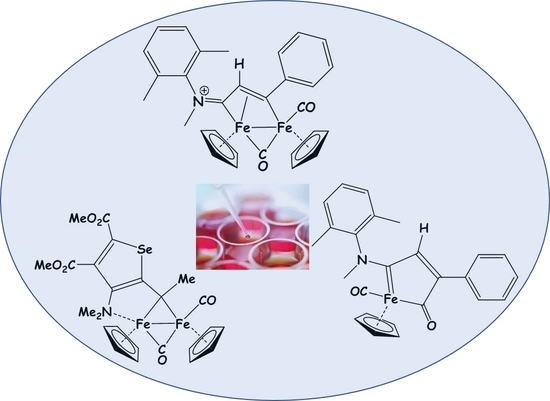
The Cytotoxic Activity of Diiron Bis-Cyclopentadienyl Complexes with Bridging C3-Ligands
Abstract
1. Introduction
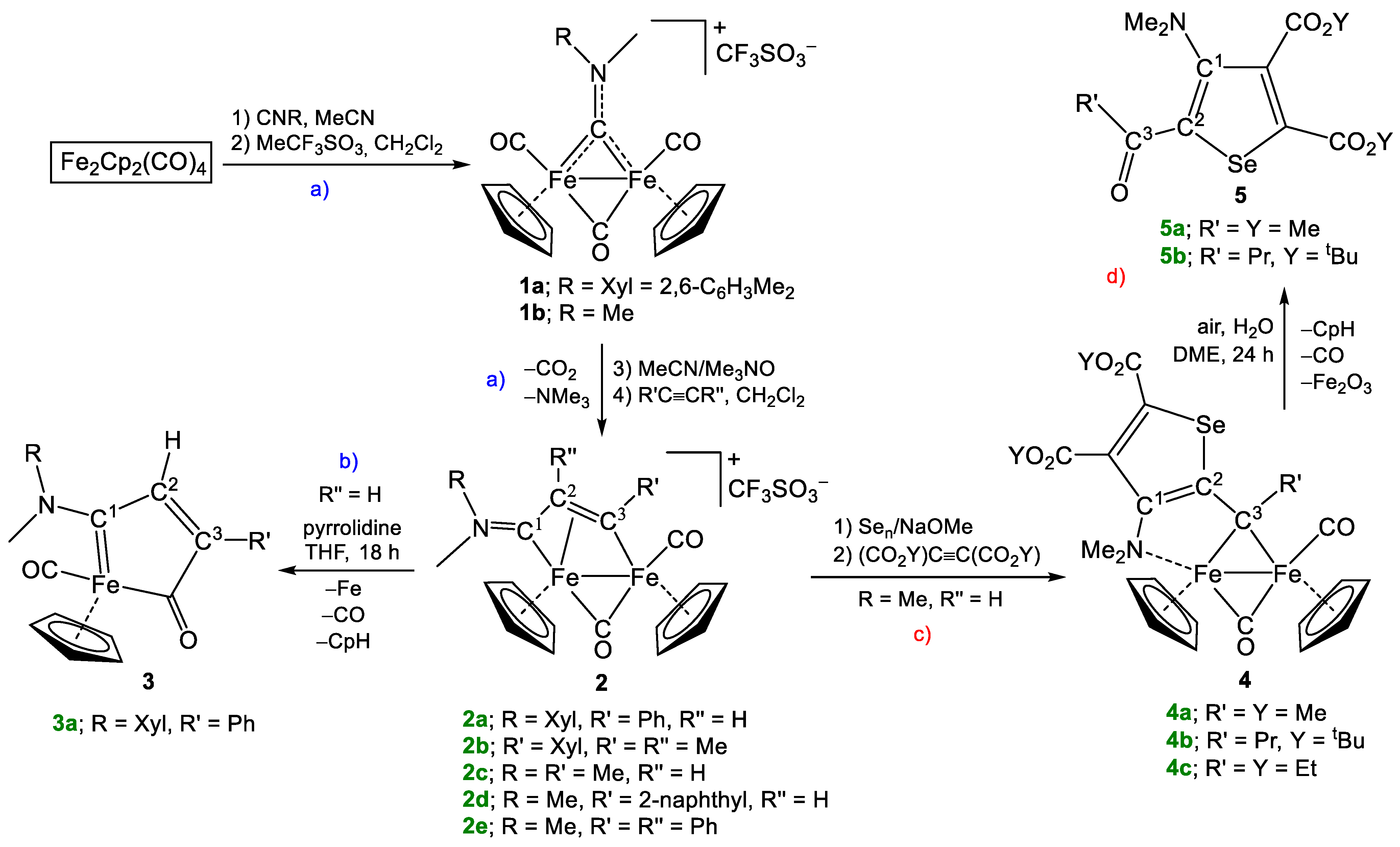
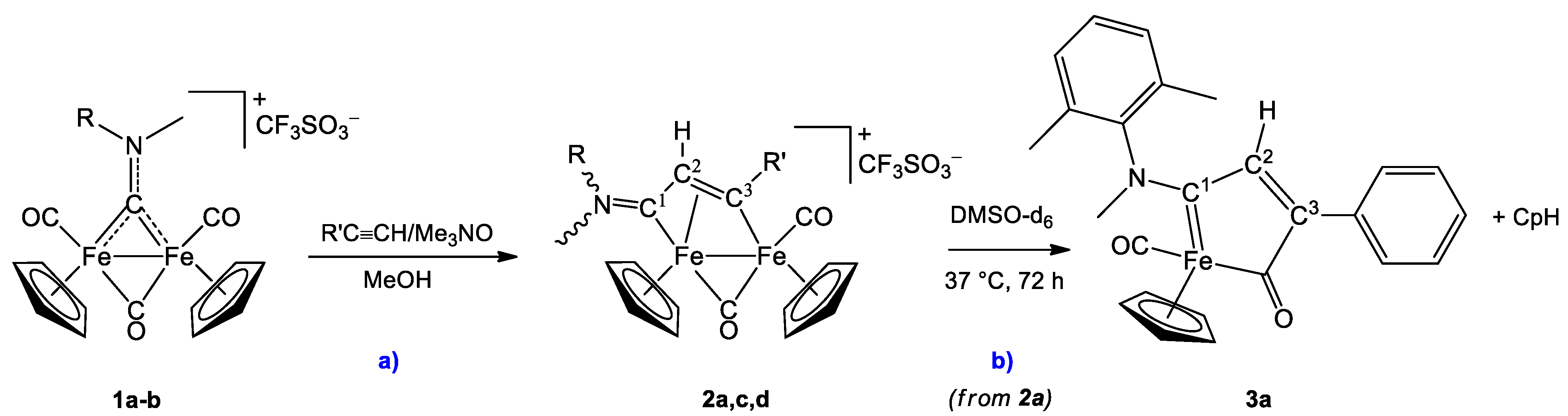
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Materials and Methods
4.2. Synthesis of [Fe2Cp2(CO)(μ-CO){μ-η1:η3-C3(R′)C2(R″)C1NMe(R)}]CF3SO3, (2a,c,d)
4.3. Stability in DMSO/Water Mixtures
4.3.1. Formation of [FeCp(CO){C1(NMeXyl)C2HC3(Ph)C(O)}], 3a, in DMSO-d6
4.3.2. Stability in DMSO-d6/D2O
4.3.3. Stability in DMSO-d6/DMEM-d Cell Culture Medium
4.4. In Vitro Cytotoxicity Investigation
4.4.1. Cell Lines
4.4.2. Cytotoxicity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, B.S.; Dyson, P.J. Recent progress in the development of organometallics for the treatment of cancer. Curr. Opinion Chem. Biol. 2020, 56, 28–34. [Google Scholar] [CrossRef]
- Štarha, P.; Trávníček, Z. Non-platinum complexes containing releasable biologically active ligands. Coord. Chem. Rev. 2019, 395, 130–145. [Google Scholar] [CrossRef]
- Bratsos, I.; Gianferrara, T.; Alessio, E.; Hartinger, C.G.; Jakupec, M.A.; Keppler, B.K. Ruthenium and Other Non-Platinum Anticancer Compounds. In Bioinorganic Medicinal Chemistry; Alessio, E., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 151–174. [Google Scholar]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life, 2nd ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Chem. Rev. 2017, 1. [Google Scholar] [CrossRef]
- Sansook, S.; Hassell-Hart, S.; Ocasio, C.; Spencer, J. Ferrocenes in medicinal chemistry; a personal perspective. J. Organomet. Chem. 2020, 905, 121017. [Google Scholar] [CrossRef]
- Pilon, A.; Brás, A.R.; Côrte-Real, L.; Avecilla, F.; Costa, P.J.; Preto, A.; Garcia, M.H.; Valente, A. A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells. Molecules 2020, 25, 1592. [Google Scholar] [CrossRef]
- Buriez, O.; Heldt, J.M.; Labb, E.; Vessières, A.; Jaouen, G.; Amatore, C. Reactivity and Antiproliferative Activity of Ferrocenyl–Tamoxifen Adducts with Cyclodextrins against Hormone-Independent Breast-Cancer Cell Lines. Chem. Eur. J. 2008, 14, 8195–8203. [Google Scholar] [CrossRef]
