Study on the Compatibility of Eco-Friendly Insulating Gas C5F10O/N2 and C5F10O/Air with Copper Materials in Gas-Insulated Switchgears
Abstract
:1. Introduction
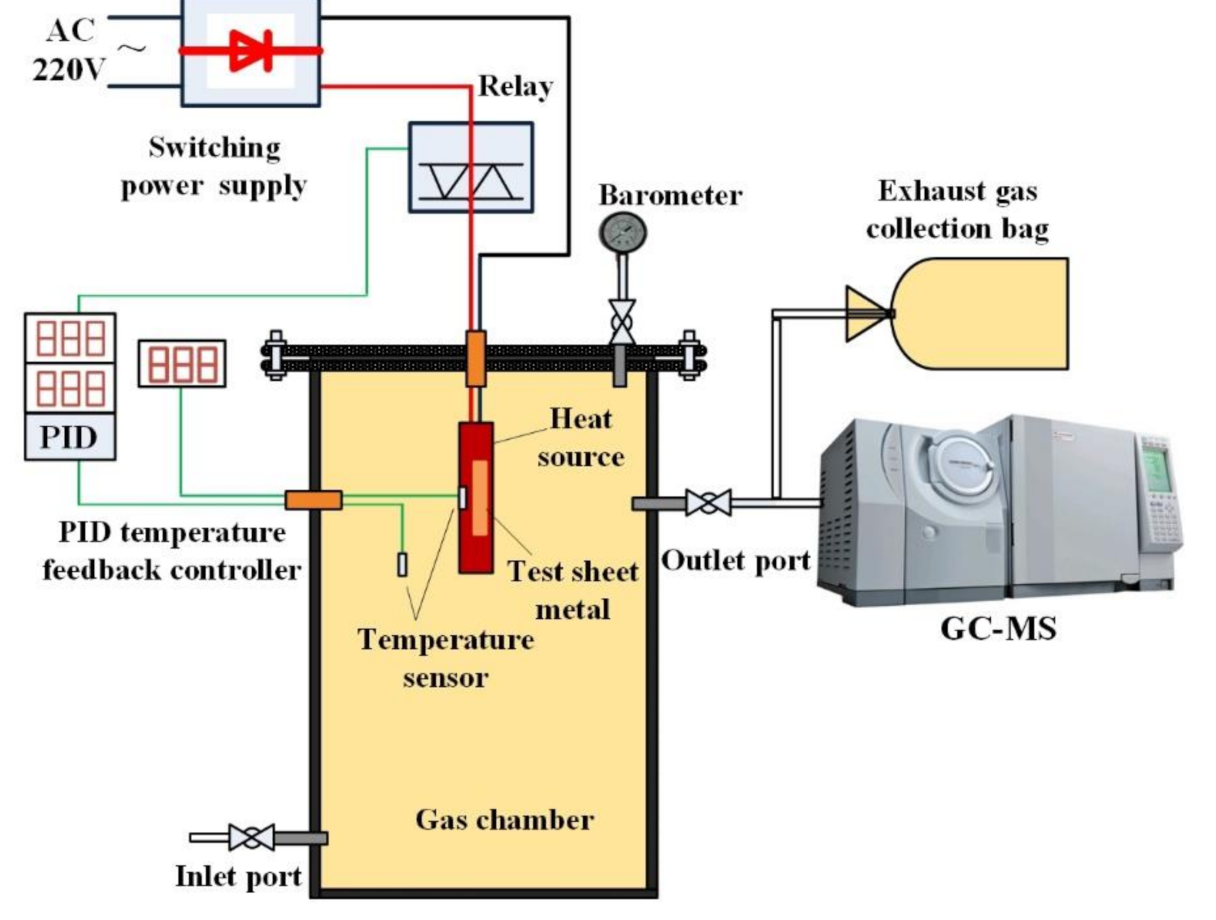
2. Experiment Platform
3. Experiment
4. Results Characterization
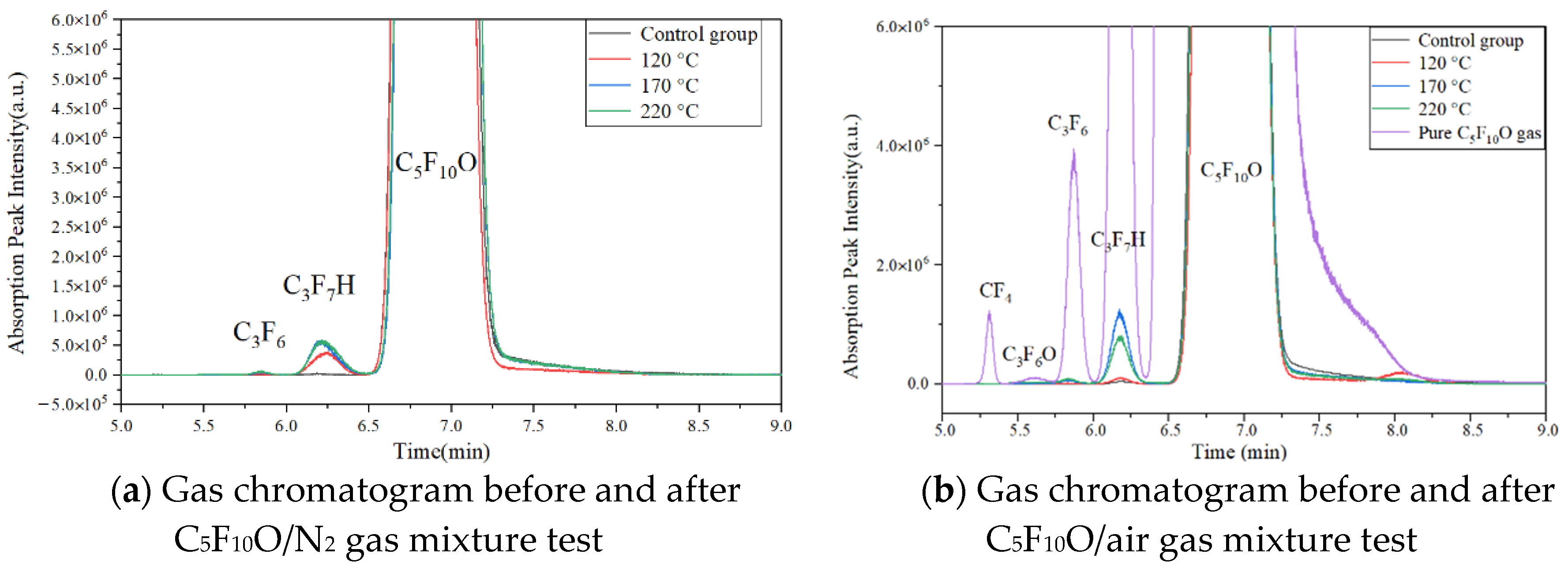
4.1. GC–MS Results
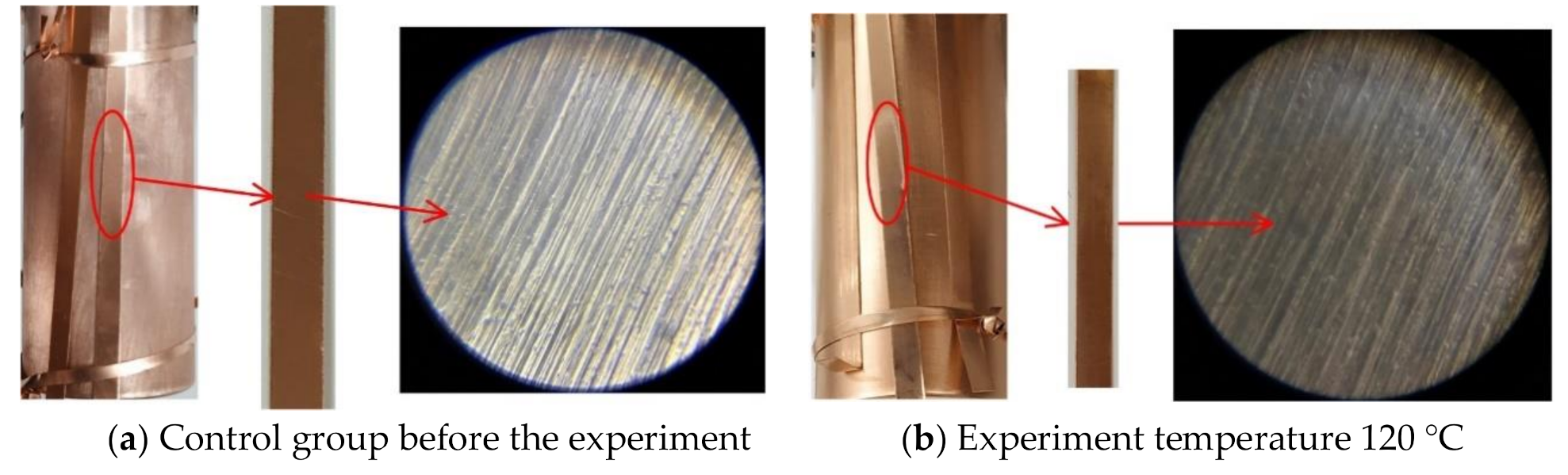
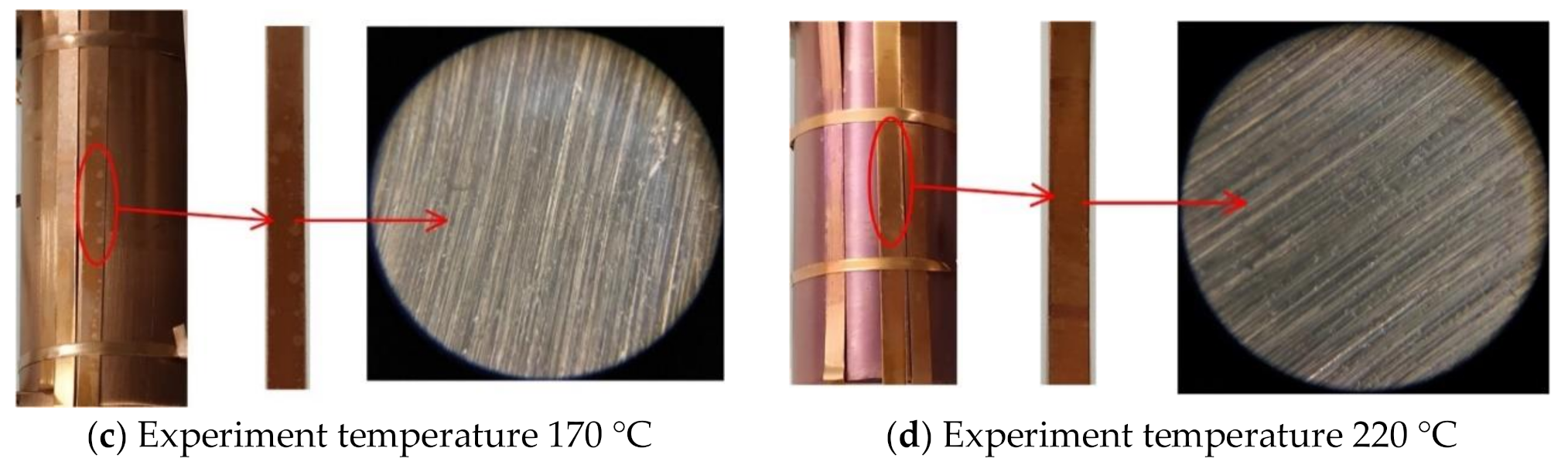
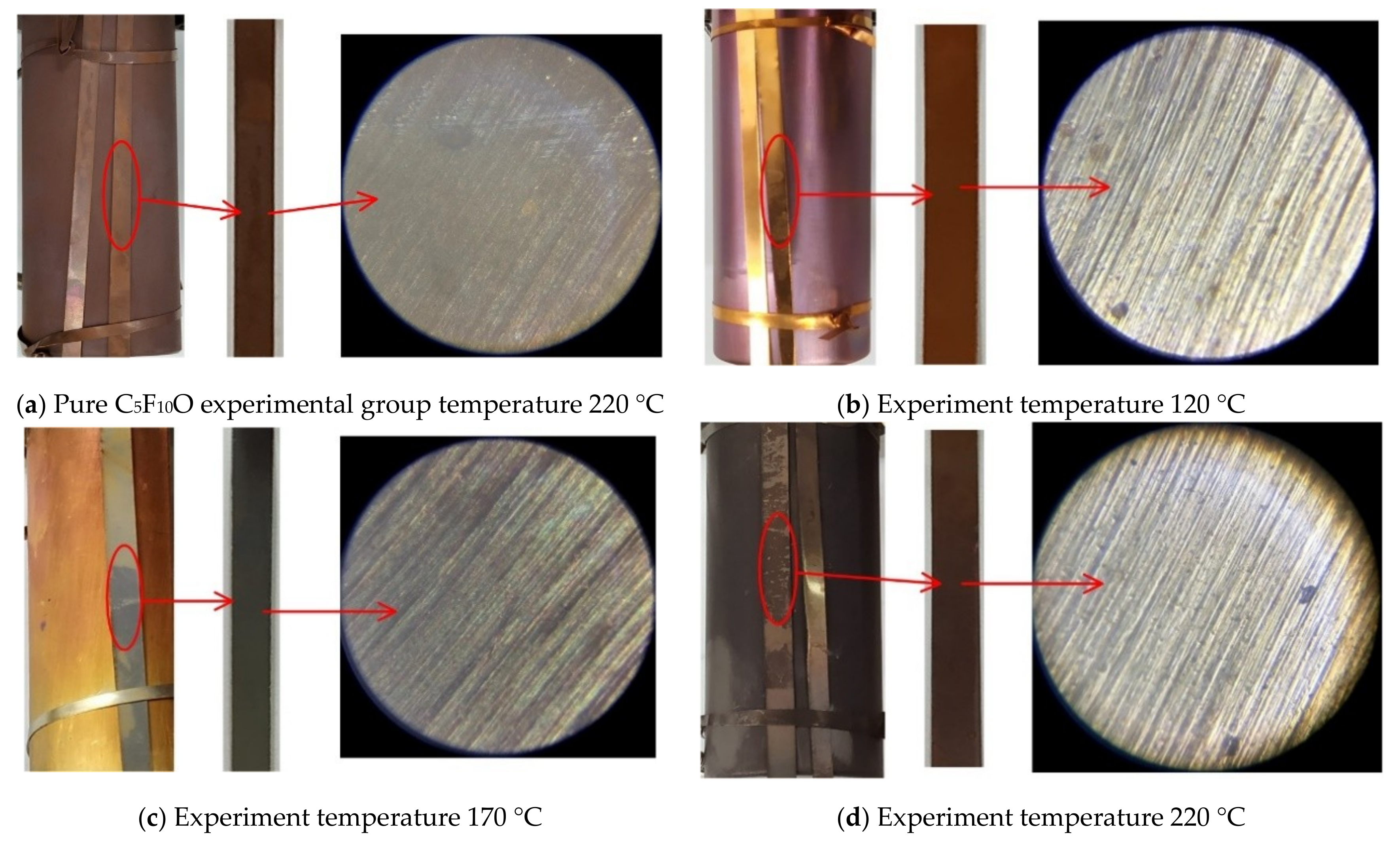
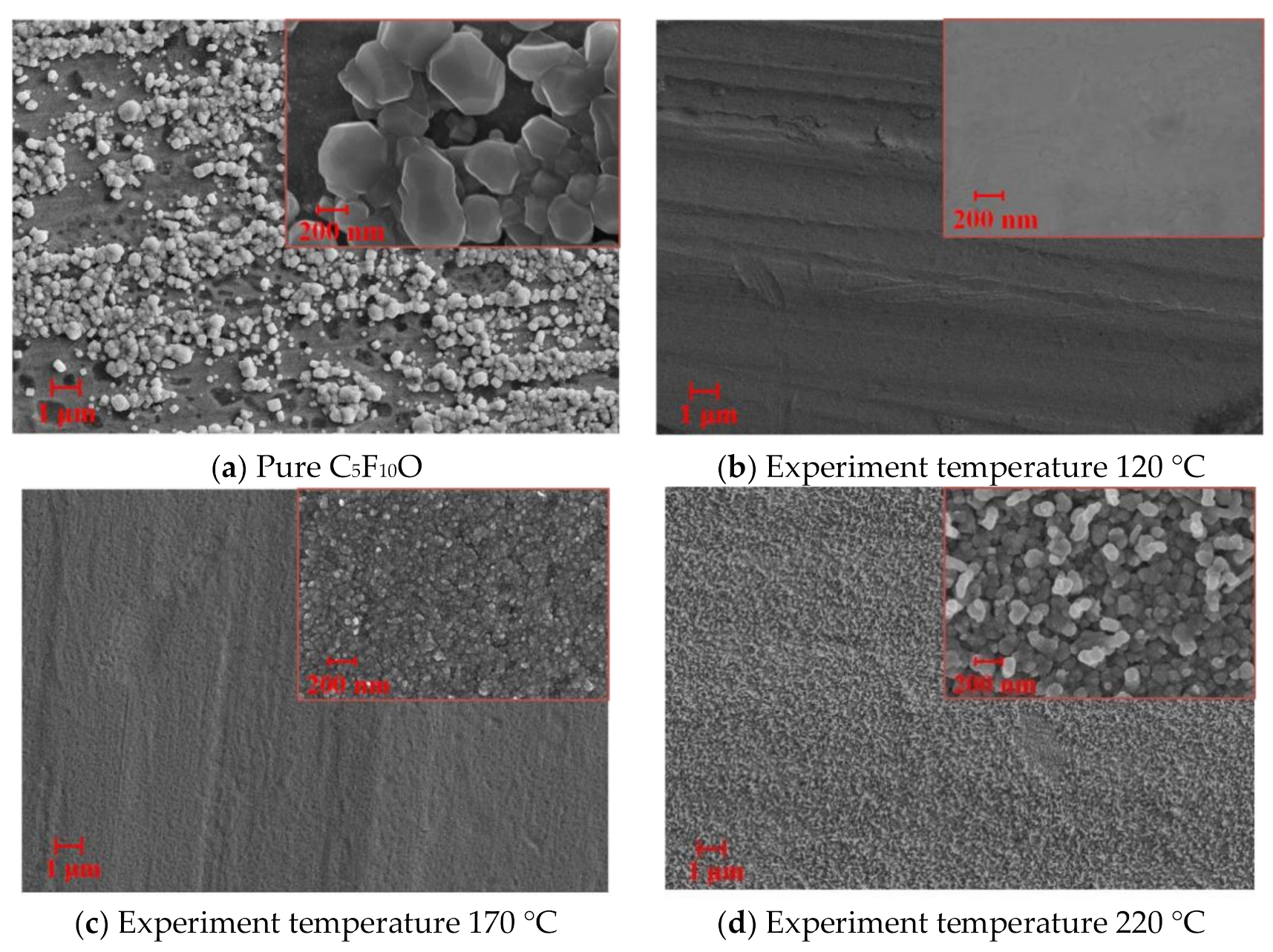
4.2. FESEM Characterization
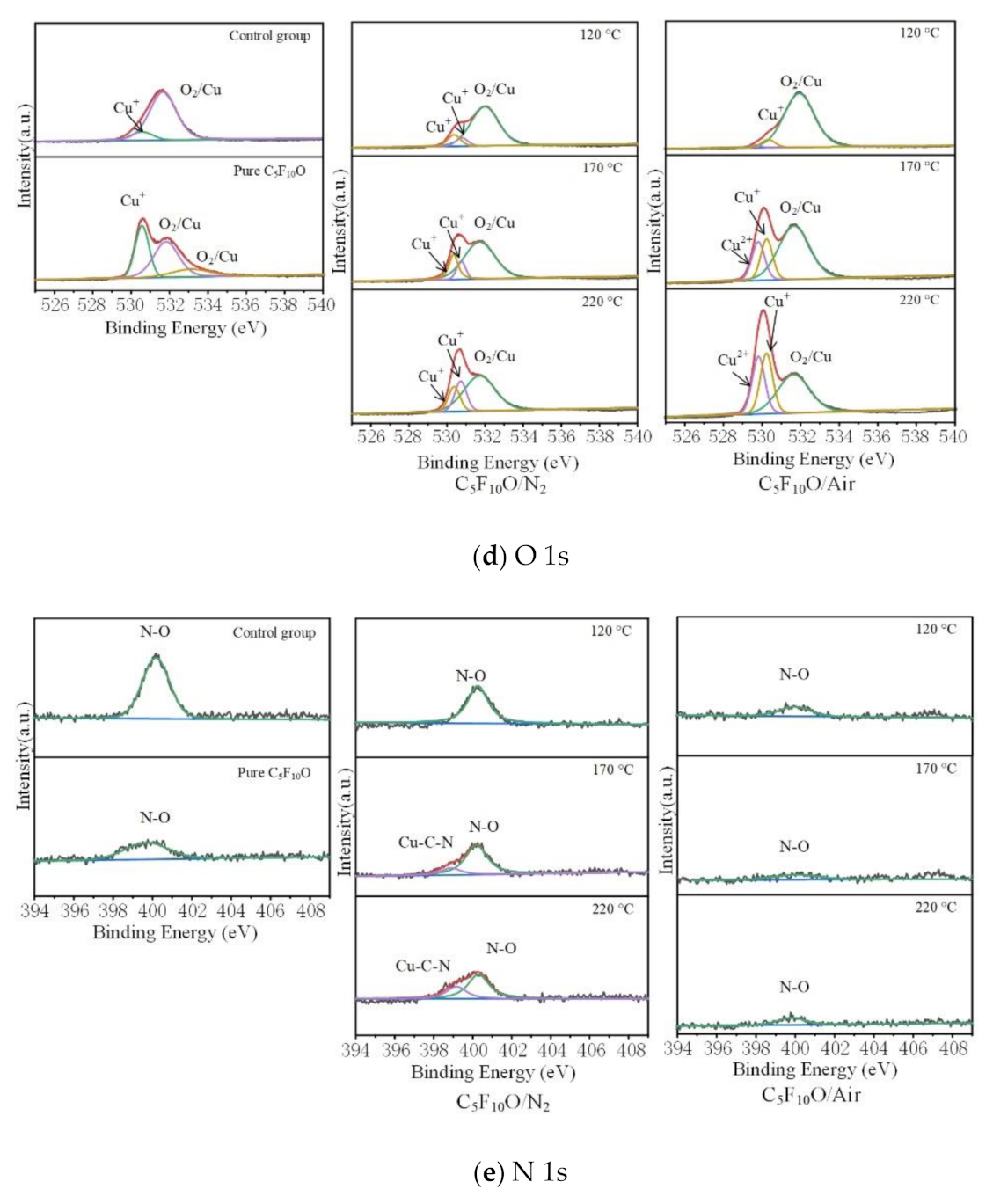
4.3. XPS Characterization
5. Simulations
6. Conclusions
- (1)
- When the experiment temperature varies between 120 °C and 220 °C, the surface color of the copper material of the C5F10O/N2 gas mixture experimental group changes from purple to golden yellow and then to pink. In the C5F10O/air gas mixture experimental group, the surface color of the copper material changes from purple to brown and then to dark brown as irritating gases are emitted.
- (2)
- The GC–MS characterization results show that the C5F10O/N2 and C5F10O/air gas mixtures have a small amount of C5F10O decomposition. The C5F10O/N2 gas mixture decomposes to produce C3F6 and C3F7H, and the C5F10O/air gas mixture decomposes to produce C3F6, C3F7H and C3F6O.
- (3)
