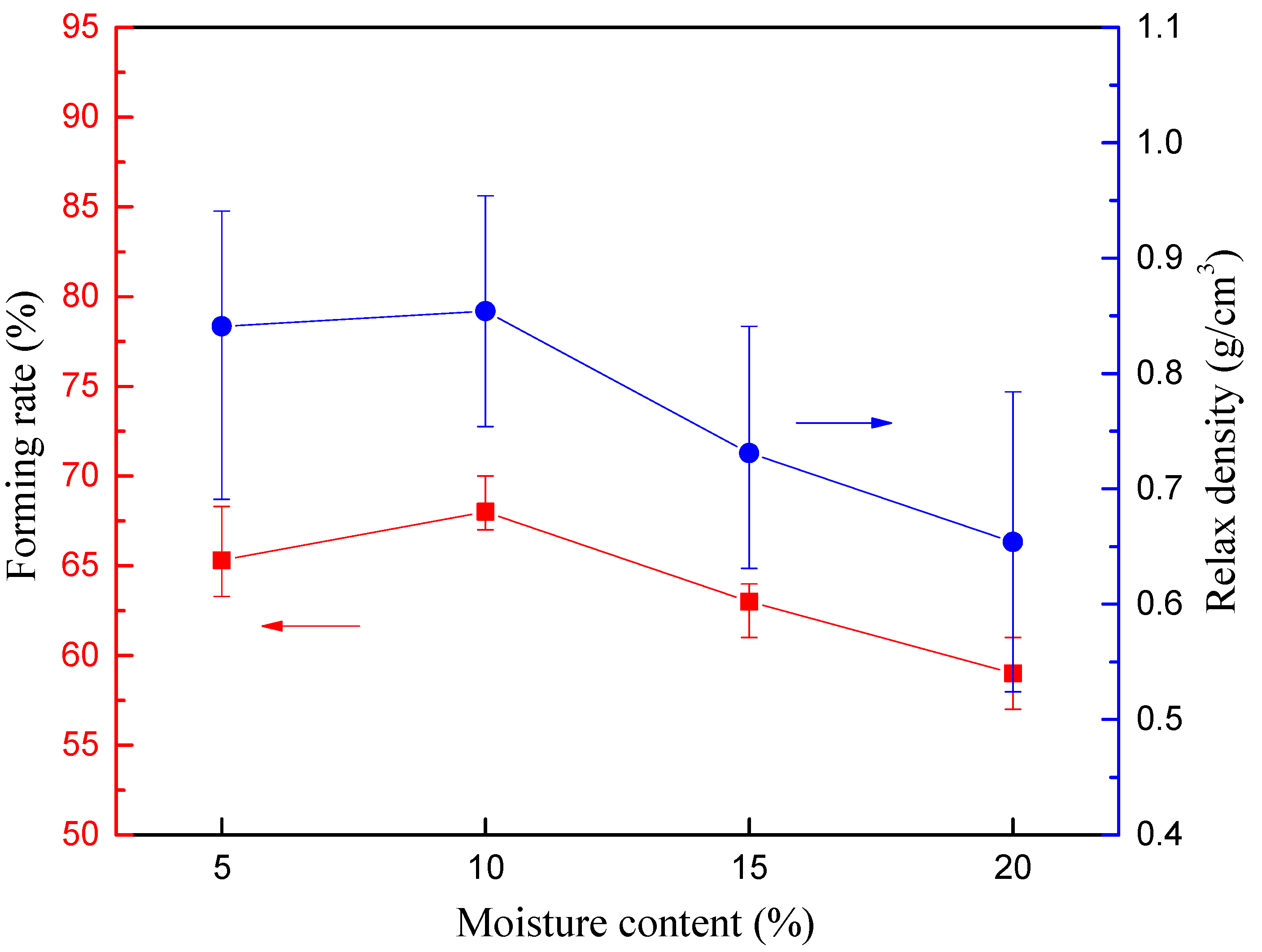
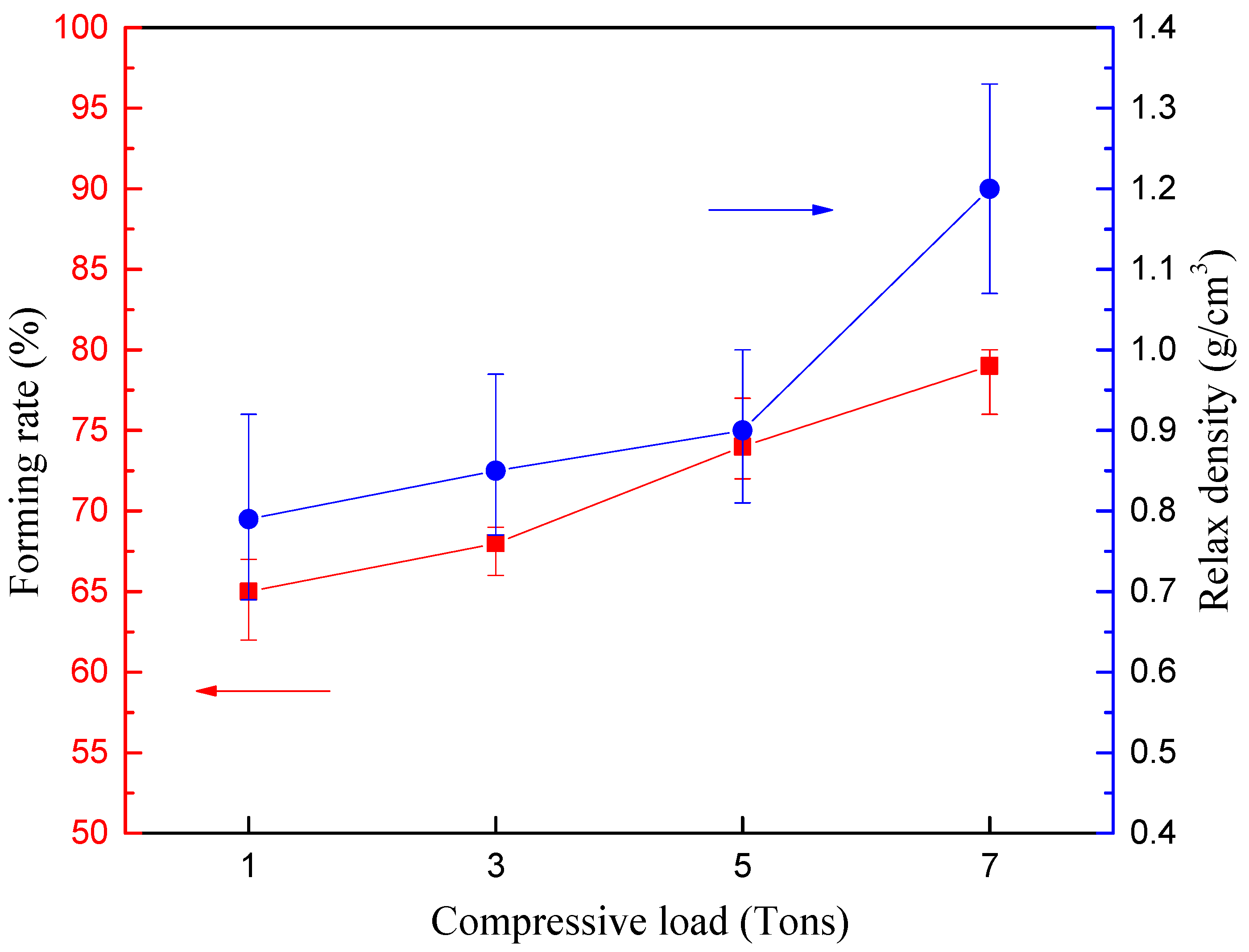
2.4.1. Forming Rate
2.4.2. Relax Density
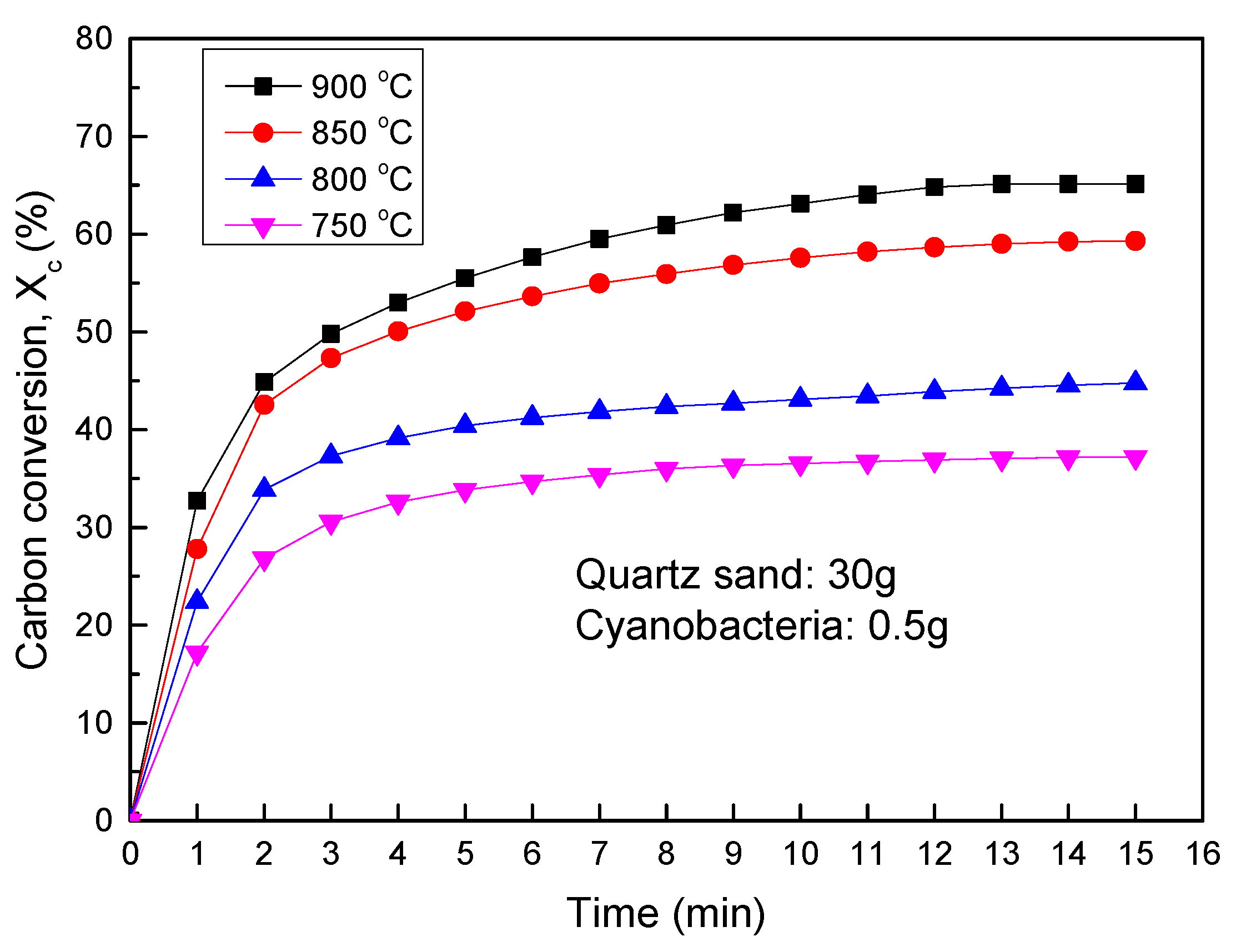
2.4.3. Carbon Conversion
3. Results
3.1. Characteristic of Briquette Cyanobacteria during Densification Process
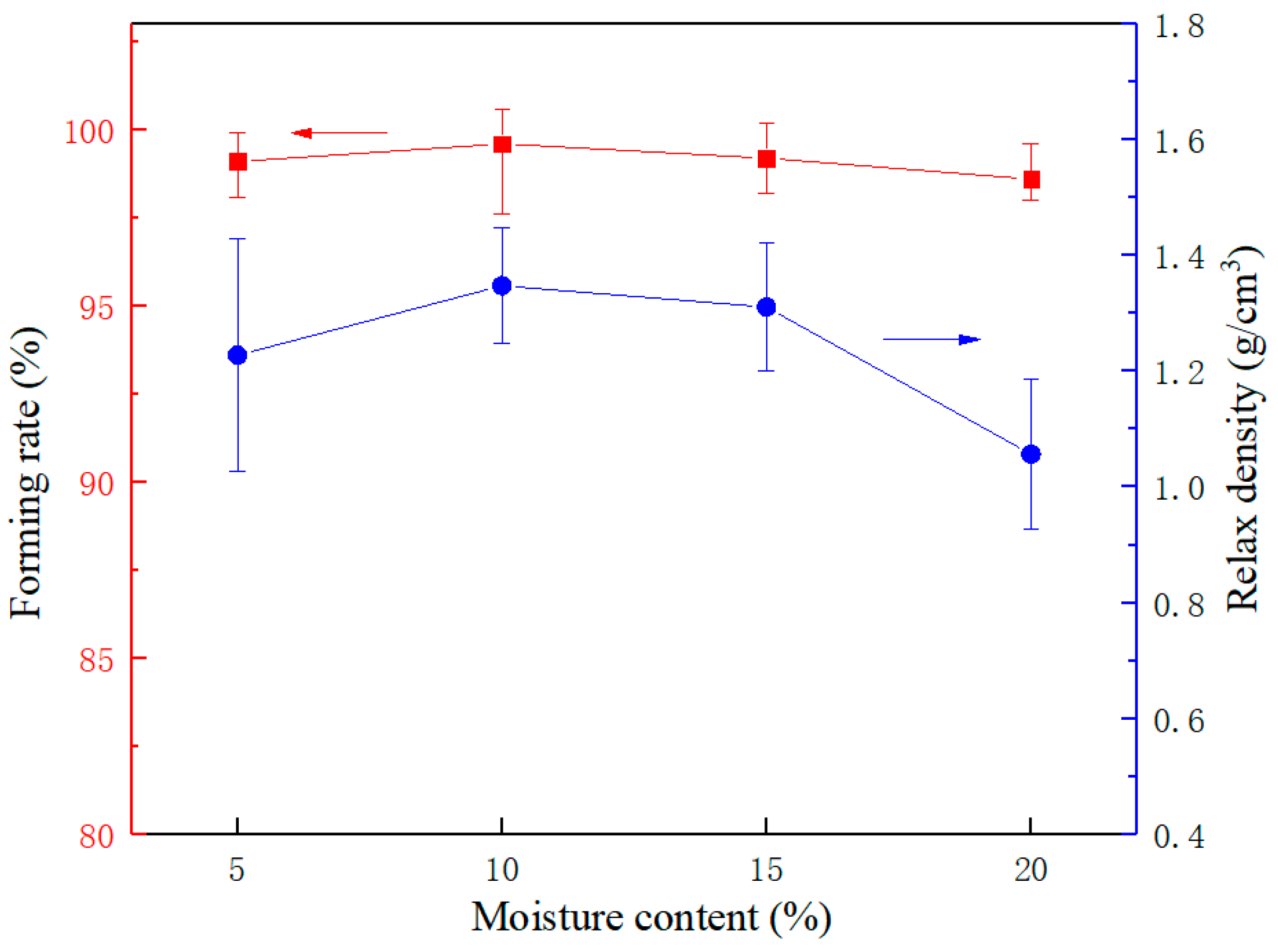
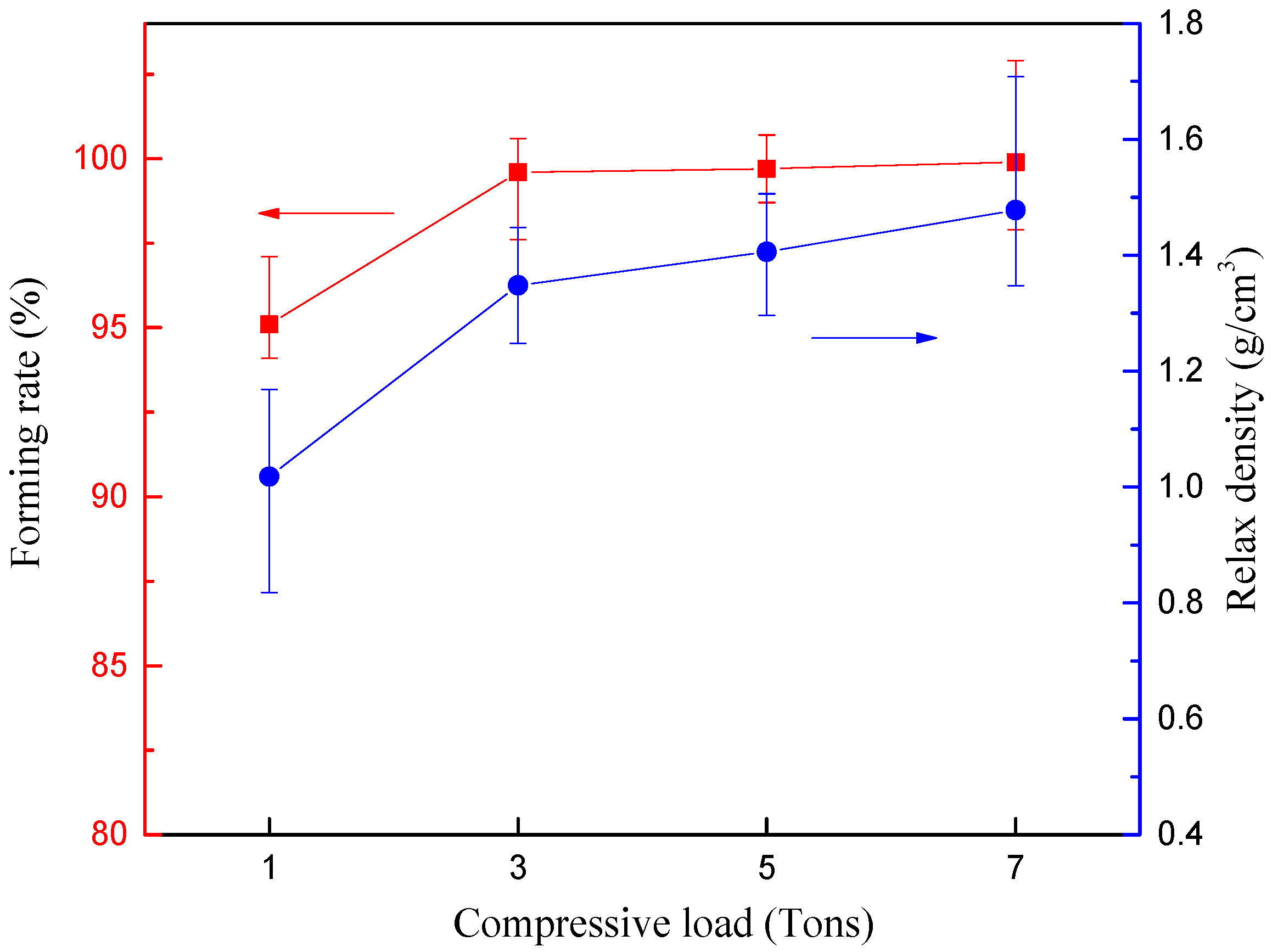
3.1.1. Mechanical Strength Analysis Based on Single Briquetting Unit
3.1.2. Mechanical Strength Analysis Based on Pressure Crushing Detector
3.2. Characteristic of Briquette Cyanobacteria in TGA Reactor
3.2.1. Drying Process of Briquette Cyanobacteria in TGA Reactor
3.2.2. Devolatilization Process of Briquette Cyanobacteria in TGA Reactor
3.3. Thermochemical Transformation of Briquette Cyanobacteria in Single Fluidized Bed
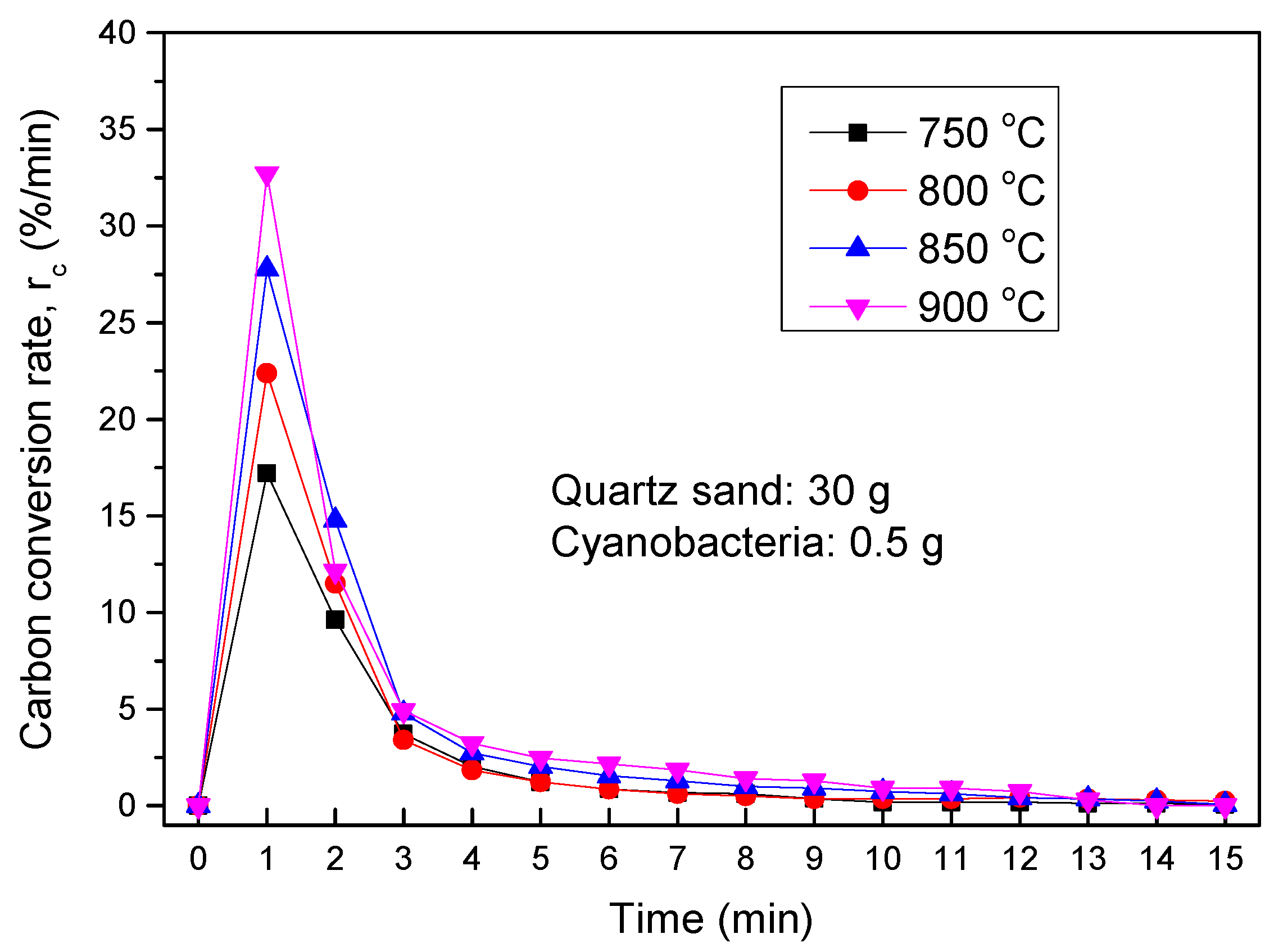
3.3.1. Thermochemical Transformation with Quartz Sand as Bed Materials
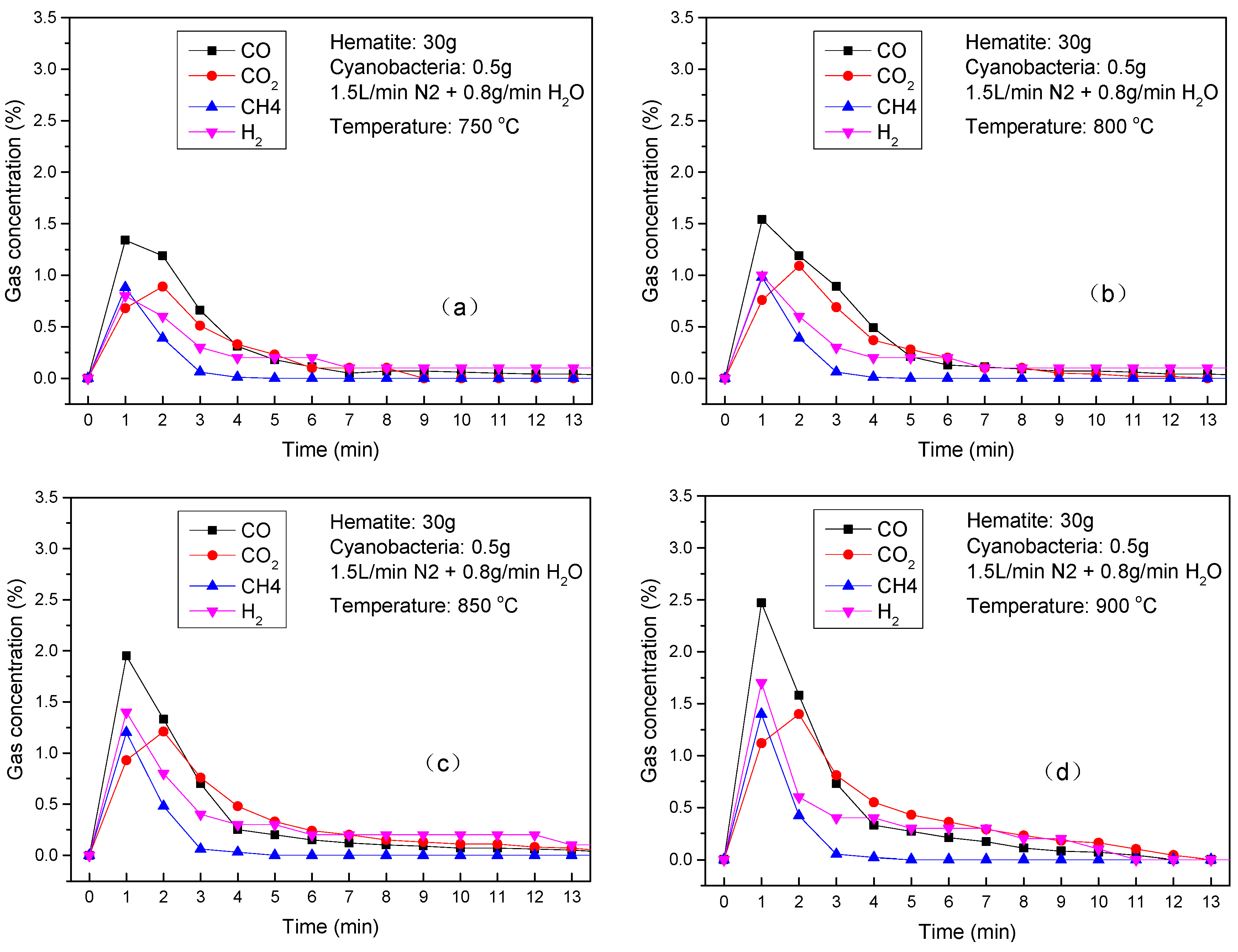
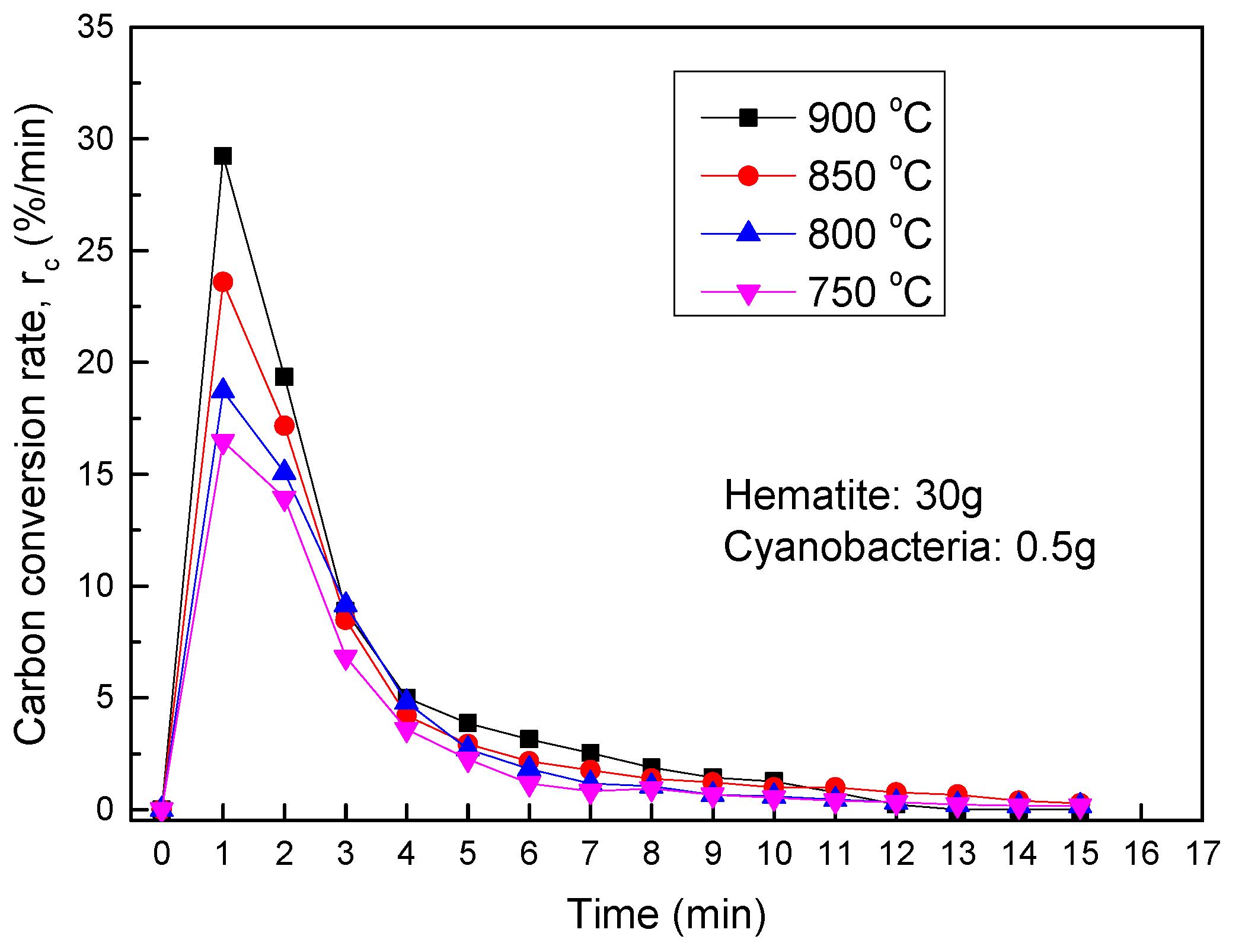
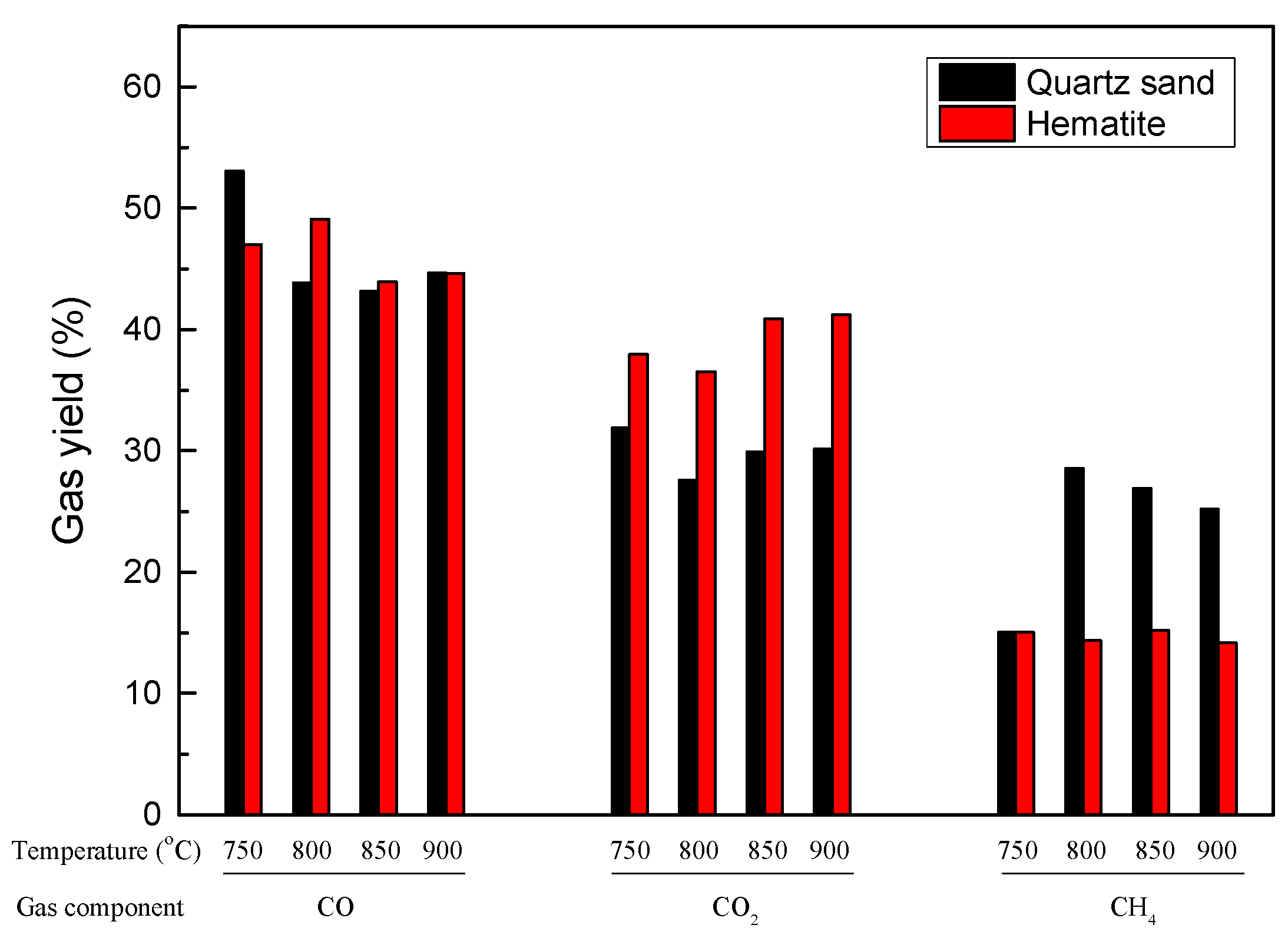
3.3.2. Thermochemical Transformation with Hematite as Bed Materials
3.4. Effect of CLC Process on Nitrogen Contaminations
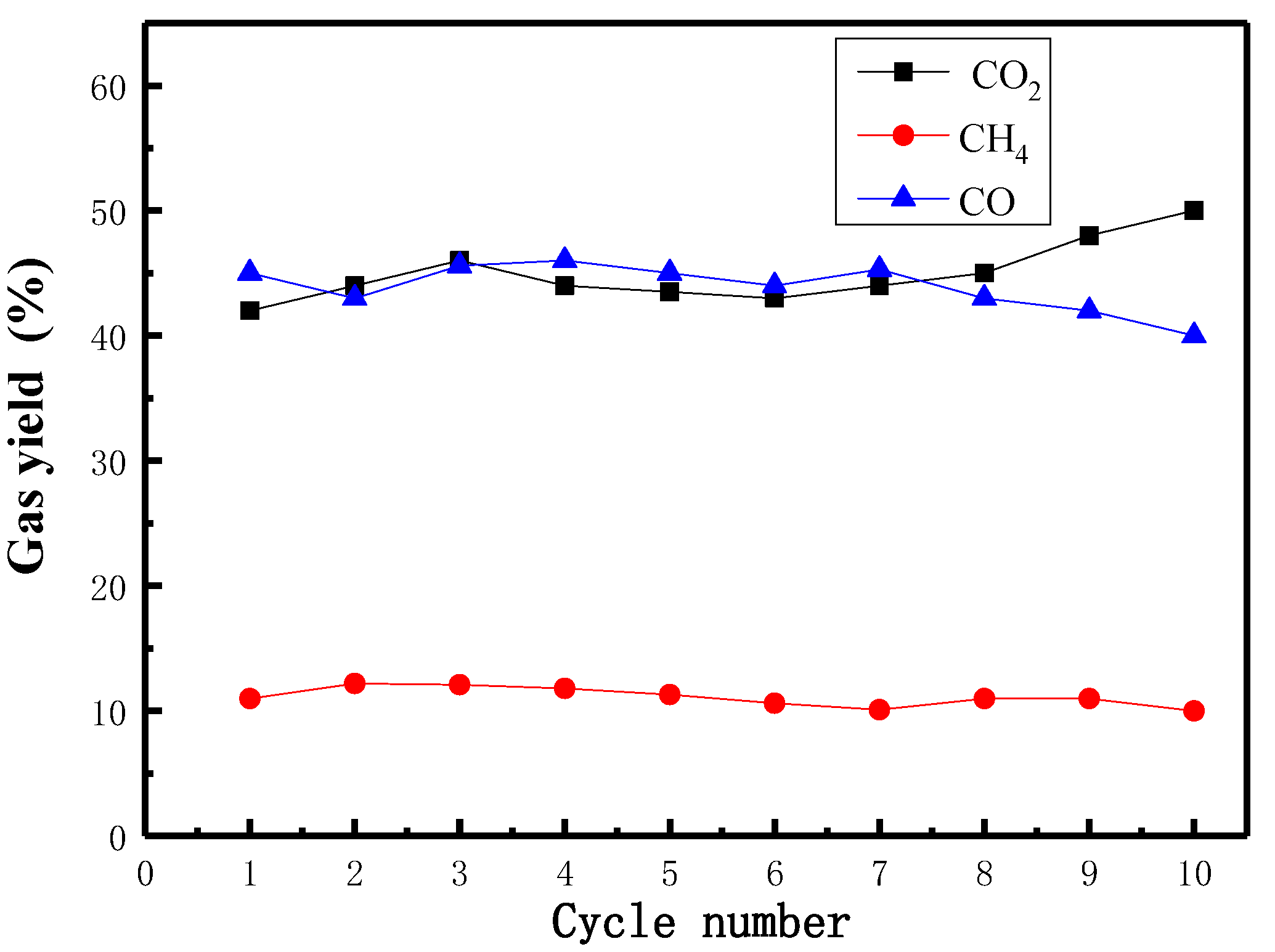
