Continuous-Wave THz Imaging for Biomedical Samples
Abstract
1. Introduction
2. CW THz Single-Point Scanning Imaging
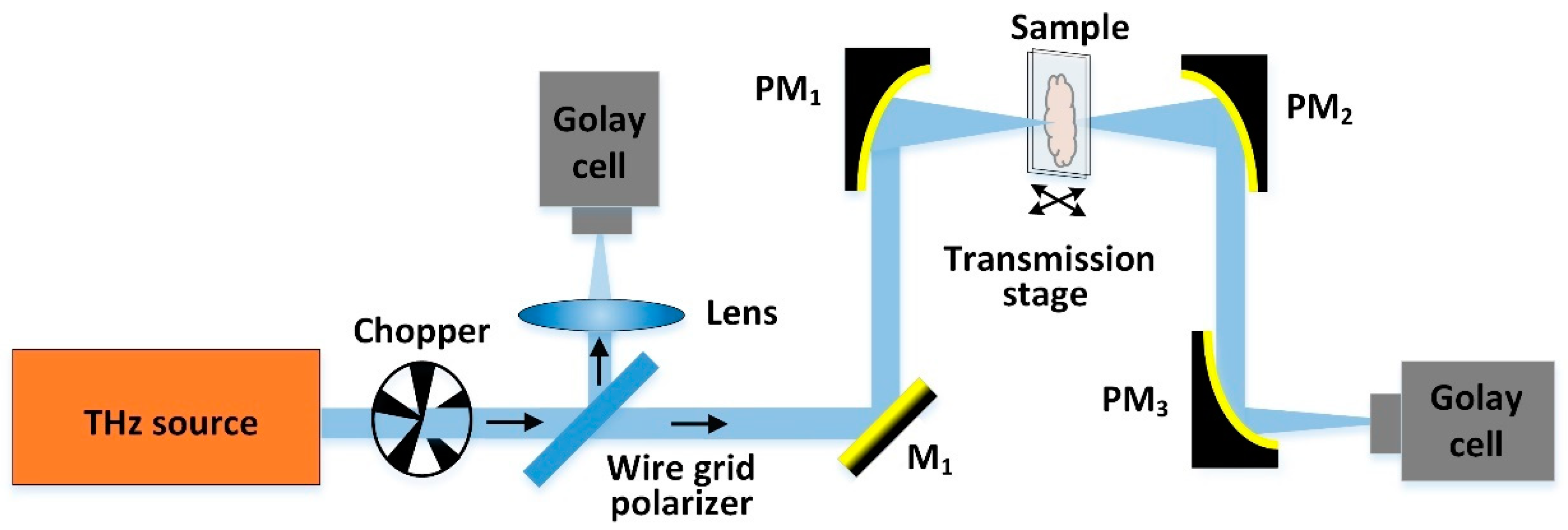
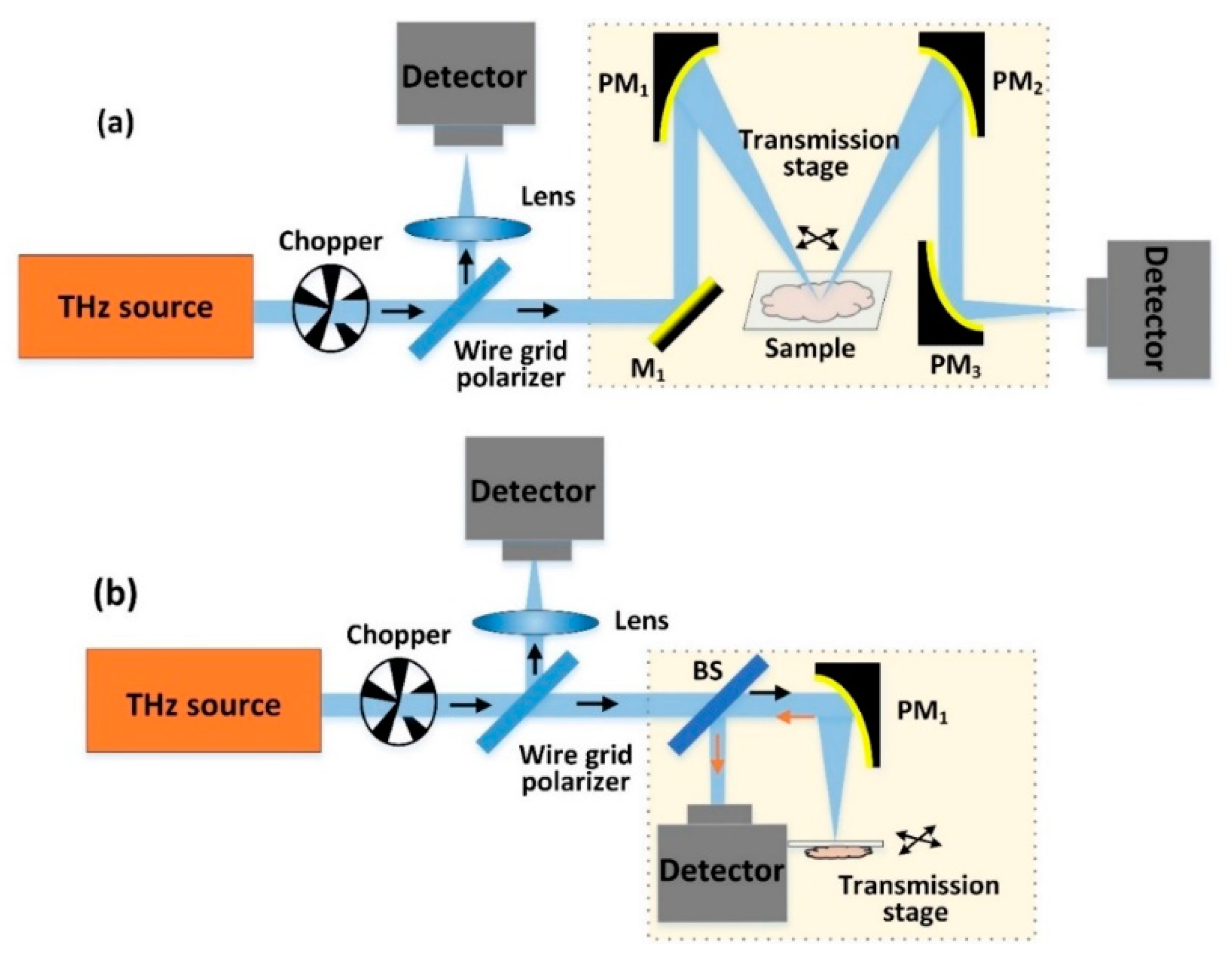
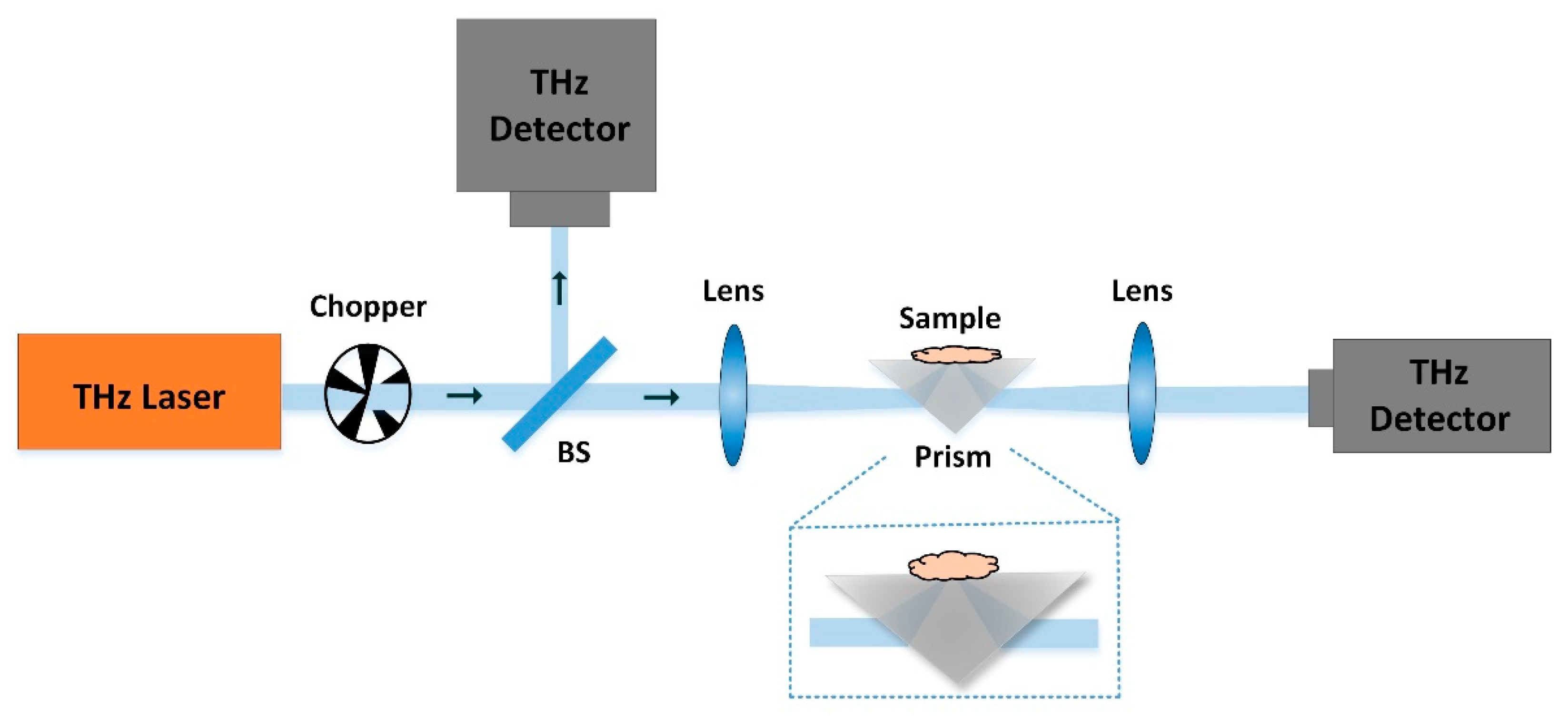
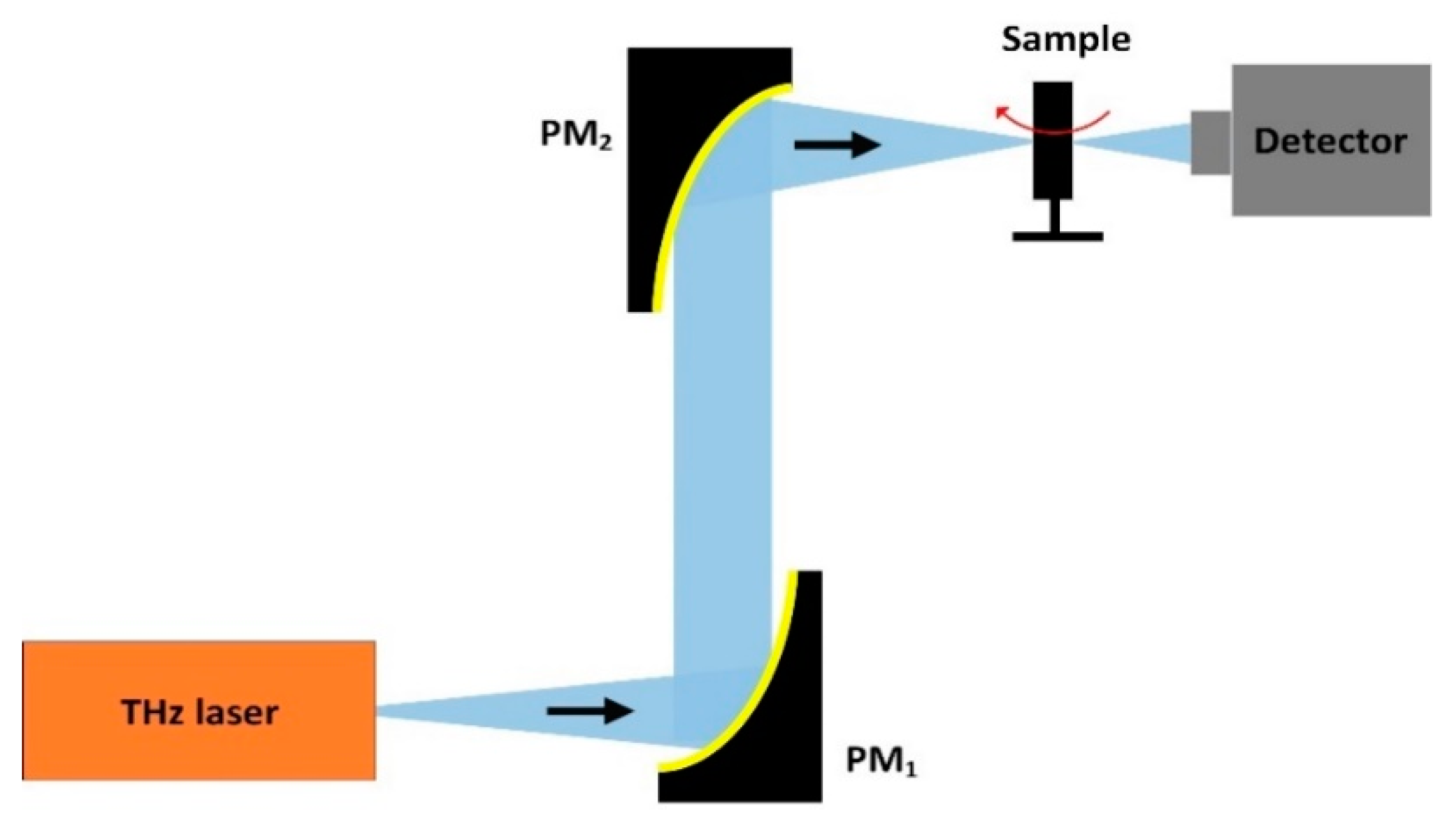
2.1. CW-THz Transmission Single-Point Scanning Imaging
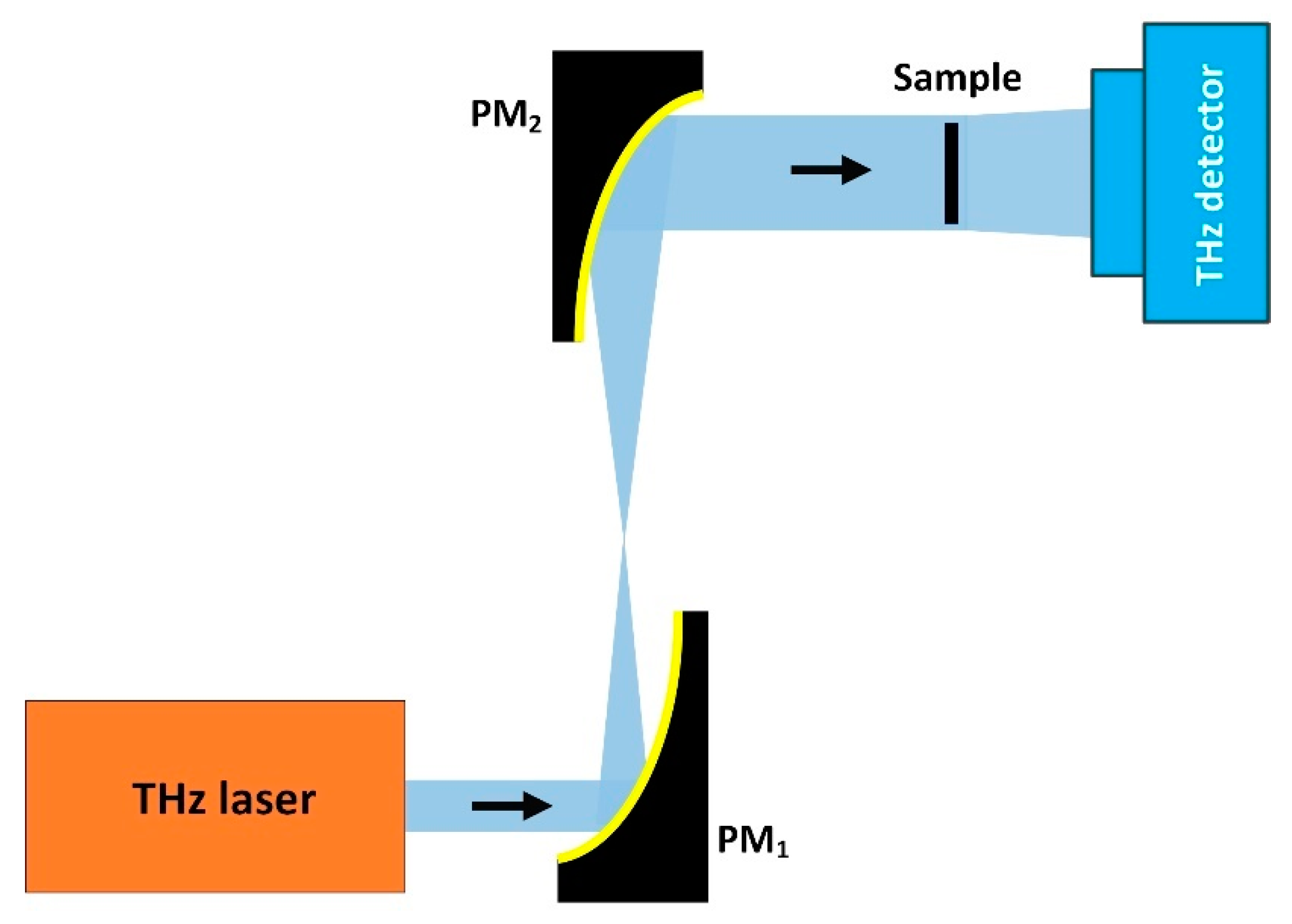
