Prediction of Neural Space Narrowing and Soft Tissue Injury of the Cervical Spine Concerning Head Restraint Arrangements in Traffic Collisions
Abstract
1. Introduction
2. Methodology
2.1. Model Development
2.2. Parametric Study
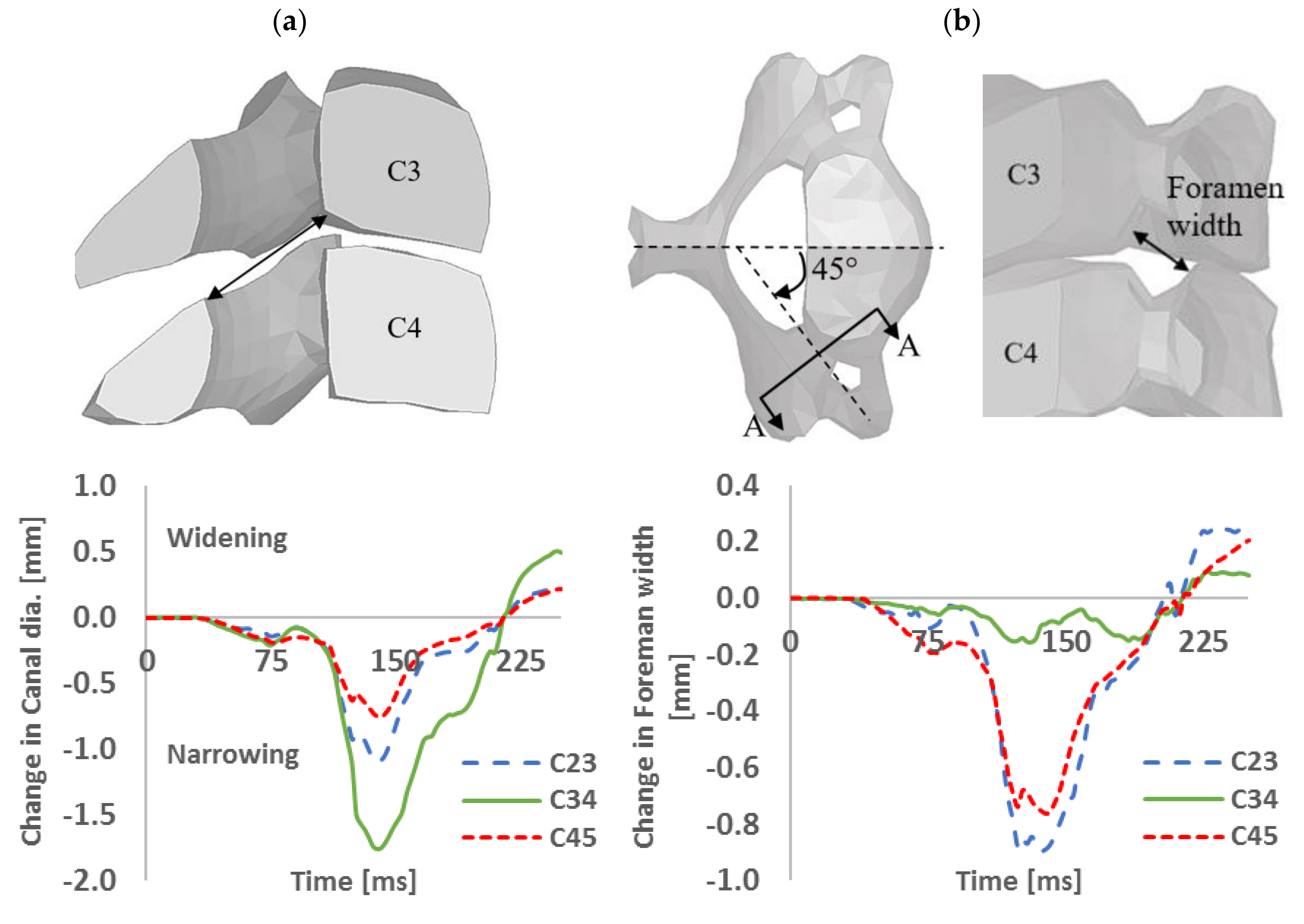
3. Results and Discussion
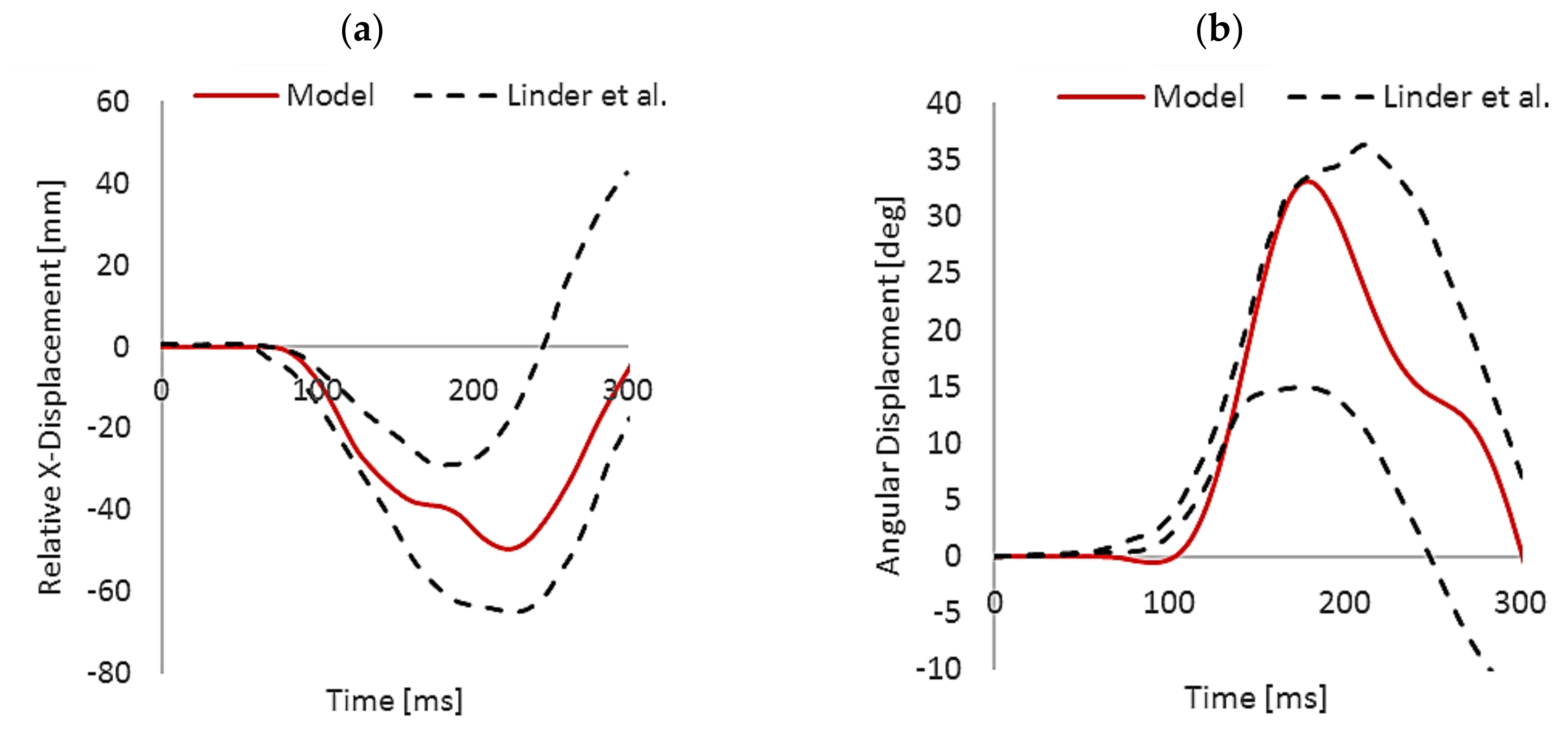
3.1. Model Validation
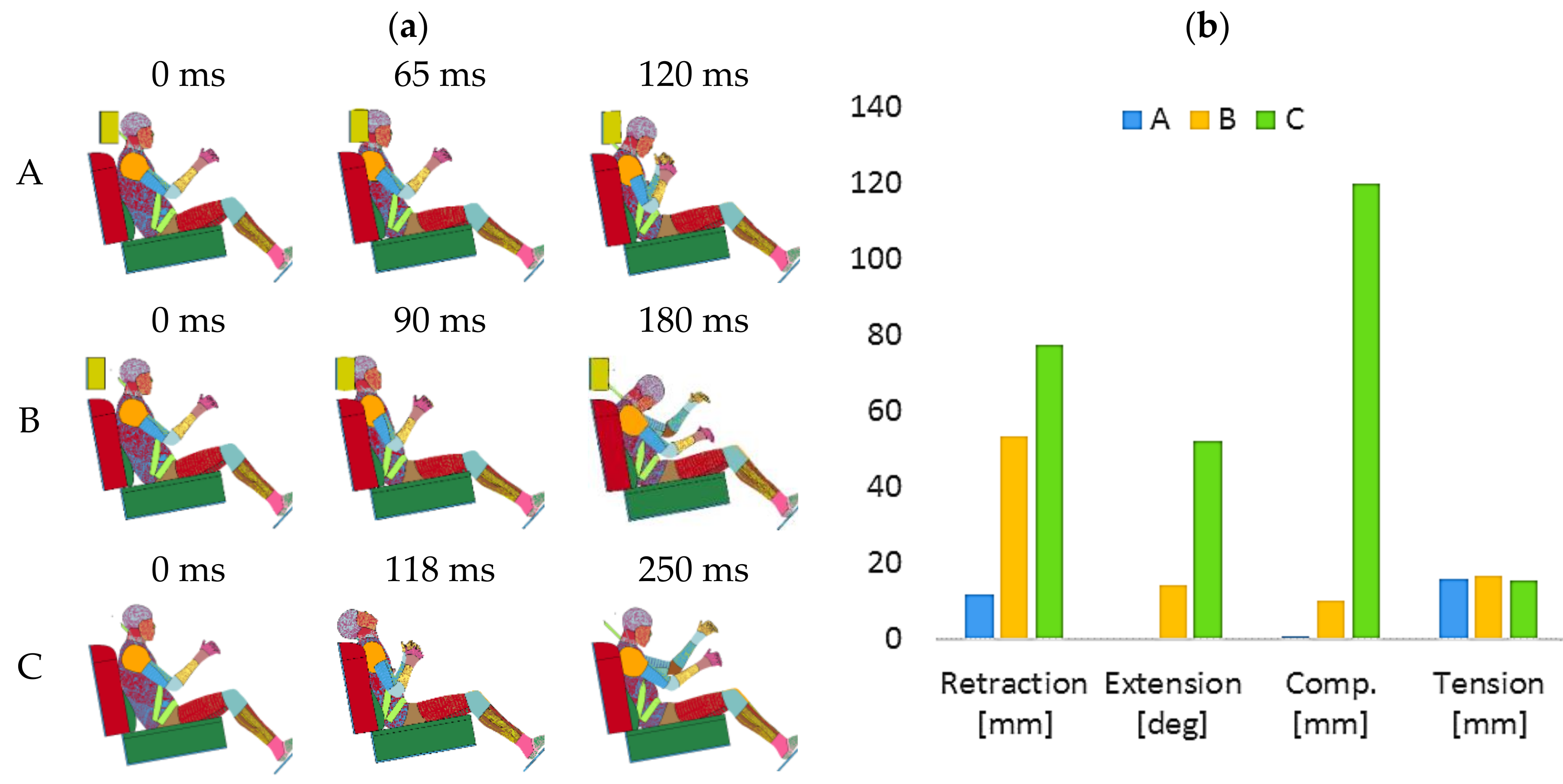
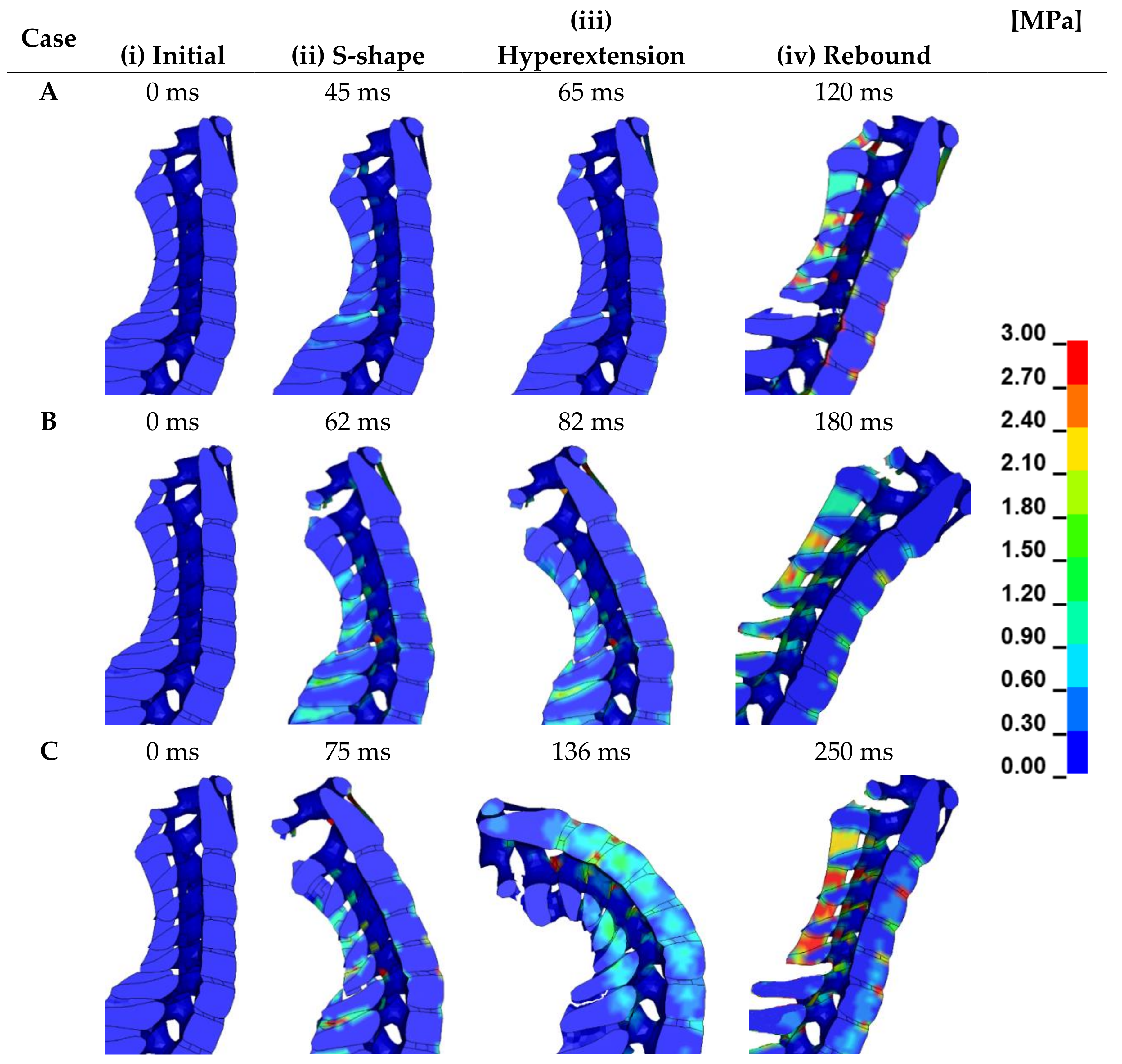
3.2. Head Retraction Phase
3.3. Hyperextension Phase
3.4. Neck Flexion Phase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.; Liu, N.; Li, H.; Zhang, B.; Tian, S.; Tan, M.; Sandoz, B. A review of neck injury and protection in vehicle accidents. Transp. Saf. Environ. 2019, 1, 89–105. [Google Scholar] [CrossRef]
- Oliphant, K. The Whiplash Capital of the World”: Genealogy of a Compensation Myth. In Damages and Compensation Culture: Comparative Perspectives; Hart Publishing: London, UK, 2016; pp. 15–36. [Google Scholar]