- Vessières, A.; Wang, Y.; McGlinchey, M.J.; Jaouen, G. Multifaceted chemical behaviour of metallocene (M = Fe, Os) quinone methides. Their contribution to biology. Coord. Chem. Rev. 2021, 430, 213658. [Google Scholar] [CrossRef]
- Marchetti, F. Constructing Organometallic Architectures from Aminoalkylidyne Diiron Complexes. Eur. J. Inorg. Chem. 2018, 3987–4003. [Google Scholar] [CrossRef]
- Mazzoni, R.; Marchetti, F.; Cingolani, A.; Zanotti, V. Bond Forming Reactions Involving Isocyanides at Diiron Complexes. Inorganics 2019, 7, 25. [Google Scholar] [CrossRef]
- Biancalana, L.; Ciancaleoni, G.; Zacchini, S.; Pampaloni, G.; Marchetti, F. Carbonyl-isocyanide mono-substitution in [Fe2Cp2(CO)4]: A re-visitation. Inorg. Chim. Acta 2020, 517, 120181. [Google Scholar] [CrossRef]
- Agonigi, G.; Biancalana, L.; Lupo, M.G.; Montopoli, M.; Ferri, N.; Zacchini, S.; Binacchi, F.; Biver, T.; Campanella, B.; Pampaloni, G.; et al. Exploring the Anticancer Potential of Diiron Bis-cyclopentadienyl Complexes with Bridging Hydrocarbyl Ligands: Behavior in Aqueous Media and In Vitro Cytotoxicity. Organometallics 2020, 39, 645–657. [Google Scholar] [CrossRef]
- Schoch, S.; Batchelor, L.K.; Funaioli, T.; Ciancaleoni, G.; Zacchini, S.; Braccini, S.; Chiellini, F.; Biver, T.; Pampaloni, G.; Dyson, P.J.; et al. Diiron Complexes with a Bridging Functionalized Allylidene Ligand: Synthesis, Structural Aspects, and Cytotoxicity. Organometallics 2020, 39, 361–373. [Google Scholar] [CrossRef]
- Rocco, D.; Batchelor, L.K.; Agonigi, G.; Braccini, S.; Chiellini, F.; Schoch, S.; Biver, T.; Funaioli, T.; Zacchini, S.; Biancalana, L.; et al. Anticancer Potential of Diiron Vinyliminium Complexes. Chem. Eur. J. 2019, 25, 14801–14816. [Google Scholar] [CrossRef]
- Agonigi, G.; Batchelor, L.K.; Ferretti, E.; Schoch, S.; Bortoluzzi, M.; Braccini, S.; Chiellini, F.; Biancalana, L.; Zacchini, S.; Pampaloni, G.; et al. Mono-, Di- and Tetra-iron Complexes with Selenium or Sulphur Functionalized Vinyliminium Ligands: Synthesis, Structural Characterization and Antiproliferative Activity. Molecules 2020, 25, 1656. [Google Scholar] [CrossRef] [PubMed]