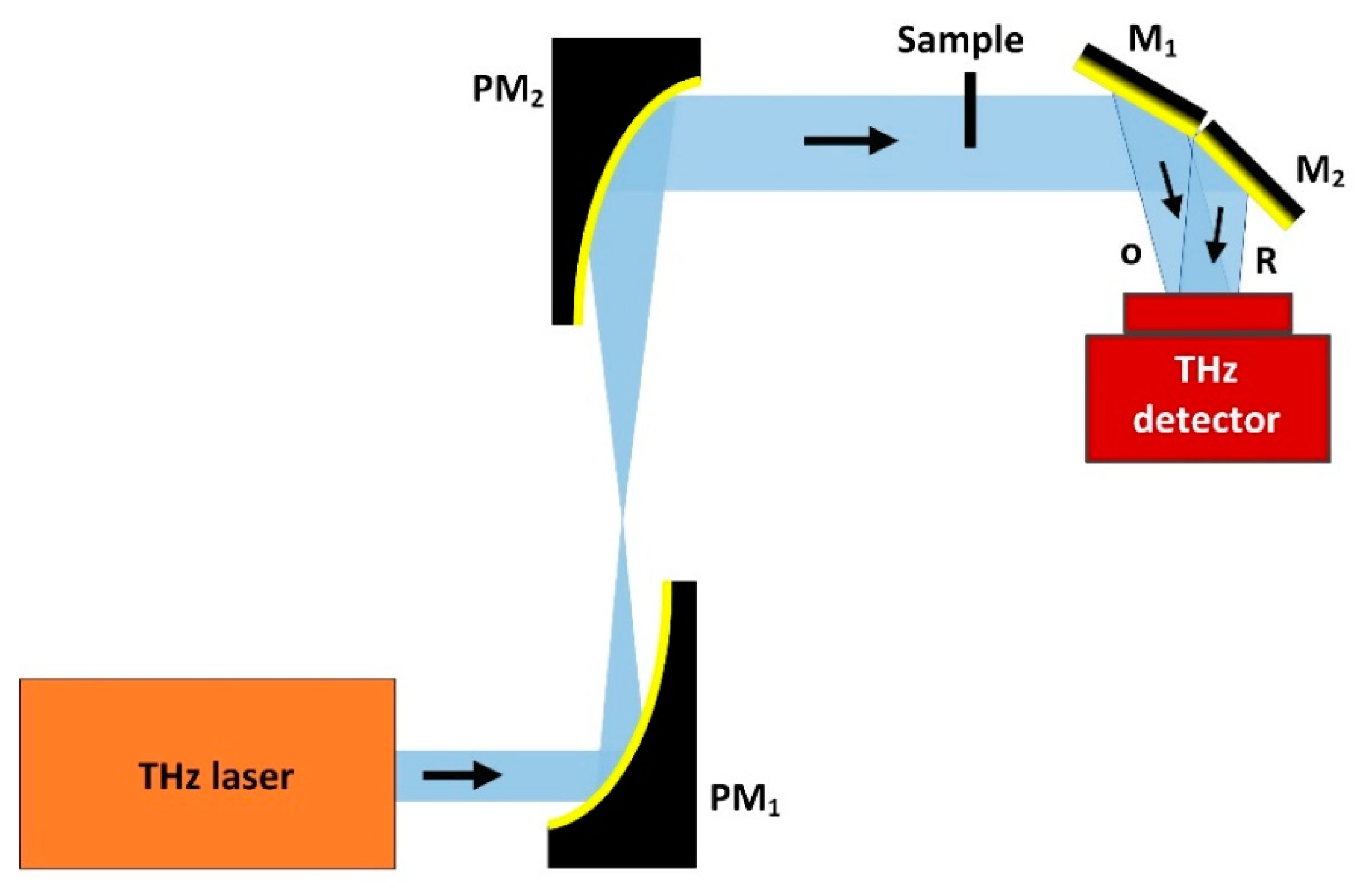
2.2. CW THz Reflection Single-Point Scanning Imaging
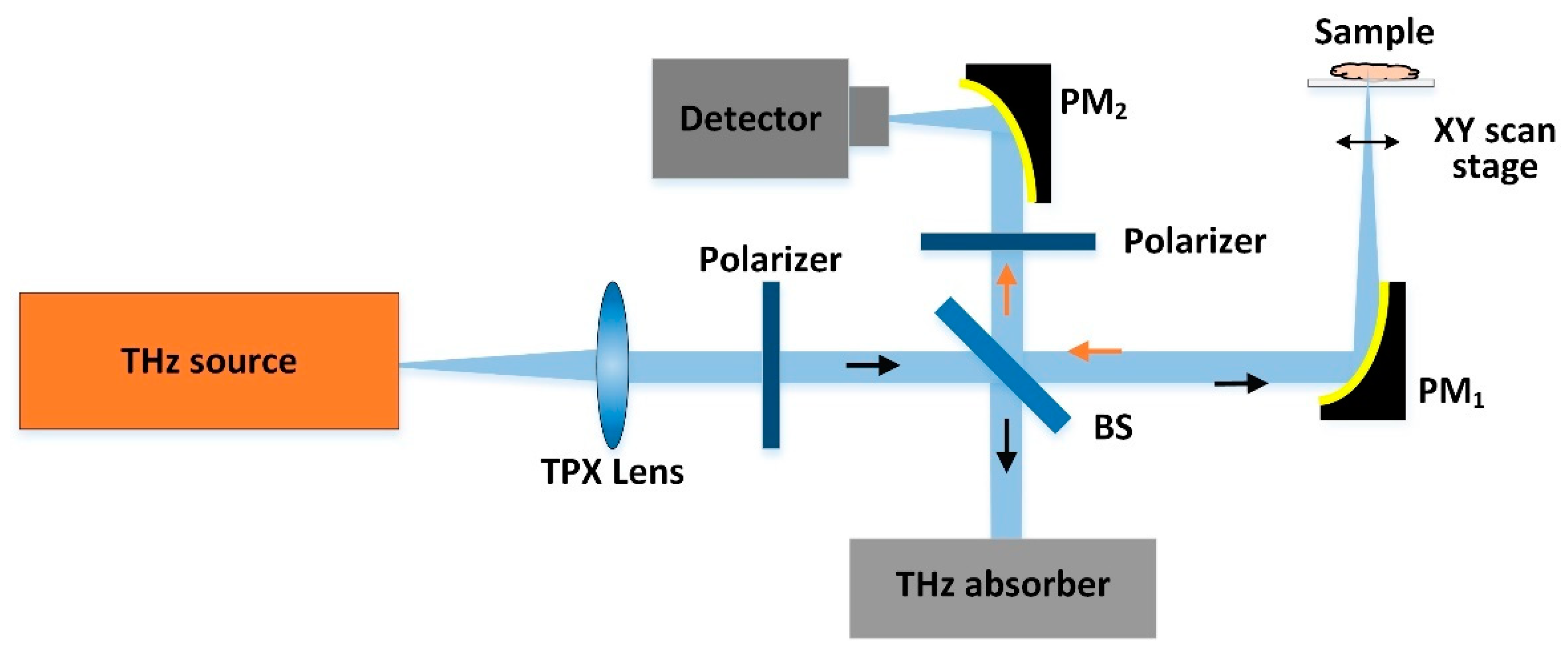
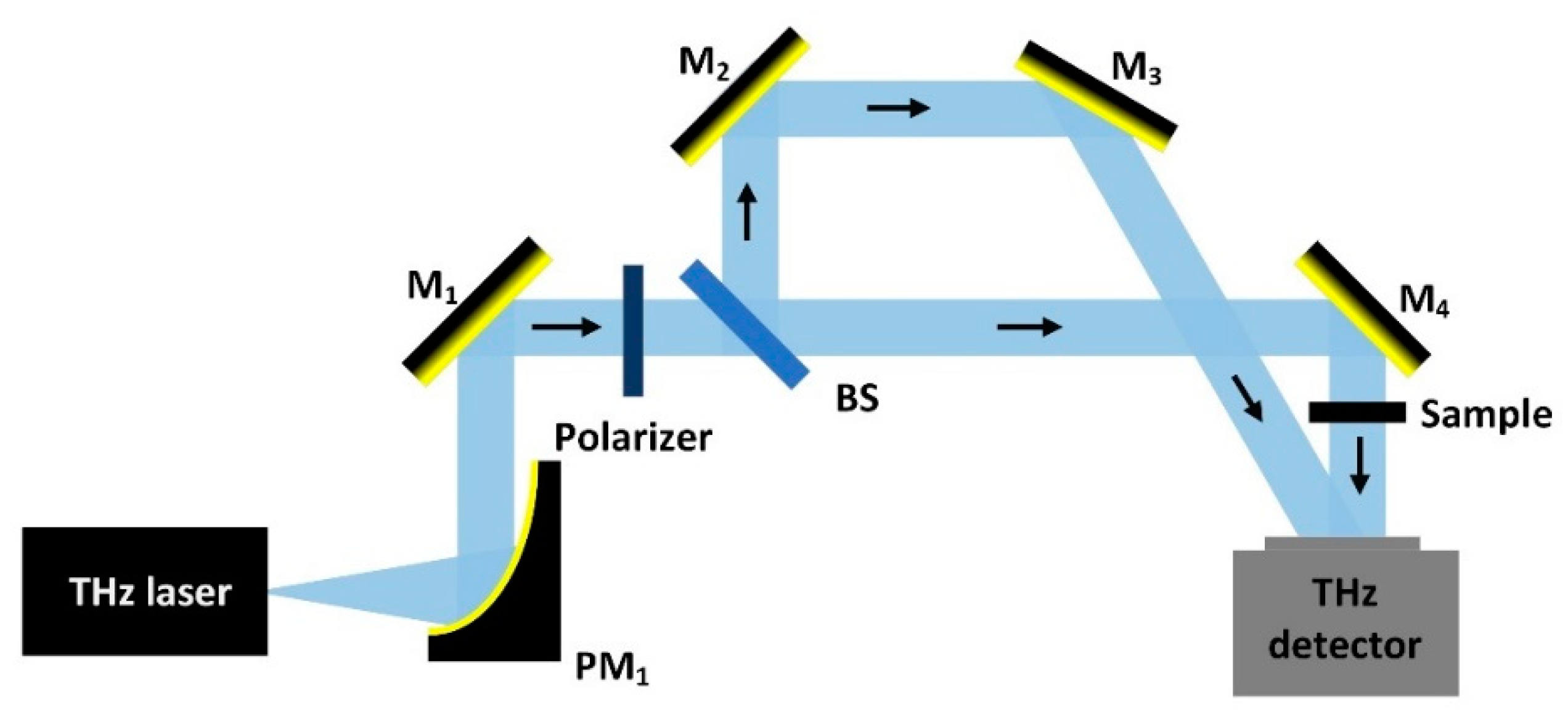
2.3. CW-THz Polarisation Single-Point Scanning Imaging
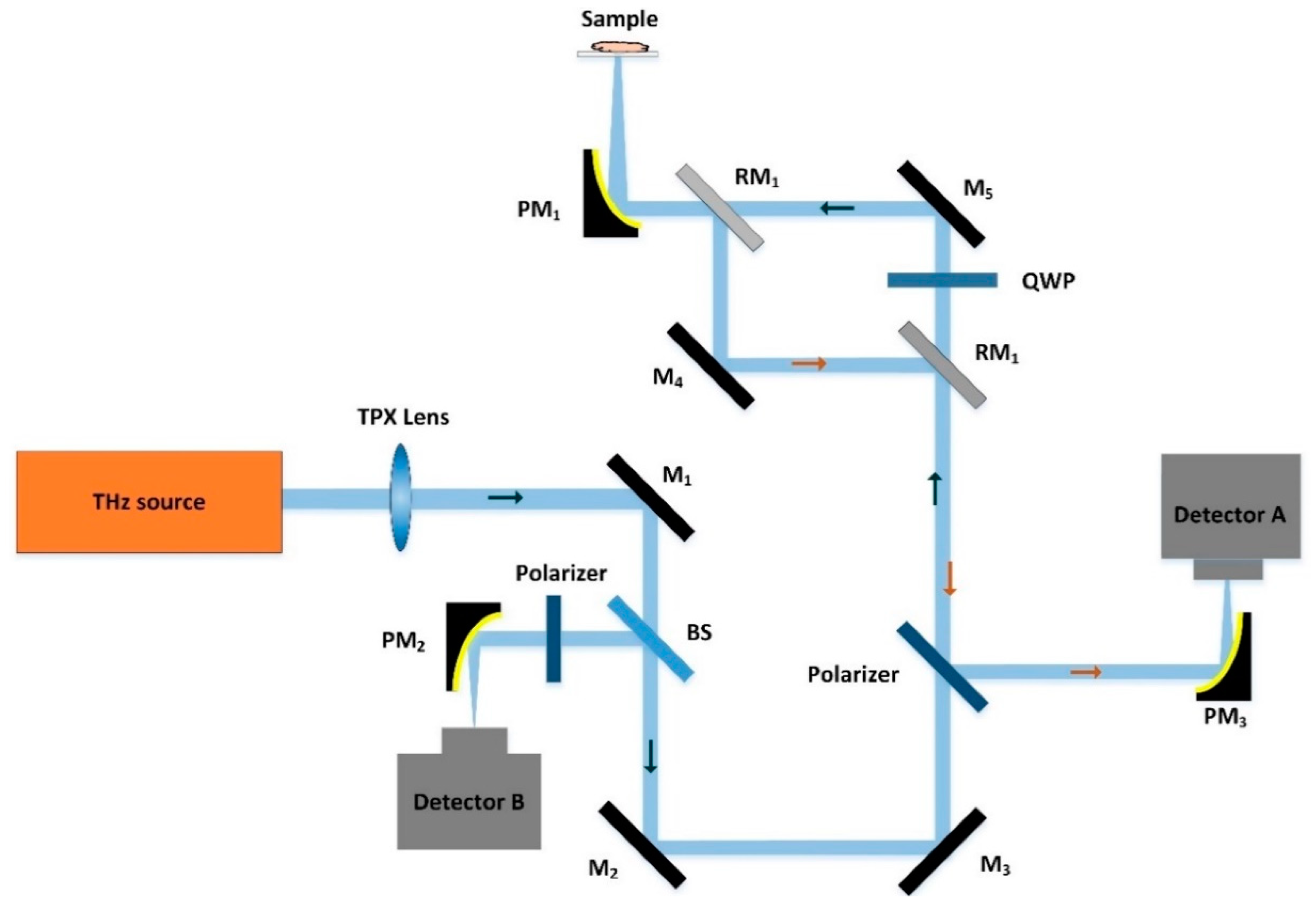
2.4. CW THz Attenuated Total Reflection Imaging
2.5. CW-THz Near-Field Microscopy Imaging