- Bener, A.; Rahman, Y.S.A.; Mitra, B. Incidence and severity of head and neck injuries in victims of road traffic crashes: In an economically developed country. Int. Emerg. Nurs. 2009, 17, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Stemper, B.D.; Yoganandan, N.; Pintar, F.A. Effect of head restraint backset on head–neck kinematics in whiplash. Accid. Anal. Prev. 2006, 38, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Trempel, R.E.; Zuby, D.S.; Edwards, M.A. IIHS head restraint ratings and insurance injury claim rates. Traffic Inj. Prev. 2016, 17, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwijck, J.; Nijs, J.; Meeus, M.; Paul, L. Evidence for central sensitization in chronic whiplash: A systematic literature review. Eur. J. Pain 2013, 17, 299–312. [Google Scholar] [CrossRef]
- Forman, J.L.; Lopez-Valdes, F.J.; Duprey, S.; Bose, D.; de Dios, E.D.; Subit, D.; Gillispie, T.; Crandall, J.R.; Segui-Gomez, M. The tolerance of the human body to automobile collision impact–a systematic review of injury biomechanics research, 1990–2009. Accid. Anal. Prev. 2015, 80, 7–17. [Google Scholar] [CrossRef]
- Ivancic, P.C.; Sha, D.; Lawrence, B.D.; Mo, F. Effect of active head restraint on residual neck instability due to rear impact. Spine 2010, 35, 2071–2078. [Google Scholar] [CrossRef]
- Iwamoto, M.; Nakahira, Y.; Kimpara, H. Development and validation of the total human model for safety (THUMS) toward further understanding of occupant injury mechanisms in precrash and during crash. Traffic Inj. Prev. 2015, 16, S36–S48. [Google Scholar] [CrossRef]
- Broos, J.; Meijer, R. Simulation Method for Whiplash Injury Prediction Using an Active Human Model. In Proceedings of the IRCOBI Conference—International Research Council on the Biomechanics of Injury, Malaga, Spain, 14–16 September 2016. [Google Scholar]
- Mustafy, T.; Moglo, K.; Adeeb, S.; El-Rich, M. Injury mechanisms of the ligamentous cervical C2–C3 functional spinal unit to complex loading modes: Finite element study. J. Mech. Behav. Biomed. Mater. 2016, 53, 384–396. [Google Scholar] [CrossRef]
