Complications of Teeth Affected by Molar-Incisor Malformation and Pathogenesis According to Microbiome Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Clinical Progress
2.3. Sampling
- Perio group
- ①
- Supragingival plaque: The patient was requested not to brush her mouth 24 h prior to sample collection. Supragingival dental plaque samples were collected by rubbing a sterile cotton swab across the cervicobuccal area of the teeth twice or three times under pressure [13].
- ②
- Subgingival plaque: The subgingival plaque samples were collected by inserting sterile endodontic paper points (sized 30; two paper points per site) into the gingival sulci or periodontal pocket for 10 s right after isolation and supragingival plaque removal [14].
- ③
- Periapical abscess: The periapical abscess sample was obtained via aspiration. The swollen area was aspirated with a syringe fitted with a 16-gauge needle and expressed into a sterile vial [15].
- Endo group
- ①
- Coronal pulp chamber and ⑤ root canal: The extracted tooth was cleaned with 30% hydrogen peroxide [16]. Aseptic techniques such as sterile burs were used to access the pulp space. Bacteriological samples of the pulp chamber were collected immediately after crown access. Pulp remnant and infected dentin were collected using a sterilized spoon excavator and sterilized paper points were inserted to absorb the remaining fluid containing microorganisms. In the root canal, two sequential new paper points were placed at the same level and utilized to soak up the fluid in the canal. Each paper point was held in position for 30 s. Sterilized endodontic files were then used to collect the infected root canal dentin severally. Only the tip area was collected into the tube by cutting.