2.6. CW-THz Single-Point Phase Contrast Imaging
3. CW THz Full-Field Imaging
3.1. CW THz Full-Field Amplitude Imaging
3.2. CW THz Digital Holography
3.3. CW THz Ptychography Imaging
4. CW THz Tomography Imaging
5. The Application of CW Imaging with Biological Tissues
5.1. Tumour Tissues
5.1.1. Non-Melanoma Skin Cancers
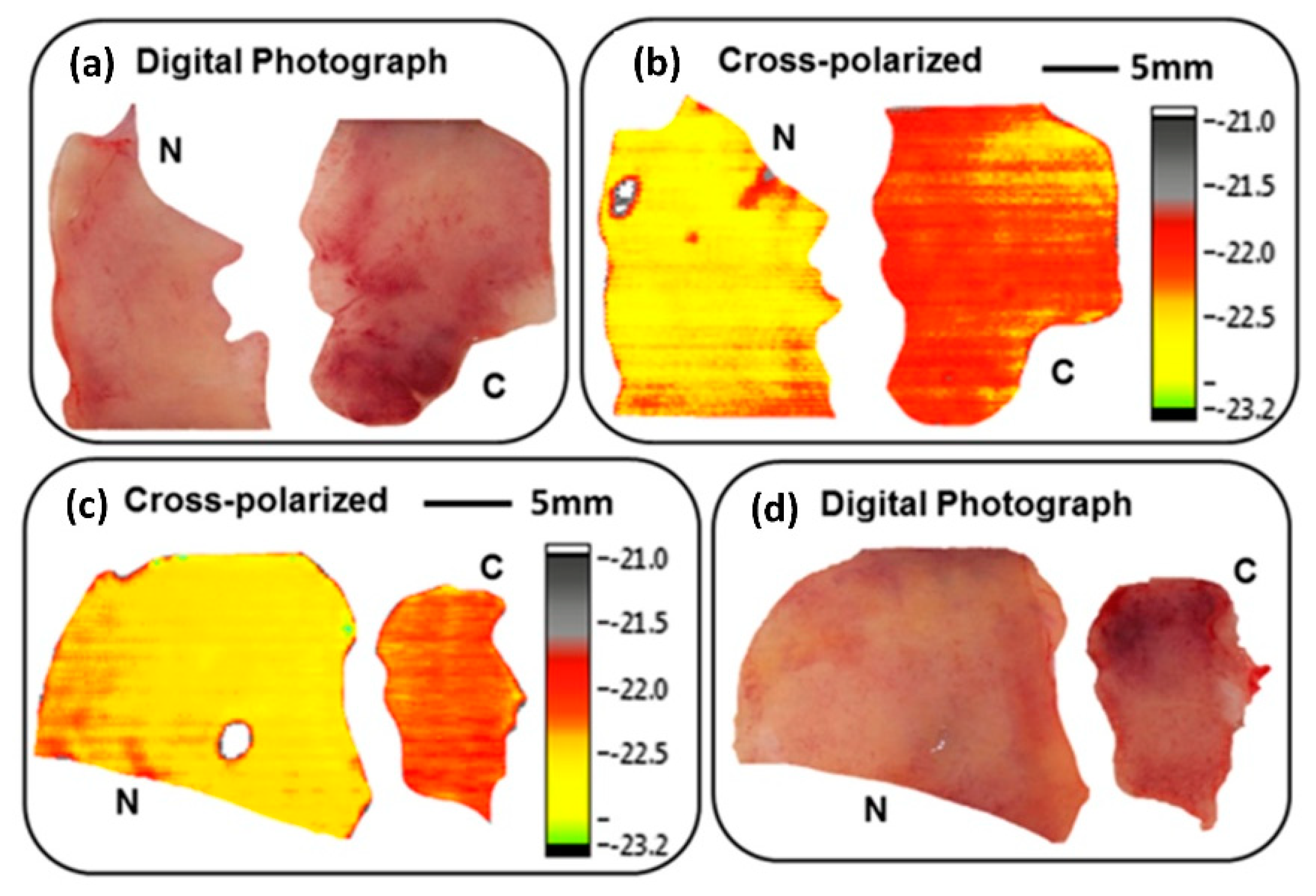