3.5. Cyclical Performance and Phosphorus Transformation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cloern, J.E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecology Prog. 2001, 210, 223–253. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Davis, J.R.; Koop, K. Eutrophication in Australian Rivers, Reservoirs and Estuaries—A Southern Hemisphere Perspective on the Science and its Implications. Hydrobiologia 2006, 559, 23–76. [Google Scholar] [CrossRef]
- Robarts, R.D.; Zohary, T. Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom forming cyanobacteria. N. Z. J. Mar. Freshw. Res. 1987, 21, 391–399. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar]
- Shao, J.; Gu, J.; Peng, L.; Luo, S.; Luo, H.; Yan, Z.; Wu, G. Modification of cyanobacterial bloom-derived biomass using potassium permanganate enhanced the removal of microcystins and adsorption capacity toward cadmium (II). J. Hazard. Mater. 2014, 272, 83–88. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Gibbons, H.L.; Stoops, K.B.; Bouchard, D.D. Response of a Shallow, Polymictic Lake to Buffered Alum Treatment. Lake Reserv. Manag. 1994, 10, 103–112. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, L.; Li, L. Effect of shading on the algal blooms during spring Lake Dianchi. Environ. Sci. Technol. 2015, 38, 53–59. [Google Scholar]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Alba, L.G.; Torri, C.; Fabbri, D.; Kersten, S.R.; Brilman, D.W. Microalgae growth on the aqueous phase from hydrothermal liquefaction of the same microalgae. Chem. Eng. J. 2013, 228, 214223. [Google Scholar]