2.4. PCR Amplification and Illumina Sequencing
2.5. MiSeq Pipeline Method
3. Results
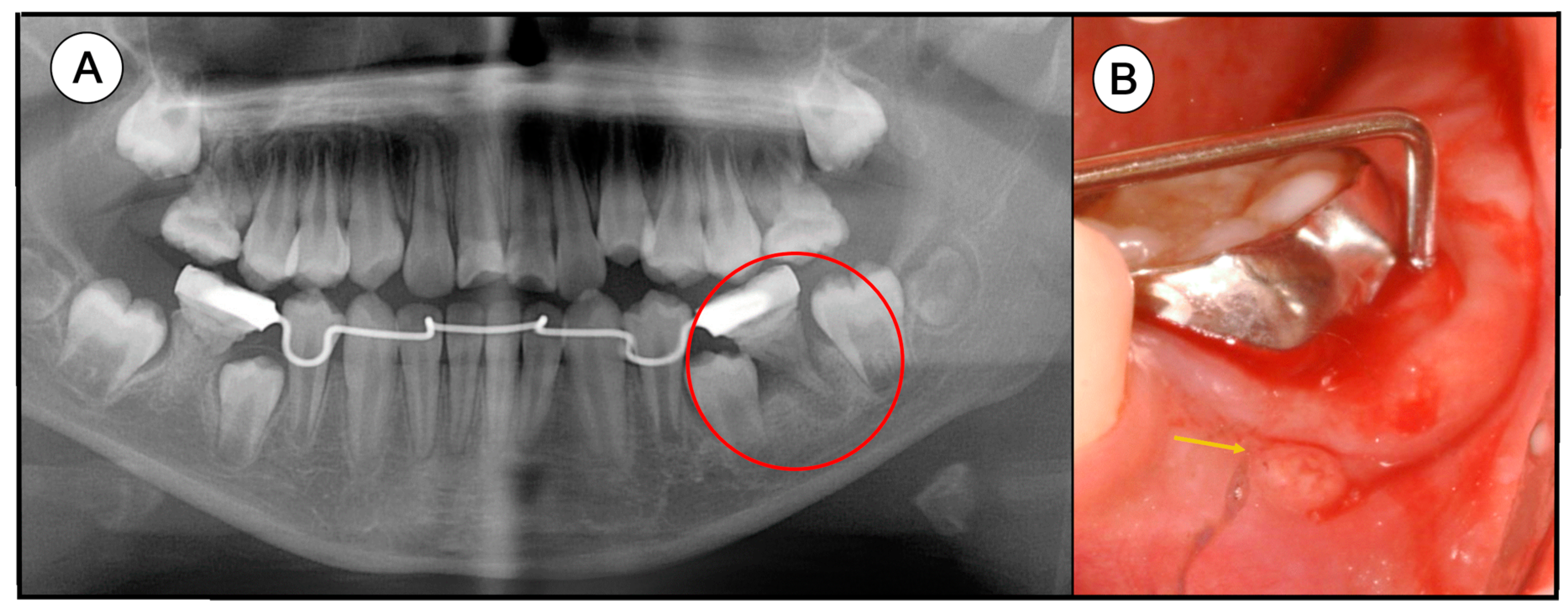
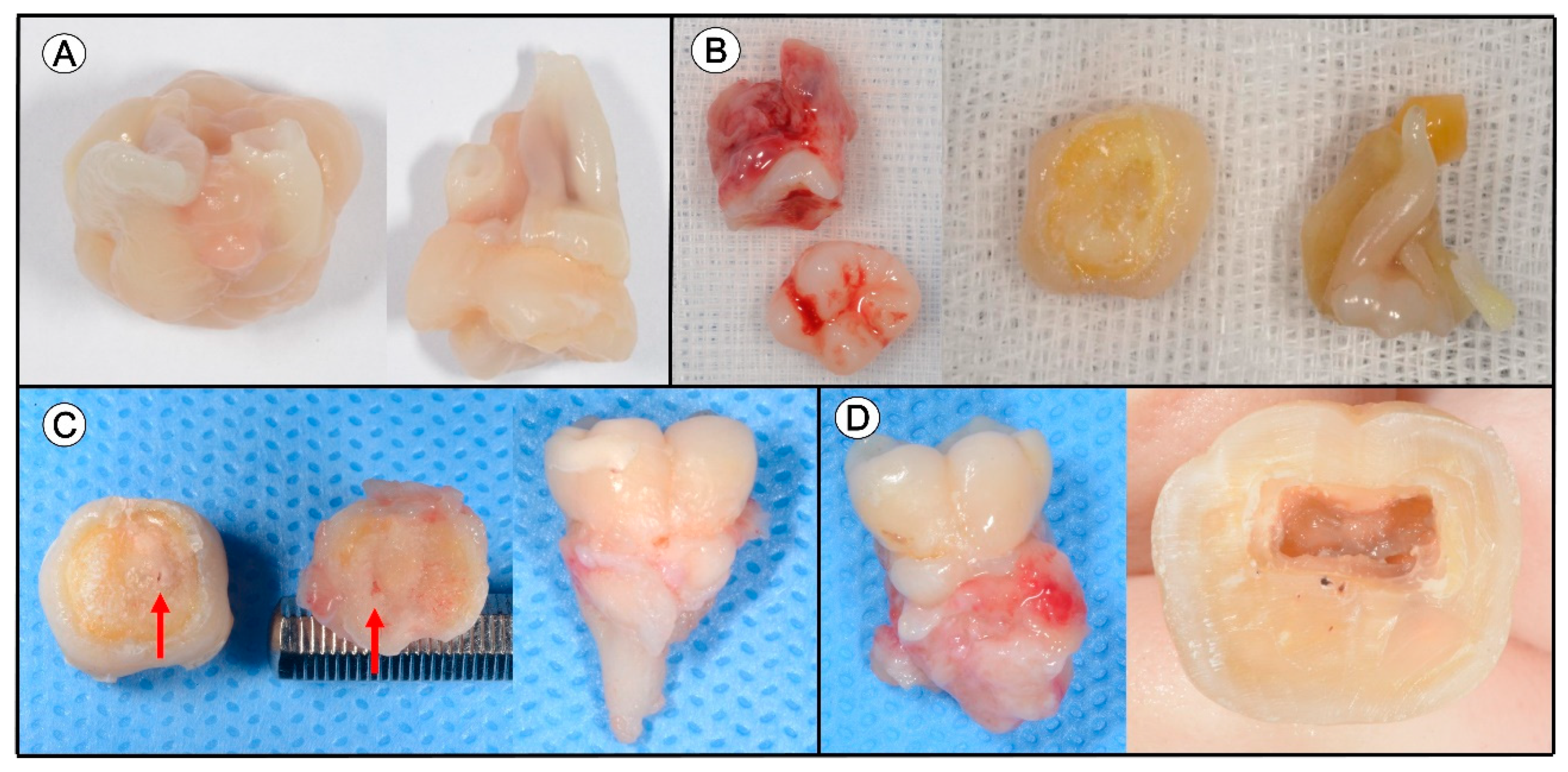
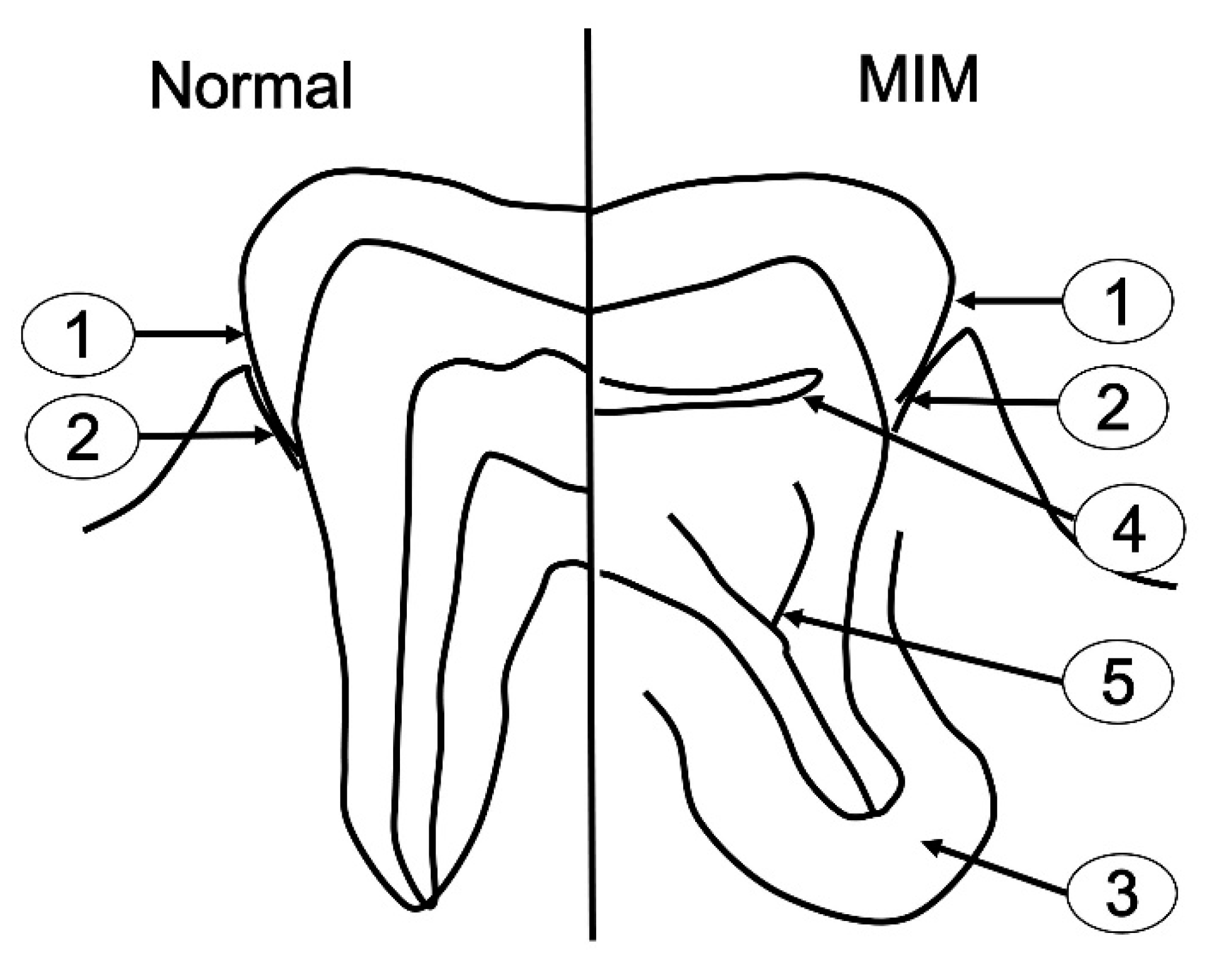
3.1. Clinical Information
3.2. Taxonomic Identification and Operational Taxonomical Unit (OTU) Assessment of Diversity
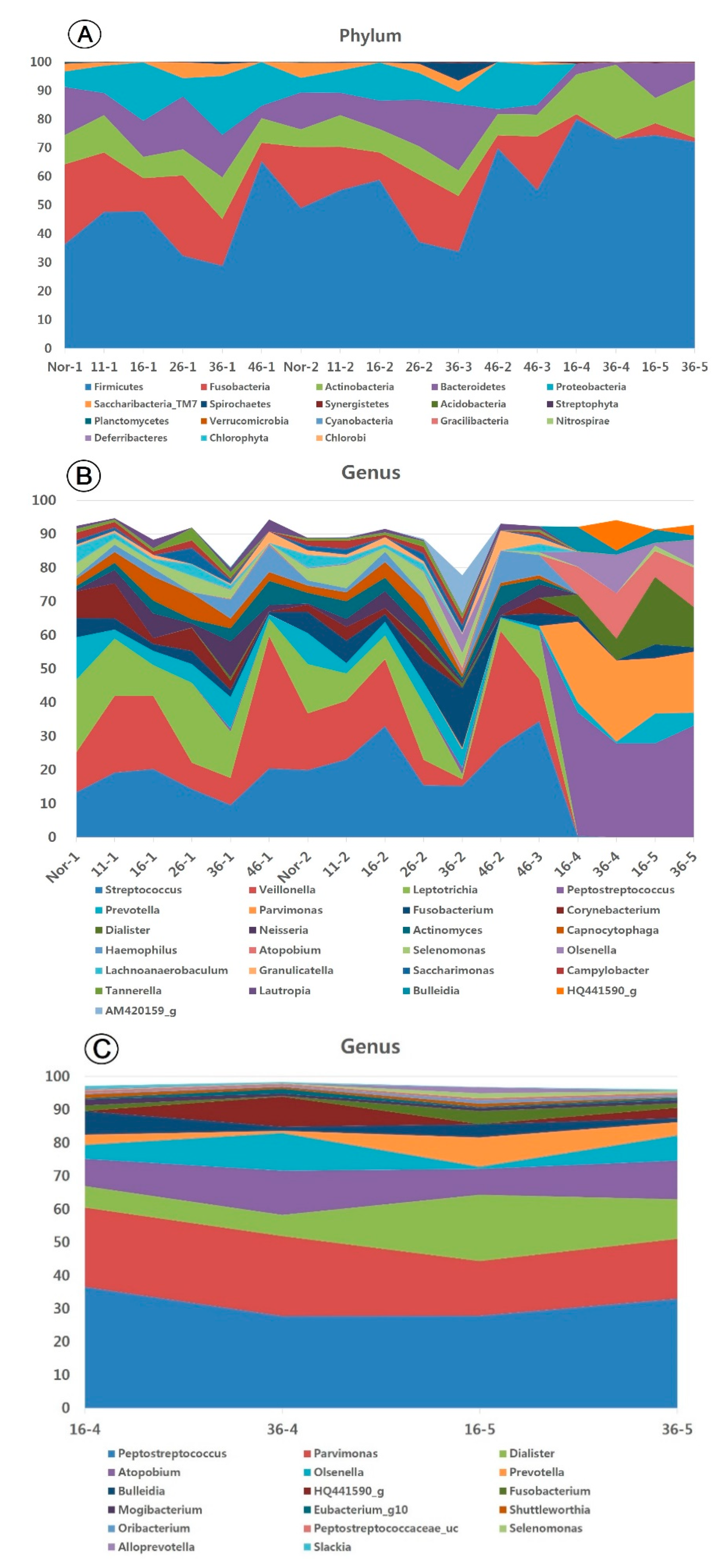
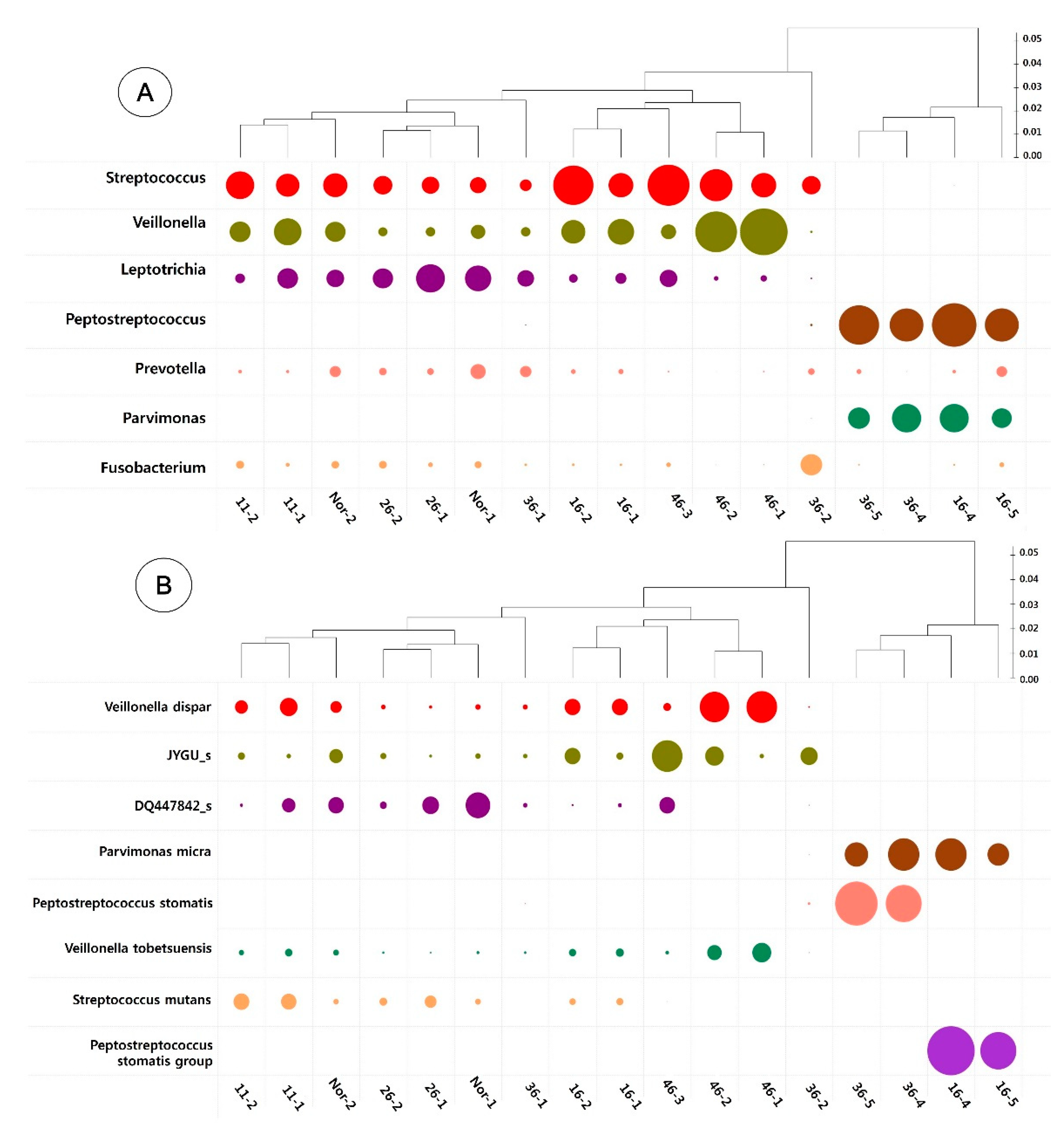
3.3. Taxonomic Identification of the Oral Microbiome
3.4. Heatmap Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, H.S.; Kim, S.H.; Kim, S.O.; Lee, J.H.; Choi, H.J.; Jung, H.S.; Song, J.S. A new type of dental anomaly: Molar-incisor malformation (MIM). Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 101–109. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, S.H.; Kim, S.O.; Choi, B.J.; Cho, S.W.; Park, W.; Song, J.S. Microscopic analysis of molar--incisor malformation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 544–552. [Google Scholar] [CrossRef]
- Vargo, R.J.; Reddy, R.; Da Costa, W.B.; Mugayar, L.R.F.; Islam, M.N.; Potluri, A. Molar-incisor malformation: Eight new cases and a review of the literature. Int. J. Paediatr. Dent. 2020, 30, 216–224. [Google Scholar] [CrossRef]
- Pavlic, A.; Vrecl, M.; Jan, J.; Bizjak, M.; Nemec, A. Case report of a molar-root incisor malformation in a patient with an autoimmune lymphoproliferative syndrome. BMC Oral Health 2019, 19, 49. [Google Scholar] [CrossRef]