5.1.2. Breast Cancer
5.1.3. Brain Glioma
5.1.4. Human Colon Tissues
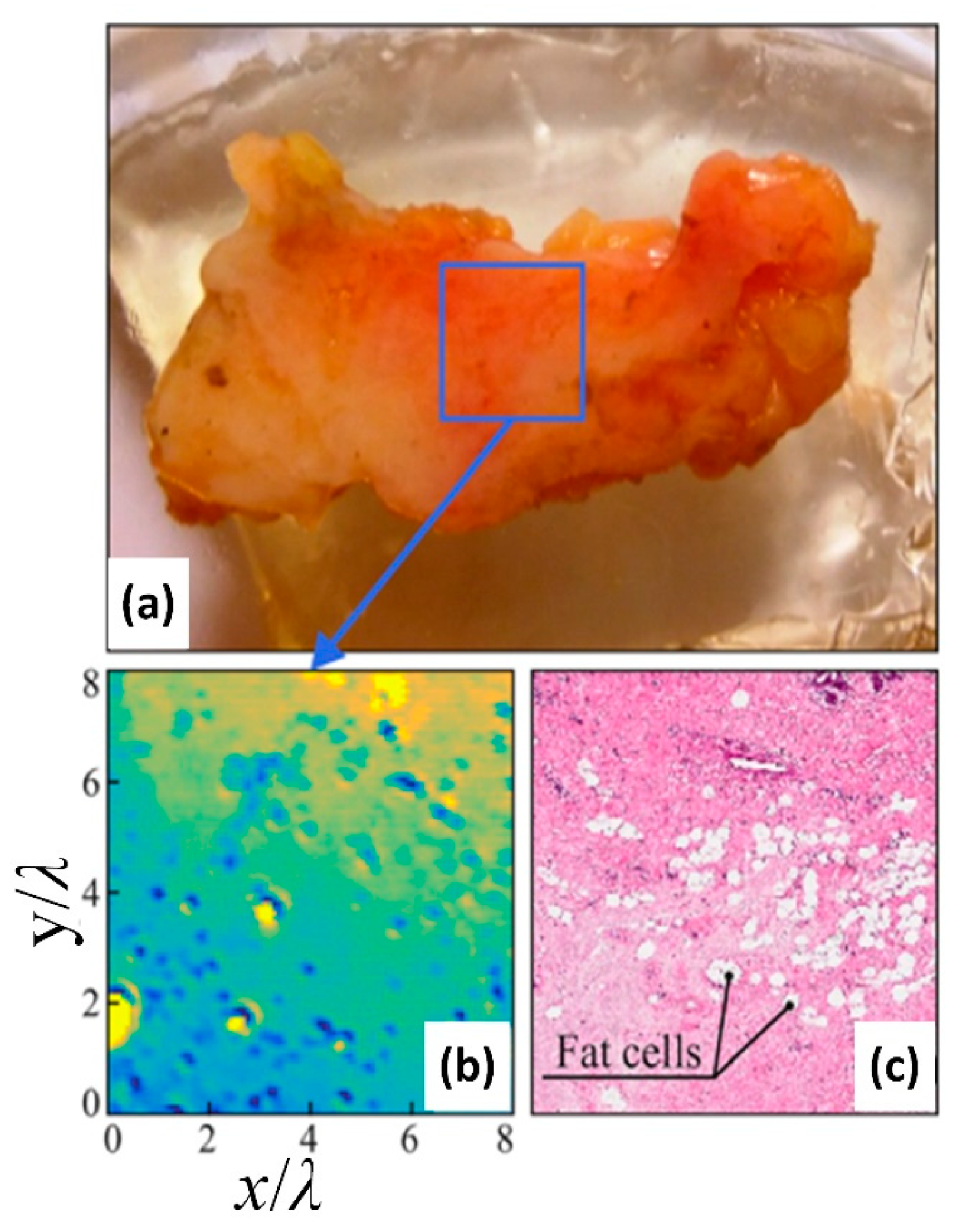
5.1.5. Liver Cancer
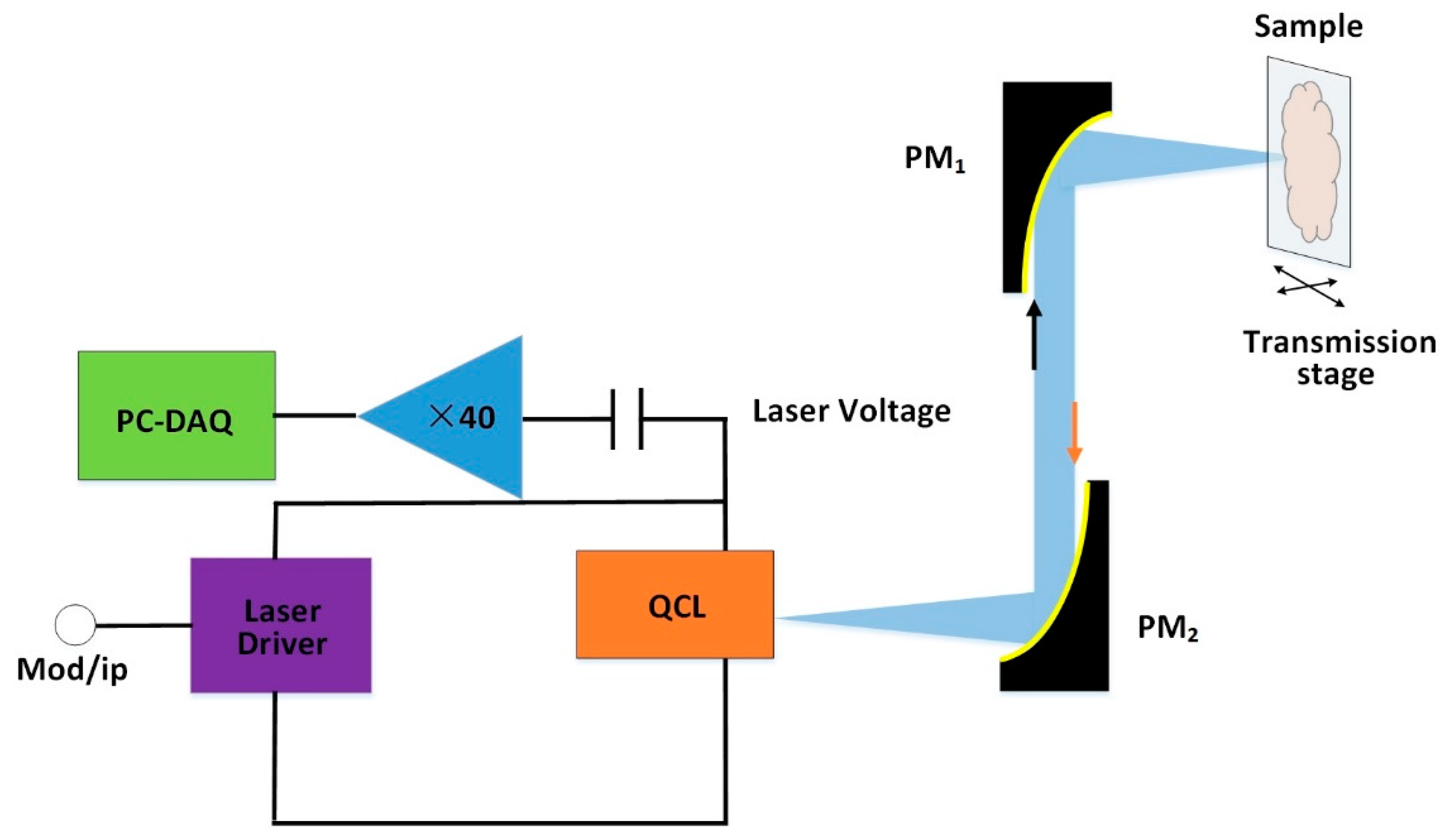
5.2. Traumatic Brain Injury of Rat
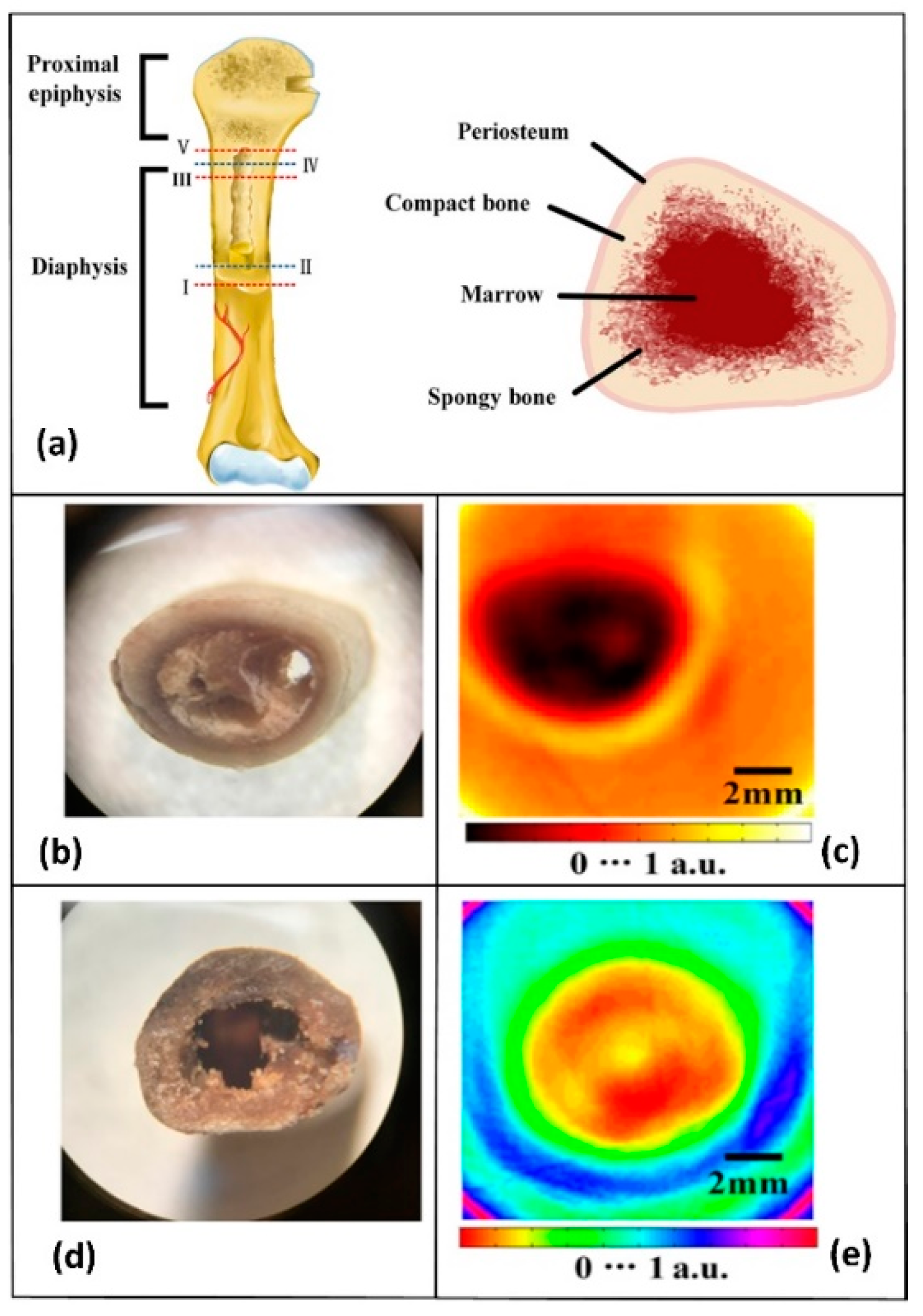
5.3. Bones
6. Conclusions and Perspectives
- (1)
- The lack of high sensitivity, large dynamic range, and large number of pixels challenge the development of THz array detectors for the observation and recording of biological samples in real-time.
- (2)
- The lack of multiple and flexible THz beam shaping devices to achieve diffraction-limited spatial resolution and high-quality THz images.
- (3)
- The lack of THz waveguides for lossless transmission to access tissues.