- Rocco, D.; Busto, N.; Pérez-Arnaiz, C.; Biancalana, L.; Zacchini, S.; Pampaloni, G.; Garcia, B.; Marchetti, F. Antiproliferative and bactericidal activity of diiron and monoiron cyclopentadienyl carbonyl complexes comprising a vinyl-aminoalkylidene unit. Appl. Organomet. Chem. 2020, 34, 5923. [Google Scholar] [CrossRef]
- Ciancaleoni, G.; Zacchini, S.; Zanotti, V.; Marchetti, F. DFT Mechanistic Insights into the Alkyne Insertion Reaction Affording Diiron μ-Vinyliminium Complexes and New Functionalization Pathways. Organometallics 2018, 37, 3718–3731. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Q.; Hu, J.; Li, X.; Zhang, X. Reactive Oxygen Species (ROS)-Responsive Prodrugs, Probes, and Theranostic Prodrugs: Applications in the ROS-Related Diseases. J. Med. Chem. 2021, 64, 298–325. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Jung, H.S. Metal–organic complex-based chemodynamic therapy agents for cancer therapy. Chem. Commun. 2020, 56, 8332–8341. [Google Scholar] [CrossRef]
- Marzenell, P.; Hagen, H.; Sellner, L.; Zenz, T.; Grinyte, R.; Pavlov, V.; Daum, S.; Mokhir, A. Aminoferrocene-Based Prodrugs and Their Effects on Human Normal and Cancer Cells as Well as Bacterial Cells. J. Med. Chem. 2013, 56, 6935–6944. [Google Scholar] [CrossRef]
- Rocco, D.; Batchelor, L.K.; Ferretti, E.; Zacchini, S.; Pampaloni, G.; Dyson, P.J.; Marchetti, F. Piano Stool Aminoalkyli-dene-Ferracyclopentenone Complexes from Bimetallic Precursors: Synthesis and Cytotoxicity, Data. ChemPlusChem 2020, 85, 110–122. [Google Scholar] [CrossRef]
- Busetto, L.; Marchetti, F.; Zacchini, S.; Zanotti, V. Unprecedented Zwitterionic Iminium−Chalcogenide Bridging Ligands in Diiron Complexes. Organometallics 2006, 25, 4808–4816. [Google Scholar] [CrossRef]
- Provinciali, G.; Bortoluzzi, M.; Funaioli, T.; Zacchini, S.; Campanella, B.; Pampaloni, G.; Marchetti, F. Tetrasubstituted Selenophenes from the Stepwise Assembly of Molecular Fragments on a Diiron Frame and Final Cleavage of a Bridging Alkylidene. Inorg. Chem. 2020, 59, 17497–17508. [Google Scholar] [CrossRef] [PubMed]