- Zschocke, J.; Schossig, A.; Bosshardt, D.D.; Karall, D.; Glueckert, R.; Kapferer-Seebacher, I. Variable expressivity of TCTEX1D2 mutations and a possible pathogenic link of molar-incisor malformation to ciliary dysfunction. Arch. Oral Biol. 2017, 80, 222–228. [Google Scholar] [CrossRef]
- Qari, H.; Kessler, H.; Narayana, N.; Premaraj, S. Symmetric multiquadrant isolated dentin dysplasia (SMIDD), a unique presentation mimicking dentin dysplasia type 1b. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, e164–e169. [Google Scholar] [CrossRef]
- Kim, M.J.; Song, J.S.; Kim, Y.J.; Kim, J.W.; Jang, K.T.; Hyun, H.K. Clinical Considerations for Dental Management of Children with Molar-Root Incisor Malformations. J. Clin. Pediatr. Dent. 2020, 44, 55–59. [Google Scholar] [CrossRef]
- Kim, J.E.; Hong, J.K.; Yi, W.J.; Heo, M.S.; Lee, S.S.; Choi, S.C.; Huh, K.-H. Clinico-radiologic features of molar-incisor malformation in a case series of 38 patients: A retrospective observational study. Medicine 2019, 98, e17356. [Google Scholar] [CrossRef]
- Yue, W.; Kim, E. Nonsurgical Endodontic Management of a Molar-Incisor Malformation-affected Mandibular First Molar: A Case Report. J. Endod. 2016, 42, 664–668. [Google Scholar] [CrossRef]