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the human intervertebral disc: A review of testing techniques and results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434. [Google Scholar] [CrossRef]
- Panzer, M.B.; Cronin, D.S. C4–C5 segment finite element model development, validation, and load-sharing investigation. J. Biomech. 2009, 42, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Mattucci, S.F.; Moulton, J.A.; Chandrashekar, N.; Cronin, D.S. Strain rate-dependent properties of younger human cervical spine ligaments. J. Mech. Behav. Biomed. Mater. 2012, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- El-Rich, M.; Arnoux, P.; Wagnaca, E.; Brunet, C.; Aubin, C. Finite element investigation of the loading rate effect on the spinal load-sharing changes under impact conditions. J. Biomech. 2009, 42, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Kisanuki, Y.; Watanabe, I.; Furusu, K.; Miki, K.; Hasegawa, J. Development of a finite element model of the total human model for safety (THUMS) and application to injury reconstruction. In Proceedings of the International IRCOBI Conference, Munich, Germany, 18–20 September 2002. [Google Scholar]
- Cheng, S.; Clarke, E.C.; Bilston, L.E. Rheological properties of the tissues of the central nervous system: A review. Med. Eng. Phys. 2008, 30, 1318–1337. [Google Scholar] [CrossRef]
- Mohanty, P.P.; Mahapatra, S. A finite element approach for analyzing the effect of cushion type and thickness on pressure ulcer. Int. J. Ind. Ergon. 2014, 44, 499–509. [Google Scholar] [CrossRef]
- Briody, C.; Duignan, B.; Jerrams, S. Testing, modelling, and validation of numerical model capable of predicting stress fields throughout polyurethane foam. In Proceedings of the 7th European Conference on Constitutive models for Rubber, Munich, Bavaria, 28–31 August 2011. [Google Scholar]
- RCAR-IIWPG Seat/Head Restraint Evaluation Protocol (Version 3). 2008. Available online: www.iihs.org/media/84f361de-a61a-4614-985c-c420c1d20634/1846913147/Ratings/Protocols/current (accessed on 12 August 2020).