- Brayshaw, S.K.; Clarke, L.P.; Homanen, P.; Koentjoro, O.F.; Warren, J.E.; Raithby, P.R. Arene—Ruthenium(II) Complexes Containing Amino—Phosphine Ligands as Catalysts for Nitrile Hydration Reactions. Organometallics 2011, 30, 3955–3965. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Collery, C. Strategies for the development of selenium-based anticancer drugs. J. Trace Elem. Med. Biol. 2018, 50, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Tan, H.-W.; Mo, H.-J.; Lau, A.T.Y.; Xu, Y.-M. Selenium Species: Current Status and Potentials in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 75. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef]
- Albano, V.G.; Busetto, L.; Marchetti, F.; Monari, M.; Zacchini, S.; Zanotti, V. Hydride addition at μ-vinyliminium ligand obtained from disubstituted alkynes. J. Organomet. Chem. 2005, 690, 837–846. [Google Scholar] [CrossRef]
- Albano, V.G.; Busetto, L.; Marchetti, F.; Monari, M.; Zacchini, S.; Zanotti, V. Diiron µ-Vinyliminium Complexes from Acetylene Insertion into a Metal-Aminocarbyne Bond. Organometallics 2003, 22, 1326–1331. [Google Scholar] [CrossRef]
- Agonigi, G.; Ciancaleoni, G.; Funaioli, T.; Zacchini, S.; Pineider, F.; Pinzino, C.; Pampaloni, G.; Zanotti, V.; Marchetti, F. Controlled Dissociation of Iron and Cyclopentadienyl from a Diiron Complex with a Bridging C3 Ligand Triggered by One-Electron Reduction. Inorg. Chem. 2018, 57, 15172–15186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xiao, Z.; Zhong, W.; Liu, X. Brief survey of diiron and monoiron carbonyl complexes and their potentials as CO-releasing molecules (CORMs). Coord. Chem. Rev. 2021, 429, 213634. [Google Scholar] [CrossRef]
- Agonigi, G.; Bortoluzzi, M.; Marchetti, F.; Pampaloni, G.; Zacchini, S.; Zanotti, V. Regioselective Nucleophilic Additions to Diiron Carbonyl Complexes Containing a Bridging Aminocarbyne Ligand: A Synthetic, Crystallographic and DFT Study. Eur. J. Inorg. Chem. 2018, 2018, 960–971. [Google Scholar] [CrossRef]
- Menges, F. “Spectragryph—Optical Spectroscopy Software”, Version 1.2.5, @ 2016–2017. Available online: http://www.effemm2.de/spectragryph (accessed on 20 April 2021).