- Byun, C.; Kim, C.; Cho, S.; Baek, S.H.; Kim, G.; Kim, S.G.; Kim, S.-Y. Endodontic Treatment of an Anomalous Anterior Tooth with the Aid of a 3-dimensional Printed Physical Tooth Model. J. Endod. 2015, 41, 961–965. [Google Scholar] [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the oral microbiome in personalized dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J.H.; Kim, S.O.; Song, J.S.; Kim, B.I.; Kim, Y.J. Comparison of the oral microbiome of siblings using next-generation sequencing: A pilot study. Oral Dis. 2016, 22, 549–556. [Google Scholar] [CrossRef]
- Camelo-Castillo, A.J.; Mira, A.; Pico, A.; Nibali, L.; Henderson, B.; Donos, N.; Inmaculada, T. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front. Microbiol. 2015, 6, 119. [Google Scholar] [CrossRef]
- Santos, A.L.; Siqueira, J.F., Jr.; Rocas, I.N.; Jesus, E.C.; Rosado, A.S.; Tiedje, J.M. Comparing the bacterial diversity of acute and chronic dental root canal infections. PLoS ONE 2011, 6, e28088. [Google Scholar] [CrossRef]
- Hsiao, W.W.; Li, K.L.; Liu, Z.; Jones, C.; Fraser-Liggett, C.M.; Fouad, A.F. Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genom. 2012, 13, 345. [Google Scholar] [CrossRef]
- Blaxter, M.; Mann, J.; Chapman, T.; Thomas, F.; Whitton, C.; Floyd, R.; Abebe, E. Defining operational taxonomic units using DNA barcode data. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1935–1943. [Google Scholar] [CrossRef]
- McCreedy, C.; Robbins, H.; Newell, A.; Mallya, S.M. Molar-incisor Malformation: Two Cases of a Newly Described Dental Anomaly. J. Dent. Child. 2016, 83, 33–37. [Google Scholar]