- (4)
- The lack of an effective method to sustain the activity of tissue samples for a long time through THz imaging acquisition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erik, B.; Heinz-Wilhelm, H.; Maurice, F. Terahertz Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–28. [Google Scholar]
- Lee, Y. Principles of Terahertz Science and Technology; Springer: New York, NY, USA, 2009; pp. 1–41. [Google Scholar]
- Peralta, X.G.; Lipscomb, D.; Wilmink, G.J.; Echchgadda, I. Terahertz spectroscopy of human skin tissue models with different melanin content. Biomed. Opt. Express 2019, 10, 2942–2955. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.Y.; Sonawane, D.D.; Erande, K.B.; Derle, D.V. Terahertz technology and its applications. Drug Invent. Today 2013, 5, 157–163. [Google Scholar] [CrossRef]
- Fan, S.; He, Y.; Ung, B.; Pickwell-MacPherson, E. The growth of biomedical terahertz research. Appl. Phys. 2014, 47, 374009. [Google Scholar] [CrossRef]
- Ho, L.; Pepper, M.; Taday, P.F. Terahertz spectroscopy: Signatures and fingerprints. Nat. Photonics 2008, 2, 541–543. [Google Scholar] [CrossRef]
- Wang, Y.; Minamide, H.; Tang, M.; Notake, T.; Ito, H. Study of water concentration measurement in thin tissues with terahertz-wave parametric source. Opt. Express 2010, 18, 15504–15512. [Google Scholar] [CrossRef]
- Yardimci, T.; Cakmakyapan, S.; Hemmati, S.; Jarrahi, M. A High-Power Broadband Terahertz Source Enabled by Three Dimensional Light Confinement in a Plasmonic Nanocavity. Sci. Rep. 2017, 7, 4166. [Google Scholar] [CrossRef]
- Cheon, H.; Yang, H.J.; Lee, S.H.; Kim, Y.; Son, J.H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef]
- Reid, C.B.; Reese, G.; Gibson, A.; Wallace, V.P. Terahertz Time-Domain Spectroscopy of Human Blood. IEEE Trans. Terahertz Sci. Technol. 2013, 3, 363–367. [Google Scholar] [CrossRef]
- Alibadi, A.; Macgrogan, G.; Grzyb, J.; Guillet, J.P.; Mavarani, L.; Mounaix, P.; Hillger, P.; Cassar, Q.; Zimmer, T.; Pfeiffer, U.R. Pilot study of freshly excised breast tissue response in the 300–600 GHz range. Biomed. Opt. Express 2018, 9, 2930–2942. [Google Scholar]
- Hu, B.B.; Nuss, M.C. Imaging with terahertz waves. Opt. Lett. 1995, 16, 1716–1718. [Google Scholar] [CrossRef]
- Schall, M.; Helm, H.; Keiding, S.R. Far Infrared Properties of Electro-Optic Crystals Measured by THz Time-Domain Spectroscopy. Int. J. Infrared Millim. Waves 1999, 20, 595–604. [Google Scholar] [CrossRef]
- Good, J.T.; Holland, D.B.; Finneran, I.A.; Carroll, P.B.; Kelley, M.J.; Blake, G.A. A decade-spanninghigh-resolution asynchronous optical sampling terahertz time-domain and frequency comb spectrometer. Rev. Sci. Instrum. 2015, 86, 103–107. [Google Scholar] [CrossRef]
- Pavlov, S.G.; HuBers, H.W.; Riemann, H.; Zhukavin, R.K.; Orlova, E.E.; Shastin, V.N. Terahertz optically pumped Si:Sb laser. J. Appl. Phys. 2002, 92, 5632–5634. [Google Scholar] [CrossRef]
- He, W.; Zhang, L.; Bowes, D.; Yin, H.; Ronald, K.; Phelps, A.D.R.; Cross, A.W. Generation of broadband terahertz radiation using a backward wave oscillator and pseudospark-sourced electron beam. Appl. Phys. Lett. 2015, 133501. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Jiang, T.; Zhan, Z.; Deng, Q.; Li, W.; Wu, W.; Yang, N.; Chu, W.; Duan, S. High-power terahertz quantum cascade lasers with 0.23 W in continuous wave mode. Aip. Adv. 2016, 6, 075210. [Google Scholar] [CrossRef]
- Maestrini, A.; Thomas, B.; Wang, H.; Jung, C.; Treuttel, J.; Jin, Y.; Chattopadhyay, G.; Mehdi, I.; Beaudin, G. Schottky diode-based terahertz frequency multipliers and mixers. C. R. Phys. 2010, 11, 480–495. [Google Scholar] [CrossRef]
- Golay Detectors. Available online: http://www.tydexoptics.com/pdf/Golay_Detectors.pdf (accessed on 25 October 2020).
- Behnken, B.N.; Karunasiri, G.; Chamberlin, D.R.; Robrish, P.R.; Faist, J. Real-time imaging using a 2.8 THz quantum cascade laser and uncooled infrared micrometer camera. Opt. Lett. 2008, 33, 440–442. [Google Scholar] [CrossRef]
- Ophir THz Laser Measurement Products. Available online: https://www.ophiropt.com/laser-measurement/sites/default/files/Pyrocam_1.pdf (accessed on 25 October 2020).
- Bowman, T.; Campbell, L. Terahertz transmission vs. reflection imaging and model-based characterization for excised breast carcinomas. Biomed. Opt. Express 2016, 7, 3756–3783. [Google Scholar] [CrossRef]
- Salhi, M.; Pupeza, I.; Koch, M. Confocal THz Laser Microscope. J. Infrared Millim. Terahertz Waves 2010, 31, 358–366. [Google Scholar] [CrossRef]
- Lisauskas, A.; Boppel, S.; Krozer, V.; Roskos, H.G. Silicon CMOS-based THz detection. In Proceedings of the 10th IEEE Conference on Sensors, Limerick, Ireland, 28–31 October 2011; pp. 55–58. [Google Scholar]
- De Cumis, U.S.; Xu, J.; Masini, L.; Degl’Innocenti, R.; Pingue, P.; Beltram, F.; Tredicucci, A.; Vitiello, M.S.; Benedetti, P.A.; Beere, H.E.; et al. Terahertz confocal microscopy with a quantum cascade laser source. Opt. Express 2012, 20, 20924–21931. [Google Scholar]
- Doradla, P.; Alavi, K.; Joseph, C.; Giles, R. Single-channel prototype terahertz endoscopic system. J. Biomed. Opt. 2014, 19, 80501. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Yang, J.; Wu, N. A CMOS Fully Integrated 860-GHz Terahertz Sensor. IEEE Trans. Terahertz Sci. Technol. 2017, 7, 445–465. [Google Scholar] [CrossRef]
- Qin, H.; Li, X.; Sun, J.; Zhang, Z.; Sun, Y.; Yu, Y.; Li, X.; Luo., M. Detection of incoherent terahertz light using antenna-coupled high-electron-mobility field-effect transistors. Appl. Phys. Lett. 2017, 110, 171109. [Google Scholar] [CrossRef]
- Chen, G.; Pei, J.; Yang, F.; Zhou, X.Y.; Sun, Z.L.; Cui, T.J. Terahertz-Wave Imaging System Based on Backward Wave Oscillator. IEEE Trans. Terahertz Sci. Technol. 2012, 5, 504–512. [Google Scholar] [CrossRef]
- Wang, D.H.C.; Du, J.; Ji, F.; Li, X.D.; Zeng, D.; Smart, K. A cryogen-free HTS Josephson junction detector for terahertz imaging. Supercond. Sci. Tech. 2015, 28, 84001. [Google Scholar]
- Shi, J.; Wang, Y.; Xu, D.; Yan, C.; Chen, T.; He, Y.; Tang, L.; Nie, M.; Duan, P.; Yan, D.; et al. Terahertz Imaging Based on Morphological Reconstruction. IEEE J. Select. Topics Quantum Electron. 2017, 23, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Shi, J.; Wang, Y.; Yang, K.; Fu, W. Label-free bacterial colony detection and viability assessment by continuous-wave terahertz transmission imaging. J. Biophotonics 2018, 11, e201700386. [Google Scholar] [CrossRef]
- Panula, P.A.J. Handbook of Biological Confocal Microscopy, 2nd ed.; Springer: New York, NY, USA, 2003; pp. 228–229. [Google Scholar]
- Zhang, M.; Quan, R.; Su, H.; Hu, X. Investigation of optically pumped continuous terahertz laser in biological imaging. J. Shenzhen University Sci. Eng. 2014, 31, 160–163. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, J.I.; Jeon, S.G.; Kim, J.; Park, K.K.; Oh, C.H. Enhanced Continuous-Wave Terahertz Imaging with a Horn Antenna for Food Inspection. J. Infrared Millim. Terahertz Waves 2012, 33, 657–664. [Google Scholar] [CrossRef]
- Ok, G.; Park, K.; Kim, H.J.; Chun, H.S.; Choi, S.W. High-speed terahertz imaging toward food quality inspection. Appl. Opt. 2014, 53, 1406–1412. [Google Scholar] [CrossRef]
- Sung, S.; Garritano, J.; Bajwa, N.; Deng, S.; Taylor, Z.D. Preliminary results of non-contact THz imaging of cornea. In Proceedings of the Conference on Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications VIII, San Francisco, CA, USA, 10–12 February 2015; Volume 9362. [Google Scholar]
- Fucheng, Q.; Zhiyong, T.; Zhanglong, F.; Wenjian, W.; Mengqi, L.; Chang, W.; Juncheng, C. Reflective scanning imaging based on a fast terahertz photodetector. Opt. Commun. 2018, 427, 170–174. [Google Scholar]
- Wang, Y.; Sun, Z.; Xu, D.; Wu, L.; Yao, J. A hybrid method based region of interest segmentation for continuous wave terahertz imaging. J. Phys. D Appl. Phys. 2020, 53, 95403. [Google Scholar] [CrossRef]
- Joseph, C.S.; Patel, R.; Neel, V.A.; Giles, R.H.; Yaroslavsky, A.N. Imaging of ex vivo nonmelanoma skin cancers in the optical and terahertz spectral regions optical and terahertz skin cancers imaging. J. Biophotonics 2014, 7, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Doradla, P.; Alavi, K.; Joseph, C.; Giles, R. Detection of colon cancer by continuous-wave terahertz polarization imaging technique. J. Biomed. Opt. 2013, 18, 0504. [Google Scholar] [CrossRef]
- Martin, J.; Joseph, C.; Giles, R. Continuous-wave circular polarization terahertz imaging. J. Biomed. Opt. 2016, 21, 070502. [Google Scholar] [CrossRef]