- Gnansounou, E.; Raman, J. Life cycle assessment of algae biodiesel and its co-products. Appl. Energy 2016, 161, 300308. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.H.; Fennell, P.S.; Shen, L.; Fan, L.S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1865–2046. [Google Scholar] [CrossRef]
- Thiruvenkadam, S.; Izhar, S.; Yoshida, H.; Danquah, M.K.; Harun, R. Process application of Subcritical Water Extraction (SWE) for algal bioproducts and biofuels production. Appl. Energy 2015, 154, 815–828. [Google Scholar] [CrossRef]
- Sikkema, R.; Steiner, M.; Junginger, M.; Hiegl, W.; Faaij, A. The European wood pellet markets: Current status and prospects for 2020. Biofuels Bioprod. Biorefin. 2011, 5, 250–278. [Google Scholar] [CrossRef]
- Adekunle, A.; Jamiu, K.; Peter, P.; Olumuyiwa, A.; Madhurai, M.; Dayanand, P. Essential basics on biomass torrefaction, densification and utilization. Int. J. Energy Res. 2021, 45, 1375–1395. [Google Scholar]
- Gao, N.; Li, A.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef]
- Chang, L. Study on the Formation and Release of Nitrogen-Containing Compounds during Coal Pyrolysis and Gasification. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2004. [Google Scholar]
- Tilman, D.; Fargione, J.; Wolff, B.; D’antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Liu, R.; Zhao, K.; Zheng, A.; Chang, S.; Zhao, Z.; Li, H. Characteristics of biomass gasification using chemical looping with iron ore as an oxygen carrier. Int. J. Hydrogen Energy 2013, 38, 14568–14575. [Google Scholar] [CrossRef]
- Mendiara, T.; Johansen, J.M.; Utrilla, R.; Geraldo, P.; Jensen, A.D.; Glarborg, P. Evaluation of different oxygen carriers for biomass tar reforming (I): Carbon deposition in experiments with toluene. Fuel 2011, 90, 1049–1060. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F. Catalytic oxygenless steam cracking of syngas-containing benzene model tar compound over natural Fe-bearing silicate minerals. Fuel 2012, 97, 741–750. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, L.; Zou, X. Chemical-looping auto-thermal reforming of biomass using Cu-based oxygen carrier. Appl. Energy 2015, 157, 408–415. [Google Scholar] [CrossRef]
- Keller, M.; Fung, J.; Leion, H.; Mattisson, T. Cu-impregnated alumina/silica bed materials for Chemical Looping Reforming of biomass gasification gas. Fuel 2016, 180, 448–456. [Google Scholar] [CrossRef]