- Wright, J.T.; Curran, A.; Kim, K.J.; Yang, Y.M.; Nam, S.H.; Shin, T.J.; Hyun, H.-K.; Kim, Y.-J.; Lee, S.-H.; Kim, J. Molar root-incisor malformation: Considerations of diverse developmental and etiologic factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 164–172. [Google Scholar] [CrossRef]
- Qin, N.; Li, D.; Yang, R. Next-generation sequencing technologies and the application in microbiology--A review. Wei Sheng Wu Xue Bao 2011, 51, 445–457. [Google Scholar]
- Lee, S.E.; Nam, O.H.; Lee, H.S.; Choi, S.C. Diversity and homogeneity of oral microbiota in healthy Korean pre-school children using pyrosequencing. Acta Odontol. Scand. 2016, 74, 335–336. [Google Scholar] [CrossRef]
- Sakamoto, M.; Siqueira, J.F., Jr.; Rocas, I.N.; Benno, Y. Diversity of spirochetes in endodontic infections. J. Clin. Microbiol. 2009, 47, 1352–1357. [Google Scholar] [CrossRef]
- Baumgartner, J.C.; Khemaleelakul, S.U.; Xia, T. Identification of spirochetes (treponemes) in endodontic infections. J. Endod. 2003, 29, 794–797. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Gomes, B.P.; Berber, V.B.; Kokaras, A.S.; Chen, T.; Paster, B.J. Microbiomes of Endodontic-Periodontal Lesions before and after Chemomechanical Preparation. J. Endod. 2015, 41, 1975–1984. [Google Scholar] [CrossRef]
- Needleman, I.; Garcia, R.; Gkranias, N.; Kirkwood, K.L.; Kocher, T.; Iorio, A.D.; Moreno, F.; Petrie, A. Mean annual attachment, bone level, and tooth loss: A systematic review. J. Periodontol. 2018, 89 (Suppl. S1), S120–S139. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Periodontol. 2018, 89 (Suppl. S1), S103–S119. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Ercoli, C.; Caton, J.G. Dental prostheses and tooth-related factors. J. Periodontol. 2018, 89 (Suppl. S1), S223–S236. [Google Scholar] [CrossRef]