- Beck, B.; Brown, J.; Bilston, L.E. Variations in rear seat cushion properties and the effects on submarining. Traffic Inj. Prev. 2011, 12, 54–61. [Google Scholar] [CrossRef]
- Linder, A.; Schick, S.; Hell, W.; Svensson, M.; Carlsson, A.; Lemmen, P.; Schmitt, K.; Gutsche, A.; Tomasch, E. ADSEAT–Adaptive seat to reduce neck injuries for female and male occupants. Accid. Anal. Prev. 2013, 60, 334–343. [Google Scholar] [CrossRef]
- Yoganandan, N.; Pintar, F.; Butler, J.; Reinartz, J.; Sances, A., Jr.; Larson, S.J. Dynamic response of human cervical spine ligaments. Spine 1989, 14, 1102–1110. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Aubry-Rozier, B.; Kaouri, S.; Lamy, O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: Systematic review and additional cases. J. Bone Miner. Res. 2017, 32, 1291–1296. [Google Scholar] [CrossRef]
- Marwan, Y.; Kombar, O.R.; Al-Saeed, O.; Aleidan, A.; Samir, A.; Esmaeel, A. The feasibility of two screws anterior fixation for Type II odontoid fracture among Arabs. Spine 2016, 41, E643–E646. [Google Scholar] [CrossRef]
- Yoganandan, N.; Stemper, B.D.; Rao, R.D. Patient mechanisms of injury in whiplash-associated disorders. Semin. Spine Surg. 2013, 25, 67–74. [Google Scholar] [CrossRef]
- Chen, H.B.; King, H.Y.; Wang, Z.G. Biomechanics of whiplash injury. Chin. J. Traumatol. 2009, 12, 305–314. [Google Scholar] [PubMed]
- Panjabi, M.M.; Pearson, A.M.; Ito, S.; Ivancic, P.C.; Gimenez, S.E.; Tominaga, Y. Cervical spine ligament injury during simulated frontal impact. Spine 2004, 29, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
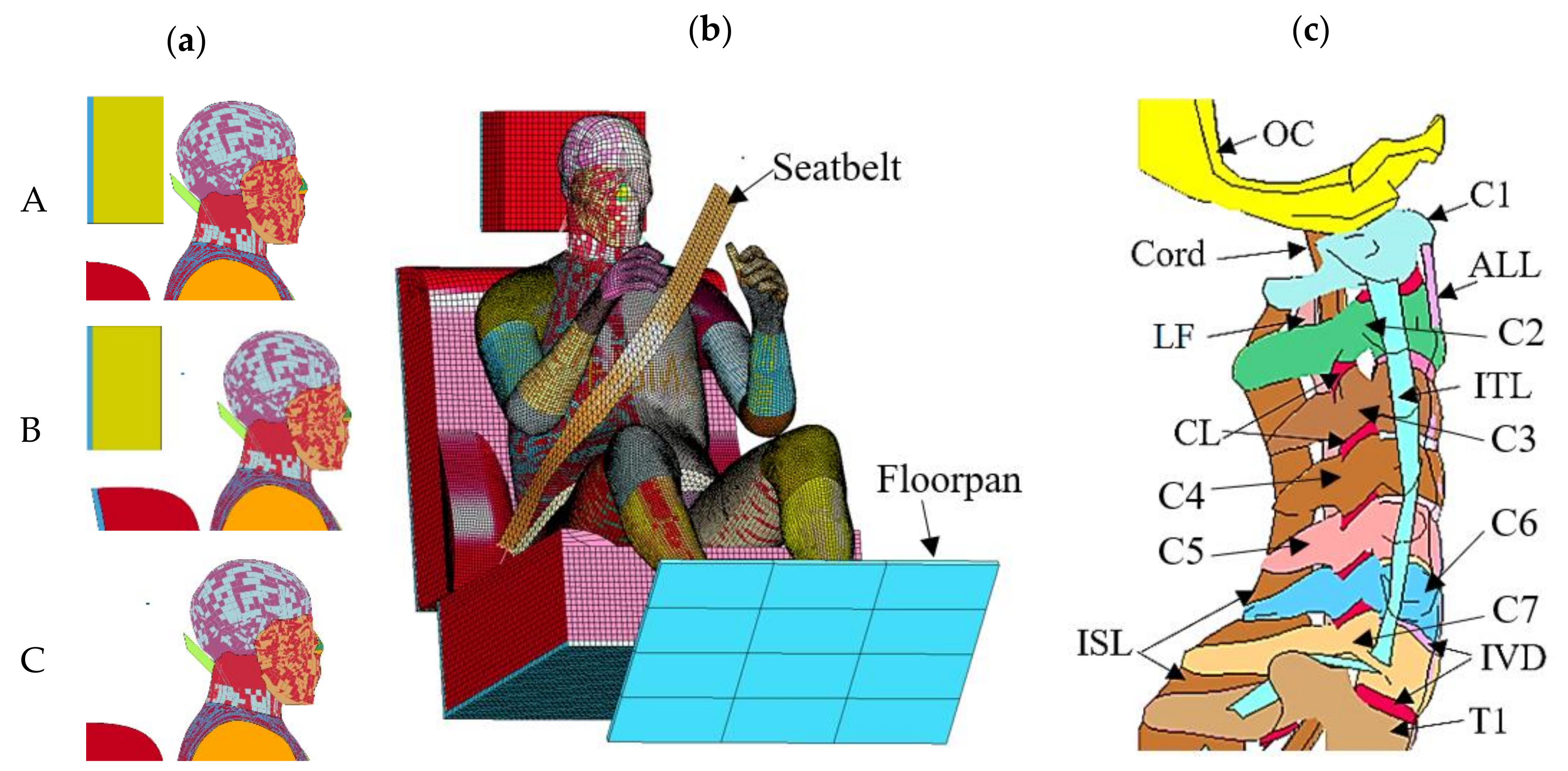


| Component | Constitutive Model | Material Properties (ρ 10−6 kg/mm3, σ MPa) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortical | Johnson and Cook Elasto-plastic | [11] | ||||||||
| Cancellous | Johnson and Cook Elasto-plastic | [11] | ||||||||
| Nucleus pulposus | Fluid | [12] | ||||||||
| Annulus fibrosus | Hill hyper-elastic | [13] | ||||||||
| Spinal cord | Quasilinear viscoelastic | [17] | ||||||||
| Muscles | 3D Feng hyper-elastic (bulky muscles), 1D nonlinear (thin muscles) | Nonlinear loading/unloading curves | [16] | |||||||