- Huang, Y.; Singh, R.; Xie, L.; Ying, Y. Attenuated Total Reflection for Terahertz Modulation, Sensing, Spectroscopy and Imaging Applications: A Review. Appl. Sci. 2020, 10, 4688. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Xu, D.; Jiang, Z.; Li, J.; Wu, L.; Yan, C.; Tang, L.; He, Y.; Yan, D.; et al. Optimization for vertically scanning terahertz attenuated total reflection imaging. Opt. Express 2018, 26, 20744–20757. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Xu, D.; Wu, L.; Yan, C.; Yan, D.; Tang, L.; He, Y.; Feng, H.; Yao, J. High-sensitivity attenuated total internal reflection continuous-wave terahertz imaging. J. Phys. D Appl. Phys. 2017, 50, 375103. [Google Scholar] [CrossRef]
- Born, M.; Wolf, E.; Bhatia, A.B.; Clemmow, P.C.; Gabor, D.; Stokes, A.R.; Taylor, A.M.; Wayman, P.A.; Wilcock, W.L. Interference and Diffraction with Partially Coherent Light; Cambridge University Press: Cambridge, UK, 1999; pp. 1–70. [Google Scholar]
- Adam, A.J.L. Review of Near-Field Terahertz Measurement Methods and Their Applications. J. Infrared Millim. Terahertz 2011, 32, 976–1019. [Google Scholar] [CrossRef]
- Chen, H.; Ma, S.H.; Yan, W.X.; Wu, X.M.; Wang, X.Z. The Diagnosis of Human Liver Cancer by using THz Fiber-Scanning Near-Field Imaging. Chin. Phy. Lett. 2013, 30, 030702. [Google Scholar] [CrossRef]
- Chen, H.; Ma, S.; Yan, W.; Wu, X.; Wang, X.C.K. All-terahertz fiber-scanning near-field microscopy. Opt. Lett. 2009, 34, 1084–1086. [Google Scholar]
- Tseng, T.F.; Yang, S.C.; Shih, Y.T.; Tsai, Y.F.; Wang, T.D.; Sun, C.K. Near-field sub-THz transmission-type image system for vessel imaging in-vivo. Opt. Express 2015, 23, 25058–25071. [Google Scholar] [CrossRef]
- McGowan, R.W.; Gallot, G.; Grischkowsky, D. Propagation of ultrawideband short pulses of terahertz radiation through submillimeter-diameter circular waveguides. Opt. Lett. 1999, 24, 1431–1433. [Google Scholar] [CrossRef]
- Rao, N. Elements of Engineering Electromagnetics, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004; pp. 527–599. [Google Scholar]
- Chernomyrdin, N.; Schadko, A.; Lebedev, S.; Tolstoguzov, V.; Kurlov, V.; Reshetov, I.; Spektor, I.; Skorobogatiy, M.; Yurchenko, S.; Zaytsev, K. Solid immersion terahertz imaging with sub-wavelength resolution. Appl. Phys. Lett. 2017, 110, 221109. [Google Scholar] [CrossRef]
- Chernomyrdin, N.; Kucheryavenko, A.; Kolontaeva, G.; Katyba, G.; Dolganova, I.; Karalkin, P.; Ponomarev, D.; Kurlov, V.; Reshetov, I.; Skorobogatiy, M.; et al. Reflection-mode continuous-wave 0.15λ-resolution terahertz solid immersion microscopy of soft biological tissues. Appl. Phys. Lett. 2018, 113, 111102. [Google Scholar] [CrossRef]
- Song, H.; Hwang, S.; An, H.; Song, H.J.; Song, J.I. Continuous-wave THz vector imaging system utilizing two-tone signal generation and self-mixing detection. Opt. Express 2017, 25, 20718. [Google Scholar] [CrossRef]
- Lim, Y.; Taimre, T.; Bertling, K.; Dean, P.; Indjin, D.; Valavanis, A.; Khanna, S.; Lachab, M.; Schaider, H.; Prow, T.; et al. High-contrast coherent terahertz imaging of porcine tissue via swept-frequency feedback interferometry. Biomed. Opt. Express 2014, 5, 3981–3989. [Google Scholar] [CrossRef]
- Wen, Y.; Jia, D.; Ma, W.; Feng, Y.; Liu, M.; Dong, L.; Zhao, Y.; Yu, X. Photomechanical meta-molecule array for real-time terahertz imaging. Microsyst. Nanoeng. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Zhang, S.; Silver, J.; Shang, X.; Del Bino, L.; Ridler, N.; Del’Haye, P. Terahertz wave generation using a soliton microcomb. Opt. Express 2019, 27, 35257–35266. [Google Scholar] [CrossRef]
- Goodman, J.W. Introduction to Fourier Optics, 3rd ed.; Roberts and Company: Placerville, CA, USA, 2005; pp. 126–165. [Google Scholar]
- Xue, K.; Li, Q.; Li, Y.D.; Wang, Q. Continuous-wave terahertz in-line digital holography. Opt. Lett. 2012, 37, 3228–3230. [Google Scholar] [CrossRef]
- Ding, S.; Li, Q.; Li, Y.D.; Wang, Q. Continuous-wave terahertz digital holography by use of a pyroelectric array camera. Opt. Lett. 2011, 36, 1993–1995. [Google Scholar] [CrossRef]
- Rong, L.; Latychevskaia, T.; Wang, D.; Zhou, X.; Huang, H.; Li, Z.; Wang, Y. Terahertz in-line digital holography of dragonfly hindwing: Amplitude and phase reconstruction at enhanced resolution by extrapolation. Opt. Express 2014, 22, 17236–17245. [Google Scholar] [CrossRef]
- Huang, H.; Rong, L.; Wang, D.; Li, W.; Deng, Q.; Li, B.; Wang, Y.; Zhan, Z.; Wang, X.; Wu, W. Synthetic aperture in terahertz in-line digital holography for resolution enhancement. Appl. Opt. 2016, 55, A43–A48. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Q.; Qin, Y.; Kong, W.; Li, G.; Zou, M.; Wang, D.; You, Z.; Zhou, X. Sparsity-based continuous wave terahertz lens-free on-chip holography with sub-wavelength resolution. Opt. Express 2019, 27, 702–713. [Google Scholar] [CrossRef]
- Li, Z.; Zou, R.; Kong, W.; Wang, X.; Deng, Q.; Yan, Q.; Qin, Y.; Wu, W.; Zhou, X. Terahertz synthetic aperture in-line holography with intensity correction and sparsity autofocusing reconstruction. Photonics Res. 2019, 7, 1391–1399. [Google Scholar] [CrossRef]
- Yamagiwa, M.; Minamikawa, T.; Minamiji, F.; Mizuno, T.; Tokizane, Y.; Oe, R.; Koresawa, H.; Mizutani, Y.; Iwata, T.; Yamamoto, H.; et al. Visualization of internal structure and internal stress in visibly opaque objects using full-field phase-shifting terahertz digital holography. Opt. Express 2019, 27, 33854–33868. [Google Scholar] [CrossRef]
- Locatelli, M.; Ravaro, M.; Bartalini, S.; Consolino, L.; Vitiello, M.; Cicchi, R.; Pavone, F.; Natale, P. Real-time terahertz digital holography with a quantum cascade laser. Sci. Rep. 2015, 5, 13566. [Google Scholar] [CrossRef]
- Huang, H.; Wang, D.; Rong, L.; Panezai, S.; Zhang, D.; Qiu, P.; Gao, L.; Gao, H.; Zheng, H.; Zheng, Z. Continuous-wave off-axis and in-line terahertz digital holography with phase unwrapping and phase autofocusing. Opt. Commun. 2018, 426, 612–622. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Rong, L.; Ma, D.; Zhao, J.; Wang, Y. Continuous-wave terahertz self-referencing digital holography based on Fresnel’s mirrors. Opt. Lett. 2020, 45, 913–916. [Google Scholar] [CrossRef]
- Rong, L.; Tang, C.; Wang, D.; Li, B.; Tan, F.; Wang, Y.; Shi, X. Probe position correction based on overlapped object wavefront cross-correlation for continuous-wave terahertz ptychography. Opt. Express 2019, 27, 938–950. [Google Scholar] [CrossRef]
- Stübling, E.; Rehn, A.; Siebrecht, T.; Bauckhage, Y.; Öhrström, L.; Eppenberger, P.; Balzer, J.; Ruhli, F.; Koch, M. Application of a robotic THz imaging system for sub-surface analysis of ancient human remains. Sci. Rep. 2019, 9, 3390. [Google Scholar]
- Kashiwagi, T.; Nakade, K.; Saiwai, Y.; Minami, H.; Kitamura, T.; Watanabe, C.; Ishida, K.; Sekimoto, S.; Asanuma, K.; Yasui, T.; et al. Computed tomography image using sub-terahertz waves generated from a high-Tc superconducting intrinsic Josephson junction oscillator. Appl. Phy. Lett. 2014, 104, 82603. [Google Scholar] [CrossRef]
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Sy, S.; Huang, S.; Wáng, Y.X.; Yu, J.; Ahuja, A.; Zhang, Y.T.; Pickwell-MacPherson, E. Terahertz spectroscopy of liver cirrhosis: Investigating the origin of contrast. Phys. Med. Biol. 2010, 55, 7587–7596. [Google Scholar] [CrossRef]
- Smolyanskaya, O.; Chernomyrdin, N.; Konovko, A.; Zaytsev, K.; Ozheredov, I.; Cherkasova, O.; Nazarov, M.; Guillet, J.P.; Kozlov, S.; Yury, K.; et al. Terahertz biophotonics as a tool for studies of dielectric and spectral properties of biological tissues and liquids. Prog. Quantum Electron. 2018, 62, 1–77. [Google Scholar] [CrossRef]
- Zaytsev, K.; Dolganova, I.; Chernomyrdin, N.; Katyba, G.; Gavdush, A.; Cherkasova, O.; Komandin, G.; Shchedrina, M.; Khodan, A.; Ponomarev, D.; et al. The progress and perspectives of terahertz technology for diagnosis of neoplasms: A review. J. Opt. 2020, 22, 13001. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.H.; Ji, Y.; Jeong, K.; Park, Y.; Yang, J.; Park, D.; Noh, S.; Kang, S.G.; Huh, Y.M.; et al. Study of freshly excised brain tissues using terahertz imaging. Biomed. Opt. Express 2014, 5, 2837–2842. [Google Scholar] [CrossRef]
- Doleshal, M.; Magotra, A.; Choudhury, B.; Cannon, B.; Labourier, E.; Szafranska, A. Evaluation and Validation of Total RNA Extraction Methods for MicroRNA Expression Analyses in Formalin-Fixed, Paraffin-Embedded Tissues. J. Mol. Diagn. 2008, 10, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.; Park, J.Y.; Ahn, K.M.; Park, C.; Son, J.H. Terahertz imaging of excised oral cancer at frozen temperature. Biomed. Opt. Express 2013, 4, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, S.H.; Jeong, K.; Park, Y.; Huh, Y.M.; Son, J.H.; Suh, J.S. Measurement depth enhancement in terahertz imaging of biological tissues. Opt. Express 2013, 21, 21299–21305. [Google Scholar] [CrossRef]