- Romeo, U.; Palaia, G.; Botti, R.; Nardi, A.; Del Vecchio, A.; Tenore, G.; Polimeni, A. Enamel pearls as a predisposing factor to localized periodontitis. Quintessence Int. 2011, 42, 69–71. [Google Scholar]
- Witt, C.V.; Hirt, T.; Rutz, G.; Luder, H.U. Root malformation associated with a cervical mineralized diaphragm--a distinct form of tooth abnormality? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, e311–e319. [Google Scholar] [CrossRef][Green Version]
- Herrera, D.; Retamal-Valdes, B.; Alonso, B.; Feres, M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J. Periodontol. 2018, 89 (Suppl. S1), S85–S102. [Google Scholar] [CrossRef]
- Neo, H.L.; Watt, E.N.; Acharya, P. Molar-incisor malformation: A case report and clinical considerations. J. Orthod. 2019, 46, 343–348. [Google Scholar] [CrossRef]
- Yoshiaki, N.; Erika, K.; Ayako, O.; Ryoko, O.; Mieko, S.; Yasuko, T.; Chieko, T.; Kazumune, A.; Hideki, D.; Tamotsu, S.; et al. Oral micobiome in Four Female Centenarians. Appl. Sci. 2020, 10, 5312. [Google Scholar]
- Yoshiaki, N.; Erika, K.; Noboru, K.; kaname, N.; Akihiro, Y.; Nobuhiro, H. The Oral Microbiome of Healthy Japanese People at the Age of 90. Appl. Sci. 2020, 10, 6450. [Google Scholar]
- Hwang, J.Y.; Lee, H.-S.; Choi, J.; Nam, O.H.; Kim, M.S.; Choi, S.C. The Oral Microbiome in Children with Black Stained Tooth. Appl. Sci. 2020, 10, 8054. [Google Scholar] [CrossRef]