- Hossain, M.; Sedor, K.; De Lasa, H. Co–Ni/Al2O3 oxygen carrier for fluidized bed chemical-looping combustion: Desorption kinetics and metal–support interaction. Chem. Eng. Sci. 2007, 62, 5464–5472. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Release and transformation of phosphorus in chemical looping combustion of sewage sludge. Chem. Eng. J. 2018, 335, 621–630. [Google Scholar] [CrossRef]
- He, F.; Huang, Z.; Liu, H. Biomass direct chemical looping conversion in a fluidized bed reactor with natural hematite as an oxygen carrier. In Proceedings of the Power and Energy Engineering Conference (APPEEC), Wuhan, China, 25–28 March 2011; pp. 1–7. [Google Scholar]
- Guo, Q.; Cheng, Y.; Liu, Y. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier. Ind. Eng. Chem. Res. 2013, 53, 78–86. [Google Scholar] [CrossRef]
- Matsuoka, K.; Shinbori, T.; Kuramoto, K. Mechanism of woody biomass pyrolysis and gasification in a fluidized bed of porous alumina particles. Energy Fuels 2006, 20, 1315–1320. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Q. Investigation into syngas generation from solid fuel using CaSO4-based chemical looping gasification process. Chin. J. Chem. Eng. 2013, 21, 127–134. [Google Scholar] [CrossRef]