- The FESEM characterization results show that the compatibility of the C5F10O/air gas mixture with copper at a low experiment temperature (120 °C) is better than that of the C5F10O/N2 gas mixture with copper. However, due to the effect of O2, when the experiment temperature is high (170 °C and 220 °C), the compatibility of the C5F10O/Air gas mixture with copper is significantly inferior when compared with that of C5F10O/N2 gas mixture and copper. Therefore, when using the C5F10O/air gas mixture as the insulating medium in engineering applications, special attention should be paid to the condition of the copper material inside the equipment when local overheating occurs. At the same time, operation and maintenance personnel should wear gas masks to prevent the inhalation of toxic decomposition products from harming their health.
- (4)
- The molecular adsorption of the C5F10O and Cu13 nanocluster is an exothermic reaction. The F atom on the α-position carbon atom of the C5F10O molecule and the oxygen atom on the carbonyl group have high chemical activity and are most likely to interact with Cu during the interaction process.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, G.; Zhang, X.; Cheng, H.; Tang, J. Ladder-Wise calculation method for z -coordinate of transformer PD source based on planar layout UHF antenna sensors. IEEJ Trans. Electr. Electron. Eng. 2020, 15, 340–345. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Li, Y.; Tang, F.; Lv, Q.; Zhang, J.; Xiao, S.; Tang, J.; Zhang, X. Experimental study on compatibility of eco-friendly insulating medium C5F10O/CO2 gas mixture with copper and aluminum. IEEE Access 2019, 7, 83994–84002. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Cui, Z.; Chen, D.; Zhang, X. Simulation and experiment on the catalytic degradation of high-concentration SF6 on TiO2 surface under UV light. AIP Adv. 2018, 8, 055215. [Google Scholar] [CrossRef]
- Andersen, M.P.S.; Kyte, M.; Andersen, S.T.; Nielsen, C.J.; Nielsen, O.J. Atmospheric chemistry of (CF3)2CF–C≡N: A replacement compound for the most potent industrial greenhouse gas, SF6. Environ. Sci. Technol. 2017, 51, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Gao, B.; Pang, X.; Zhang, X.; Li, Y.; Tian, S.; Tang, J.; Luo, Y. The sensitivity of C4F7N to electric field and its influence to environment-friendly insulating gas mixture C4F7N/CO2. J. Phys. D Appl. Phys. 2021, 54, 055501. [Google Scholar] [CrossRef]
- Reilly, J.; Prinn, R.G.; Harnisch, J.; Fitzmaurice, J.; Jacoby, H.D.; Kicklighter, D.; Melillo, J.; Stone, P.H.; Sokolov, A.P.; Wang, C. Multi-gas assessment of the Kyoto Protocol. Nature 1999, 401, 549–555. [Google Scholar] [CrossRef]
- Mong, R. Investigation of Electrical Properties of a New Low-GWP Insulation Gas-Comparison of AirPlus and Synthetic Air; NTNU: Trondheim, Norway, 2018. [Google Scholar]