- Cervino, G.; Cicciù, M.; Biondi, A.; Bocchieri, S.; Herford, A.S.; Laino, L.; Fiorillo, L. Antibiotic Prophylaxis on Third Molar Extraction: Systematic Review of Recent Data. Antibiotics 2019, 8, 53. [Google Scholar] [CrossRef]

| Group | Location | Maxillary Central Incisors | Maxillary Right First Molar | Maxillary Left First Molar | Mandibular Left First Molar | Mandibular Right First Molar | Non-MIM Teeth | |
|---|---|---|---|---|---|---|---|---|
| 11 | 16 | 26 | 36 | 46 | Nor | |||
| Perio | 1 | Supragingival plaque | 11-1 | 16-1 | 26-1 | 36-1 | 46-1 | Nor-1 |
| 2 | Subgingival plaque | 11-2 | 16-2 | 26-2 | 36-2 | 46-2 | Nor-2 | |
| 3 | Apical abscess | 46-3 | ||||||
| Endo | 4 | Coronal pulp chamber | 16-4 | * | 36-4 | ** | ||
| 5 | Root canal | 16-5 | * | 36-5 | ** | |||
| Group | Sample ID | Taxonomic Identification | α-Diversity | |||
|---|---|---|---|---|---|---|
| Number of Final Reads | Number of OTUs * | Ace | Chao 1 | Shannon | ||
| Nor-1 | 18092 | 183 | 201.84 | 193.62 | 3.52 | |
| Perio | 11-1 | 19109 | 155 | 181.94 | 184 | 3.32 |
| 16-1 | 18517 | 161 | 178.61 | 171.12 | 3.71 | |
| 26-1 | 18293 | 196 | 219.54 | 206.50 | 3.67 | |
| 36-1 | 18317 | 193 | 212.38 | 206.04 | 3.88 | |
| 46-1 | 19395 | 104 | 119.42 | 130.25 | 2.74 | |
| Average | 18621 | 165 | 185.62 | 181.92 | 3.47 | |
| Nor-2 | 19134 | 206 | 250.28 | 243.27 | 3.70 | |
| 11-2 | 18492 | 262 | 319.99 | 304.78 | 3.84 | |
| 16-2 | 19032 | 185 | 217.45 | 216.95 | 3.54 | |
| 26-2 | 18528 | 201 | 213.69 | 207.56 | 4.01 | |