- Kosutic, D.; Haw, W.; Ghura, V. Current Concepts in the Surgical Management of Non-melanoma Skin Cancers. Clin. Oncol. 2019, 31, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Yaroslavsky, A.; Neel, V.; Goyette, T.; Giles, R. Dual Frequency Continuous Wave Terahertz Transmission Imaging of Nonmelanoma Skin Cancers. Laser. Surg. Med. 2011, 43, 457–462. [Google Scholar] [CrossRef]
- Chen, H.; Lee, W.J.; Chiu, C.M.; Tsai, Y.F.; Tseng, T.F.; Lu, J.T.; Lai, W.L.; Sun, C.K. Performance of THz fiber-scanning near-field microscopy to diagnose breast tumors. Opt. Express 2011, 19, 19523–19531. [Google Scholar] [CrossRef]
- Peter, B.; Yngvesson, S.; Siqueira, P.; Kelly, P.; Khan, A.; Glick, S.; Karellas, A. Development and Testing of a Single Frequency Terahertz Imaging System for Breast Cancer Detection. IEEE Trans. Terahertz Sci. Technol. 2013, 3, 374–386. [Google Scholar] [CrossRef]
- Chernomyrdin, N.; Zhelnov, V.; Kucheryavenko, A.; Dolganova, I.; Katyba, G.; Karasik, V.; Reshetov, I.; Zaytsev, K. Numerical analysis and experimental study of terahertz solid immersion microscopy. Opt. Eng. 2019, 59, 61605. [Google Scholar] [CrossRef]
- Van Meir, E.; Hadjipanayis, C.; Norden, A.; Shu, H.K.; Wen, P.; Olson, J. Exciting New Advances in Neuro-Oncology: The Avenue to a Cure for Malignant Glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, D.G.; Wang, Y.; Liao, B.; Jiang, Z.; Zhao, L.; Sun, Z.; Wu, N.; Chen, T.; Feng, H.; et al. Study of in vivo brain glioma in a mouse model using continuous-wave terahertz reflection imaging. Biomed. Opt. Express 2019, 10, 3953–3962. [Google Scholar] [CrossRef]
- Wang, Y.; Notake, T.; Tang, M.; Nawata, K.; Ito, H.; Minamide, H. Terahertz-wave water concentration and distribution measurement in thin biotissue based on a novel sample preparation. Phys. Med. Biol. 2011, 56, 4517–4527. [Google Scholar] [CrossRef]
- Jakobsen, A.; Andersen, F.; Fischer, A.; Jensen, L.; Jørgensen, J.; Larsen, O.; Lindebjerg, J.; Pløen, J.; Rafaelsen, S.; Vilandt, J. Neoadjuvant chemotherapy in locally advanced colon cancer. A phase II trial. Acta Oncol. 2015, 54, 1747–1753. [Google Scholar] [CrossRef]
- Wahaia, F.; Kašalynas, I.; Venckevicius, R.; Seliuta, D.; Valušis, G.; Urbanowicz, A.; Molis, G.; Carneiro, F.; Silva, C.; Granja, P. Terahertz absorption and reflection imaging of carcinoma-affected colon tissues embedded in paraffin. Mol. Struct. 2015, 1107, 214–219. [Google Scholar] [CrossRef]
- Doradla, P.; Alavi, K.; Joseph, C.S.; Giles, R.H. Development of terahertz endoscopic system for cancer detection. In Proceedings of the Conference on Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications IX, San Francisco, CA, USA, 15–18 February 2016; Volume 9747. [Google Scholar]
- Coon, C.; Berger, N.; Eastwood, D.; Tsai, S.; Christians, K.; Mogal, H.; Clarke, C.; Gamblin, T. Primary Liver Cancer: An NCDB Analysis of Overall Survival and Margins After Hepatectomy. Ann. Surg. Oncol. 2020, 27, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Latychevskaia, T.; Chen, C.; Wang, D.; Yu, Z.; Zhou, X.; Zeyu, L.; Huang, H.; Wang, Y.; Zhou, Z. Terahertz in-line digital holography of human hepatocellular carcinoma tissue. Sci. Rep. 2015, 5, 8445. [Google Scholar] [CrossRef] [PubMed]
- Mejia, J.; Pasko, J. Primary Liver Cancers: Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma. Surg. Clin. N. Am. 2020, 100, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Chen, T.; Xu, D.; Zhao, H.; Lin, C.; Yan, C.; Tang, L.; Yi, H.; Feng, H.; et al. Automatic evaluation of traumatic brain injury based on terahertz imaging with machine learning. Opt. Express 2018, 26, 6371–6381. [Google Scholar] [CrossRef] [PubMed]
- Bessou, M.; Chassagne, B.; Caumes, J.P.; Pradere, C.; Maire, P.; Tondusson, M.; Abraham, E. Three-dimensional terahertz computed tomography of human bones. Appl. Opt. 2012, 51, 6738–6744. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, D.; Rong, L.; Zhai, C.; Wang, Y.; Zhao, J. Application of continuous-wave terahertz computed tomography for the analysis of chicken bone structure. Opt. Eng. 2018, 57, 23105. [Google Scholar] [CrossRef]



| Imaging Target | THz System | Results | Reference |
|---|---|---|---|
| Basal cell carcinoma (BCC) | CW THz transmission imaging mode at two frequencies of 1.39 and 1.63 THz | Good contrast between cancerous and normal tissue was found with spatial resolution of 390 μm at 1.4 THz and 490 μm at 1.6 THz | Joseph et al., 2011 [82] |
| Non-melanoma skin cancer (NMSC) delineation | Cross-polarised CW THz reflective imaging mode working at 584 GHz | The location and the different morphological features were presented by cross-polarised and polarisation optical images with resolution of 670 μm | Joseph et al., 2014 [40] |
| Fresh human tissue of NMSC | Reflective CW THz imaging system working at 584 GHz with either LP or CP radiation at 584 GHz | Contrast between cancerous and normal tissues were found with a resolution of 150 μm | Martin et al., 2016 [42] |
| Frozen sliced breast tumours | CW THz fibre-scanning near-field microscopy transmission imaging at 320 GHz | Breast tumour tissues could be clearly distinguished from normal tissues without H&E staining with a resolution of 240 μm | Chen et al., 2011 [83] |
| Human breast cancer tissue | Reflectivity CW THz imaging mode working at 1.89 THz | Obtaining the absolute refractive index values of the samples | Peter et al., 2013 [84] |
| Human breast specimen | CW THz SI microscopy reflectivity imaging system working at 10.6 THz | A fragment of the stroma of the breast ex vivo could be observed, which was formed by the dense fibrous connective tissues containing single fat cells and their agglomerates with 0.15 λ resolution | Chernomyrd et al., 2018 [54] |
| Fresh brain tissues of mouse | CW THz reflection imaging system at the frequency of 2.52 THz | The tumour regions of in vivo and ex vivo brain tissues could be well distinguished and corresponded closely with H&E-stained images results with a resolution of 600 μm | Wu et al., 2019 [87] |
| Dehydrated human colon tissues | Transmission and reflection CW THz imaging system working at 590 GHz | A contrast of 23% between the neoplastic and control tissues. The possibility of distinguishing adenocarcinoma-affected areas even without water in the tissue | Wahaia et al., 2016 [90] |
| Fresh human colonic excisions | Reflection CW THz polarisation imaging system working at 584 GHz | Good contrast between normal and tumorous tissues with a resolution of 600 μm | Doradla et al., 2013 [41] |
| Human hepatocellular carcinoma tissue | CW THz digital in-line holography imaging system working at 2.52 THz | The indication of fibrosis in the liver cancer tissue could be observed from the THz phase image, and the appearance of healthy liver tissue was more homogeneous than that of the cancerous tissue with a resolution of 158 μm | Rong et al., 2015 [93] |
| Traumatic brain injury tissues | CW THz transmission imaging mode working at 2.52 THz based on machine learning | The highest classification of different degrees of TBI accuracy was up to 87.5% | Shi et al., 2018 [95] |
| Human lumbar vertebra, coxal bone, and skull | CW THz CT imaging system working at 110 GHz | Compact bone exhibits higher THz absorption than spongy bone at 110 GHz. The THz radiation was absorbed by the vault bones | Bessou et al., 2012 [96] |
| Chicken ulna | CW THz CT imaging system working at 0.279 THz | The complex internal structure of the chicken ulna at different sections could be obtained | Li et al., 2018 [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, C.; Huai, B.; Wang, S.; Zhang, Y.; Wang, D.; Rong, L.; Zheng, Y. Continuous-Wave THz Imaging for Biomedical Samples. Appl. Sci. 2021, 11, 71. https://doi.org/10.3390/app11010071
Zhang Y, Wang C, Huai B, Wang S, Zhang Y, Wang D, Rong L, Zheng Y. Continuous-Wave THz Imaging for Biomedical Samples. Applied Sciences. 2021; 11(1):71. https://doi.org/10.3390/app11010071
Chicago/Turabian StyleZhang, Yaya, Chuting Wang, Bingxin Huai, Shiyu Wang, Yating Zhang, Dayong Wang, Lu Rong, and Yongchang Zheng. 2021. "Continuous-Wave THz Imaging for Biomedical Samples" Applied Sciences 11, no. 1: 71. https://doi.org/10.3390/app11010071
APA StyleZhang, Y., Wang, C., Huai, B., Wang, S., Zhang, Y., Wang, D., Rong, L., & Zheng, Y. (2021). Continuous-Wave THz Imaging for Biomedical Samples. Applied Sciences, 11(1), 71. https://doi.org/10.3390/app11010071