- Romero, A.; Racz, L.; Matrai, A.; Bokor, T.; Cselko, R. A review of sulfur-hexafluoride reduction by dielectric coatings and alternative gases. In Proceedings of the 2017 6th International Youth Conference on Energy (IYCE), Budapest, Hungary, 21–24 June 2017; pp. 1–5. [Google Scholar]
- Mantilla, J.; Claessens, M.; Kriegel, M. Environmentally Friendly Perfluoroketones-Based Mixture as Switching Medium in High Voltage Circuit Breakers; Paper A3-113; CIGRE: Paris, France, 2016. [Google Scholar]
- Li, Y.; Zhang, X.; Wang, Y.; Li, Y.; Zhang, Y.; Wei, Z.; Xiao, S. Experimental study on the effect of O2 on the discharge decomposition products of C5-PFK/N2 mixtures. J. Mater. Sci. Mater. Electron. 2019, 30, 19353–19361. [Google Scholar] [CrossRef]
- Simka, P.; Ranjan, N. Dielectric strength of C5 perfluoroketone. In Proceedings of the 19th International Symposium on High Voltage Engineering, Pilsen, Czech Republic, 23–28 August 2015; pp. 23–28. [Google Scholar]
- Stoller, P.C.; Doiron, C.B.; Tehlar, D.; Simka, P.; Ranjan, N. Mixtures of CO2 and C5F10O perfluoroketone for high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2712–2721. [Google Scholar] [CrossRef]
- Zhong, J.; Fu, X.; Yang, A.; Han, G.; Liu, J.; Lu, Y.; Wang, X.; Rong, M. Insulation performance and liquefaction characteristic of C5F10O/CO2 gas mixture. In Proceedings of the 2017 4th International Conference on Electric Power Equipment—Switching Technology (ICEPE-ST), Xi’an, China, 22–25 October 2017; pp. 291–294. [Google Scholar]