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
| Comp. | A2780 | A2780cisR | A549 | BxPC-3 |
|---|---|---|---|---|
| 2a | 0.50 ± 0.06 | 1.2 ± 0.2 | 43 ± 4 | 11.5 ± 1.4 |
| 2a | 5.3 ± 1.2 (*) | 25 ± 2 (*) | ||
| 2b | 0.90 ± 0.06 | 4.0 ± 0.3 | >100 | >100 |
| 2c | 35 ± 3 | 86 ± 7 | >100 | >100 |
| 2d | 2.9 ± 0.2 | 9.3 ± 0.7 | >100 | 92 ± 2 |
| 2e | 1.8 ± 0.2 | 7.5 ± 0.3 | >100 | >100 |
| 3a | 16 ± 2 | 26 ± 3 | ||
| 3a | 37 ± 2 (*) | 86 ± 4 (*) | ||
| 4a | 25.9 ± 1.6 | 29 ± 3 | ||
| 4b | 37 ± 3 | 42 ± 6 | ||
| 4c | 39 ± 2 | 50 ± 6 | ||
| 5a | 64 ± 4 | >100 | ||
| 5b | >100 | >100 | ||
| cisplatin | 19.0 ± 1.1 (*) | 71 ± 3 (*) | ||
| cisplatin | 0.40 ± 0.07 | 26 ± 3 | 9.5 ± 0.6 | 4.6 ± 0.4 |
| Comp. | DMSO-d6/D2O | DMSO-d6/DMEM-d | |||||
|---|---|---|---|---|---|---|---|
| 6 h | 24 h | 48 h | 72 h | 6 h | 24 h | 72 h | |
| 4a | 3 | 13 | 27 | 70 | 5 | 9 | 100 |
| 4b | 25 | 71 | 100 | 100 | 13 | 49 | 100 |
| 4c | 17 | 60 | 88 | 100 | 9 | 20 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braccini, S.; Provinciali, G.; Biancalana, L.; Pampaloni, G.; Chiellini, F.; Marchetti, F. The Cytotoxic Activity of Diiron Bis-Cyclopentadienyl Complexes with Bridging C3-Ligands. Appl. Sci. 2021, 11, 4351. https://doi.org/10.3390/app11104351
Braccini S, Provinciali G, Biancalana L, Pampaloni G, Chiellini F, Marchetti F. The Cytotoxic Activity of Diiron Bis-Cyclopentadienyl Complexes with Bridging C3-Ligands. Applied Sciences. 2021; 11(10):4351. https://doi.org/10.3390/app11104351
Chicago/Turabian StyleBraccini, Simona, Giacomo Provinciali, Lorenzo Biancalana, Guido Pampaloni, Federica Chiellini, and Fabio Marchetti. 2021. "The Cytotoxic Activity of Diiron Bis-Cyclopentadienyl Complexes with Bridging C3-Ligands" Applied Sciences 11, no. 10: 4351. https://doi.org/10.3390/app11104351
APA StyleBraccini, S., Provinciali, G., Biancalana, L., Pampaloni, G., Chiellini, F., & Marchetti, F. (2021). The Cytotoxic Activity of Diiron Bis-Cyclopentadienyl Complexes with Bridging C3-Ligands. Applied Sciences, 11(10), 4351. https://doi.org/10.3390/app11104351