- Cho, P.; Mattisson, T.; Lyngfelt, A. Comparison of iron-, nickel-, copper-and manganese-based oxygen carriers for chemical-looping combustion. Fuel 2004, 83, 1215–1225. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Rydèn, M. Chemical-looping combustion in a 300 W continuously operating reactor system using a manganese-based oxygen carrier. Fuel 2006, 85, 1174–1185. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Dennis, J.S.; Hayhurst, A.N.; Scott, S.A. Development and performance of Cu-based oxygen carriers for chemical-looping combustion. Combust. Flame 2008, 154, 109–121. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Zhao, Z.; Felix, L.G.; Slimane, R.B.; Choi, C.W.; Ozkan, U.S. Olivine catalysts for methane-and tar-steam reforming. Appl. Catal. B Environ. 2008, 81, 14–26. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.; Xiao, J.; Chen, D.; Gu, H.; Zhang, S. Nitrogen transfer of fuel-N in chemical looping combustion. Combust. Flame 2012, 159, 1286–1295. [Google Scholar] [CrossRef]
- Friebel, J.; Köpsel RF, W. The fate of nitrogen during pyrolysis of German low rank coals—A parameter study. Fuel 1999, 78, 923–932. [Google Scholar] [CrossRef]
- Tan, L.L.; Li, C.Z. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part I. Effects of reactor configuration on the determined yields of HCN and NH3 during pyrolysis. Fuel 2000, 79, 1883–1889. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.; Gu, H.; Jiang, S.; Xiao, J. Characteristics of hematite and fly ash during chemical looping combustion of sewage sludge. Chem. Eng. J. 2015, 268, 236–244. [Google Scholar] [CrossRef]








| Ultimate Analysis/(wt.%) | Proximate Analysis/(wt.%) | ||
|---|---|---|---|
| Cad | 40.74 | Mar | 84.73 |
| Had | 13.02 | Vd | 85.32 |
| Oad | 34.44 | FCd | 7.55 |
| Nad | 11.41 | Ad | 7.13 |
| Sad | 0.39 | ||
| Components | Fe2O3 | Al2O3 | SiO2 | TiO2 | P2O5 | CaO | K2O | Others |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 83.21 | 5.37 | 7.06 | 0.08 | 0.38 | 0.23 | 0.03 | 3.64 |
| Reactor | Stage | Factors | Condition | Quality Attributes |
|---|---|---|---|---|
| Single briquetting unit | Densification process | Moisture content Compressive load | (5–20%) (1–7 tons) | Forming rate Relax density Crushing pressure |
| Thermogravimetric analysis (TGA) reactor | Stage 1: Drying Stage 2: Devolatilization | Moisture content Compressive load Heating rate | (5–20%) (1–7 tons) 180 °C (drying) 500 °C (Devolatilization) (10–25 °C/min) | Forming rate Relax density |
| Single fluidized bed | Thermochemical transformation including char burnout | Temperature Hematite SEM analysis Cycle number | Batch feeding (750–900 °C) Hematite 30 g N2 1.5 L/min+steam 0.8 g/min | Carbon conversion rate Gas yield Reaction stability NO emission Phosphorus phase |