- Li, X.; Guo, X.; Murphy, A.B.; Zhao, H.; Wu, J.; Guo, Z. Calculation of thermodynamic properties and transport coefficients of C5F10O-CO2 thermal plasmas. J. Appl. Phys. 2017, 122, 143302. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Wang, D. Decomposition characteristics of C5F10O/air mixture as substitutes for SF6 to reduce global warming. J. Fluor. Chem. 2018, 208, 65–72. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, L.; Ma, Y.; Wang, D.; Zhao, S.; Xiao, D. Calculation and analysis of the thermophysical properties of C5F10O-N2 mixtures. AIP Adv. 2019, 9, 105019. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Ahn, H.-S.; Choi, J.; Oh, I.-S. An estimation technology of temperature rise in GIS bus bar using three-dimensional coupled-field multiphysics. In Proceedings of the Conference Record of the 2008 IEEE International Symposium on Electrical Insulation, Vancouver, BC, Canada, 9–12 June 2008; pp. 432–436. [Google Scholar]
- Li, Y.; Zhang, X.; Li, X.; Cui, Z.; Xiao, H. Detection of ozone and nitric oxide in decomposition products of air-insulated switchgear using ultraviolet differential optical absorption spectroscopy (UV-DOAS). Appl. Spectrosc. 2018, 72, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Dervos, C.T.; Vassiliou, P.; Mergos, J.A. Thermal stability of SF6associated with metallic conductors incorporated in gas insulated switchgear power substations. J. Phys. D Appl. Phys. 2007, 40, 6942–6952. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Li, Y.; Wei, Z.; Wang, Y.; Ye, F.; Xiao, S. Effect of oxygen on power frequency breakdown characteristics and decomposition properties of C5-PFK/CO2 gas mixture. IEEE Trans. Dielectr. Electr. Insul. 2021. [Google Scholar]
- Hyrenbach, M.; Hintzen, T.; Müller, P.; Owens, J. Alternative gas insulation in medium-voltage switchgear. In Proceedings of the 23rd International Conference on Electricity Distribution, Lyon, France, 15–18 June 2015; pp. 15–18. [Google Scholar]
- Miranda, F.M.; Miranda, F.M.; Caporali, S. SERS and DFT study of copper surfaces coated with corrosion inhibitor. Beilstein J. Nanotechnol. 2014, 5, 2489–2497. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Chen, Q.; Chen, D.; Cui, Z.; Tang, J. Insight into the compatibility between C6F12O and metal materials: Experiment and theory. IEEE Access 2018, 6, 58154–58160. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, Y.; Zhan, W.; Xie, Z.; Kuang, Q.; Zheng, L. Synthesis of porous Cu2O/CuO cages using Cu-based metal–organic frameworks as templates and their gas-sensing properties. J. Mater. Chem. A 2015, 3, 12796–12803. [Google Scholar] [CrossRef]