| Ligaments | Generalized Maxwell and Kelvin–Voigt viscoelastic | [14,15] | ||||||||
| ALL | 50 | 0.4 | 10 | 0.42 | 28 | 1e6 | 31.9 | |||
| PLL | 63 | 0.49 | 9.0 | 0.42 | 28 | 1e6 | 29.3 | |||
| ITL | 11.4 | 0.4 | 11.0 | 0.42 | 28 | 1e6 | - | |||
| ISL | 13.7 | 0.39 | 4.0 | 0.42 | 28 | 1e6 | 4.5 | |||
| LF | 24.6 | 0.39 | 5.0 | 0.42 | 28 | 1e6 | 5.6 | |||
| CL | 6.9 | 0.39 | 22.0 | 0.42 | 28 | 1e6 | 3.5 | |||
| Dimensions | Backrest | Base | Head Restraint |
|---|---|---|---|
| Cushion length (mm) | 585 | 200 | 640 |
| Cushion width (mm) | 530 | 250 | 530 |
| Cushion Thickness (mm) | 151 | 120 | 160 |
| Wing width (mm) | 130 | - | 100 |
| Wing Thickness (mm) | 53 | - | 30 |
| Material Properties | Backrest/Base | Head Restraint | |
| Density, (kg/m3) | 60 | 40 | |
| Stiffness, E (kPa) | 200 | 20 | |
| Relaxation modulus, | 0.3003, 0.1997 | 0.0973, 0.174 | |
| Relaxation time, (sec) | 0.010014, 0.1002 | 0.30639, 11.21 | |
| Shear modulus (kPa) | 4.81, 3.6 | 44.185, 0.0037 | |
| Exponent coefficient | 19.8, 19.8 | 21.4556, −6.89 | |
| Decay constant (10−2) | 0.0145, 0.65 | 0, 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laban, O.; Mahdi, E.; Cabibihan, J.-J. Prediction of Neural Space Narrowing and Soft Tissue Injury of the Cervical Spine Concerning Head Restraint Arrangements in Traffic Collisions. Appl. Sci. 2021, 11, 145. https://doi.org/10.3390/app11010145
Laban O, Mahdi E, Cabibihan J-J. Prediction of Neural Space Narrowing and Soft Tissue Injury of the Cervical Spine Concerning Head Restraint Arrangements in Traffic Collisions. Applied Sciences. 2021; 11(1):145. https://doi.org/10.3390/app11010145
Chicago/Turabian StyleLaban, Othman, Elsadig Mahdi, and John-John Cabibihan. 2021. "Prediction of Neural Space Narrowing and Soft Tissue Injury of the Cervical Spine Concerning Head Restraint Arrangements in Traffic Collisions" Applied Sciences 11, no. 1: 145. https://doi.org/10.3390/app11010145
APA StyleLaban, O., Mahdi, E., & Cabibihan, J.-J. (2021). Prediction of Neural Space Narrowing and Soft Tissue Injury of the Cervical Spine Concerning Head Restraint Arrangements in Traffic Collisions. Applied Sciences, 11(1), 145. https://doi.org/10.3390/app11010145