| Composition | |
|---|---|
| Incineration ash | SiO2(major), CaAl2Si2O8(major), Ca2P2O7(trace) |
| CLC fly ash | Fe3O4(major), SiO2(major), CaAl2Si2O8(major), CaH2P2O7(trace), CaHPO4(trace) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shen, L.; Ge, H.; Bai, H. Characteristic Study of Briquette Cyanobacteria as Fuel in Chemical Looping Combustion with Hematite as Oxygen Carrier. Appl. Sci. 2021, 11, 4388. https://doi.org/10.3390/app11104388
Zhang H, Shen L, Ge H, Bai H. Characteristic Study of Briquette Cyanobacteria as Fuel in Chemical Looping Combustion with Hematite as Oxygen Carrier. Applied Sciences. 2021; 11(10):4388. https://doi.org/10.3390/app11104388
Chicago/Turabian StyleZhang, Haifeng, Laihong Shen, Huijun Ge, and Hongcun Bai. 2021. "Characteristic Study of Briquette Cyanobacteria as Fuel in Chemical Looping Combustion with Hematite as Oxygen Carrier" Applied Sciences 11, no. 10: 4388. https://doi.org/10.3390/app11104388
APA StyleZhang, H., Shen, L., Ge, H., & Bai, H. (2021). Characteristic Study of Briquette Cyanobacteria as Fuel in Chemical Looping Combustion with Hematite as Oxygen Carrier. Applied Sciences, 11(10), 4388. https://doi.org/10.3390/app11104388