- Zeng, J.; Zhuang, J.; He, T.; Chen, Q.; Liu, Y. Microstructure and dielectric response of Mg doped Cu3Ti2Ta2O12 ceramics. J. Mater. Sci. Mater. Electron. 2018, 30, 2652–2658. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, D.; Xiao, S.; Tian, S.; Tang, J.; Wang, D. Dissociative adsorption of environment-friendly insulating medium C3F7CN on Cu(111) and Al(111) surface: A theoretical evaluation. Appl. Surf. Sci. 2018, 434, 549–560. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, M.; Duan, T.; Wang, B. Insight into size dependence of C2 oxygenate synthesis from syngas on Cu cluster: The effect of cluster size on the selectivity. Appl. Surf. Sci. 2017, 407, 282–296. [Google Scholar] [CrossRef]
- Özçelik, S.; Güvenç, Z.B. Structures and melting of Cun (n = 13, 14, 19, 55, 56) clusters. Surf. Sci. 2003, 532, 312–316. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.H.; Yeo, S.C.; Lee, H.M. Structural stability of AgCu bimetallic nanoparticles and their application as a catalyst: A DFT study. Catal. Today 2012, 185, 94–98. [Google Scholar] [CrossRef]
- Govind, N.; Petersen, M.; Fitzgerald, G.; King-Smith, D.; Andzelm, J. A generalized synchronous transit method for transition state location. Comput. Mater. Sci. 2003, 28, 250–258. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]





| Gas Type | Mass-to-Charge Ratios (m/z) | Separation Time (min) |