| 36-2 | 19629 | 206 | 238.47 | 248.50 | 3.58 | |
| 46-2 | 19491 | 133 | 176.67 | 163.00 | 2.78 | |
| Average | 19051 | 199 | 236.09 | 230.68 | 3.58 | |
| 46-3 | 19952 | 185 | 215.64 | 224.18 | 3.24 | |
| Endo | 16-4 | 17033 | 52 | 67.61 | 61.43 | 2.01 |
| 36-4 | 19238 | 59 | 90.32 | 94 | 1.98 | |
| Average | 18136 | 56 | 78.97 | 77.72 | 2.00 | |
| 16-5 | 16233 | 62 | 73.68 | 69.80 | 2.23 | |
| 36-5 | 18570 | 68 | 150.03 | 98.00 | 2.17 | |
| Average | 17402 | 65 | 111.86 | 83.9 | 2.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Kim, H.J.; Lee, K.; Kim, M.S.; Nam, O.H.; Choi, S.-C. Complications of Teeth Affected by Molar-Incisor Malformation and Pathogenesis According to Microbiome Analysis. Appl. Sci. 2021, 11, 4. https://doi.org/10.3390/app11010004
Lee H-S, Kim HJ, Lee K, Kim MS, Nam OH, Choi S-C. Complications of Teeth Affected by Molar-Incisor Malformation and Pathogenesis According to Microbiome Analysis. Applied Sciences. 2021; 11(1):4. https://doi.org/10.3390/app11010004
Chicago/Turabian StyleLee, Hyo-Seol, Hee Jin Kim, Koeun Lee, Mi Sun Kim, Ok Hyung Nam, and Sung-Chul Choi. 2021. "Complications of Teeth Affected by Molar-Incisor Malformation and Pathogenesis According to Microbiome Analysis" Applied Sciences 11, no. 1: 4. https://doi.org/10.3390/app11010004
APA StyleLee, H.-S., Kim, H. J., Lee, K., Kim, M. S., Nam, O. H., & Choi, S.-C. (2021). Complications of Teeth Affected by Molar-Incisor Malformation and Pathogenesis According to Microbiome Analysis. Applied Sciences, 11(1), 4. https://doi.org/10.3390/app11010004