|---|---|---|
| C5F10O | 69, 97, 169, 197, 266 | 6.423–7.254 |
| C3F7H | 69, 151 | 6.013–6.373 |
| C3F6 | 69, 100, 131, 150 | 5.762–5.964 |
| CF4 | 51, 69 | 5.106–5.295 |
| C3F6O | 69, 97, 147 | 5.339–5.631 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, X.; Xia, Y.; Li, Y.; Wei, Z.; Wang, Y.; Xiao, S. Study on the Compatibility of Eco-Friendly Insulating Gas C5F10O/N2 and C5F10O/Air with Copper Materials in Gas-Insulated Switchgears. Appl. Sci. 2021, 11, 197. https://doi.org/10.3390/app11010197
Li Y, Zhang X, Xia Y, Li Y, Wei Z, Wang Y, Xiao S. Study on the Compatibility of Eco-Friendly Insulating Gas C5F10O/N2 and C5F10O/Air with Copper Materials in Gas-Insulated Switchgears. Applied Sciences. 2021; 11(1):197. https://doi.org/10.3390/app11010197
Chicago/Turabian StyleLi, Yalong, Xiaoxing Zhang, Yalong Xia, Yi Li, Zhuo Wei, Yi Wang, and Song Xiao. 2021. "Study on the Compatibility of Eco-Friendly Insulating Gas C5F10O/N2 and C5F10O/Air with Copper Materials in Gas-Insulated Switchgears" Applied Sciences 11, no. 1: 197. https://doi.org/10.3390/app11010197
APA StyleLi, Y., Zhang, X., Xia, Y., Li, Y., Wei, Z., Wang, Y., & Xiao, S. (2021). Study on the Compatibility of Eco-Friendly Insulating Gas C5F10O/N2 and C5F10O/Air with Copper Materials in Gas-Insulated Switchgears. Applied Sciences, 11(1), 197. https://doi.org/10.3390/app11010197






