SHS Produced TiB2-Si Powders for Selective Laser Melting of Ceramic-Based Composite
Abstract
1. Introduction
2. Materials and Methods
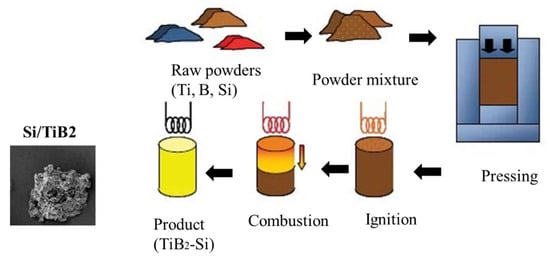
2.1. Self-Propagating High Temperature Synthesis of TiB2-Si Composite Powder
2.2. Selective Laser Melting of TiB2-Si
2.3. Characterization of Powders and Printed Parts
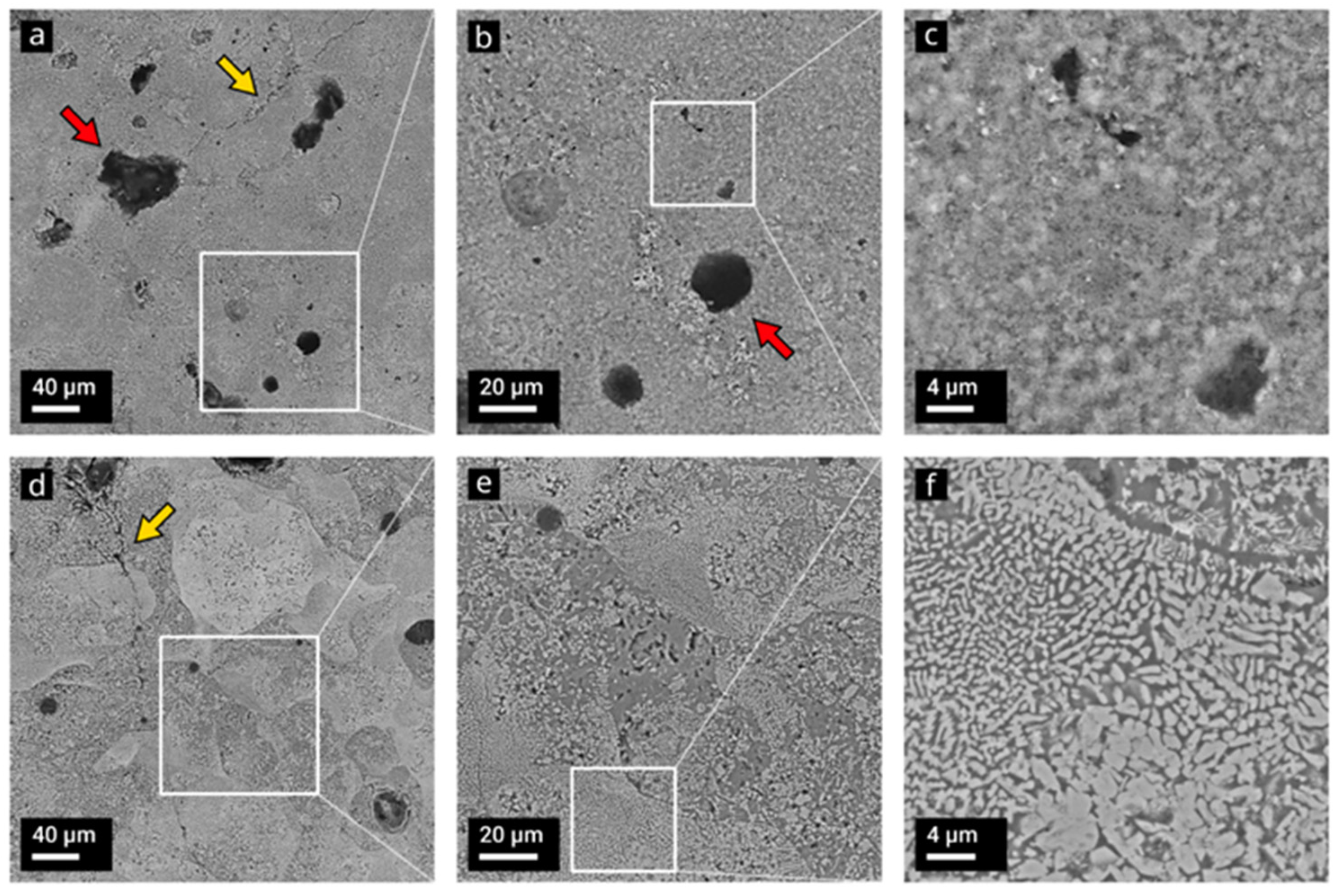
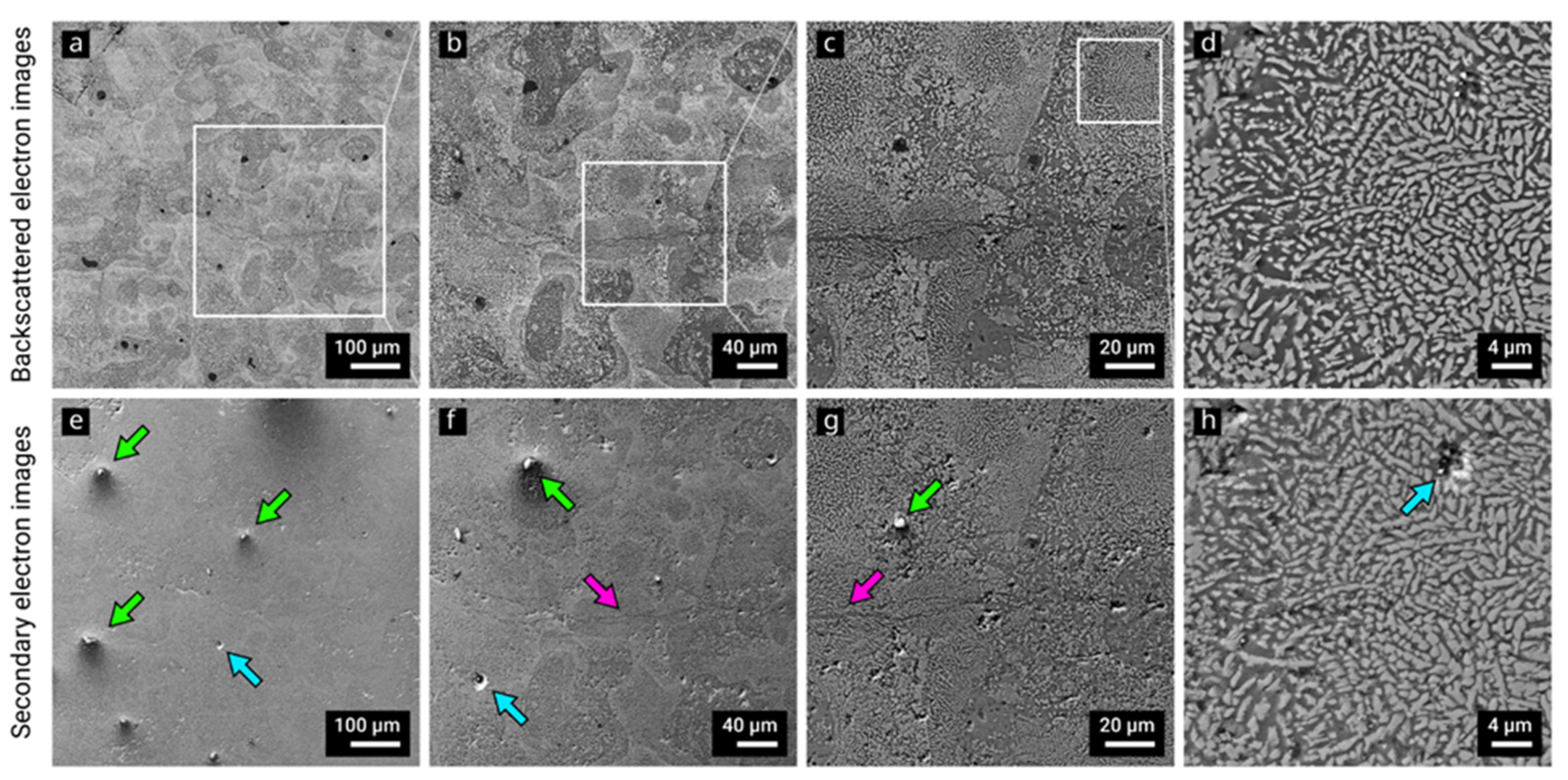
3. Results and Discussion
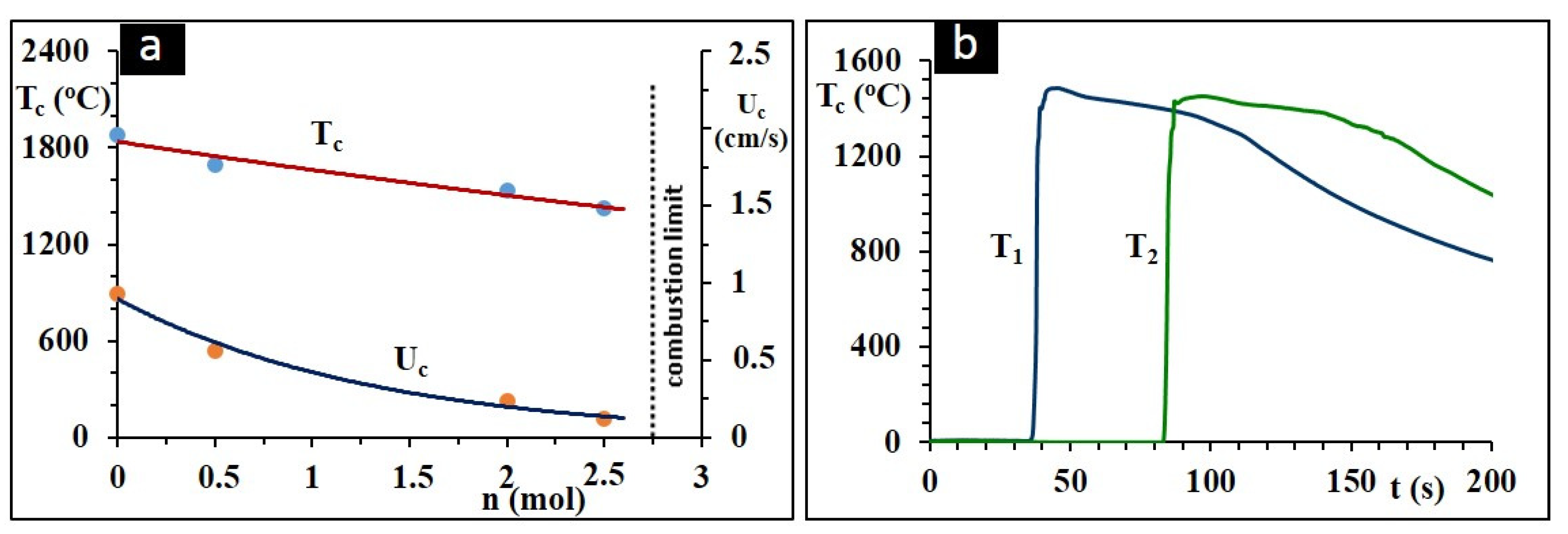
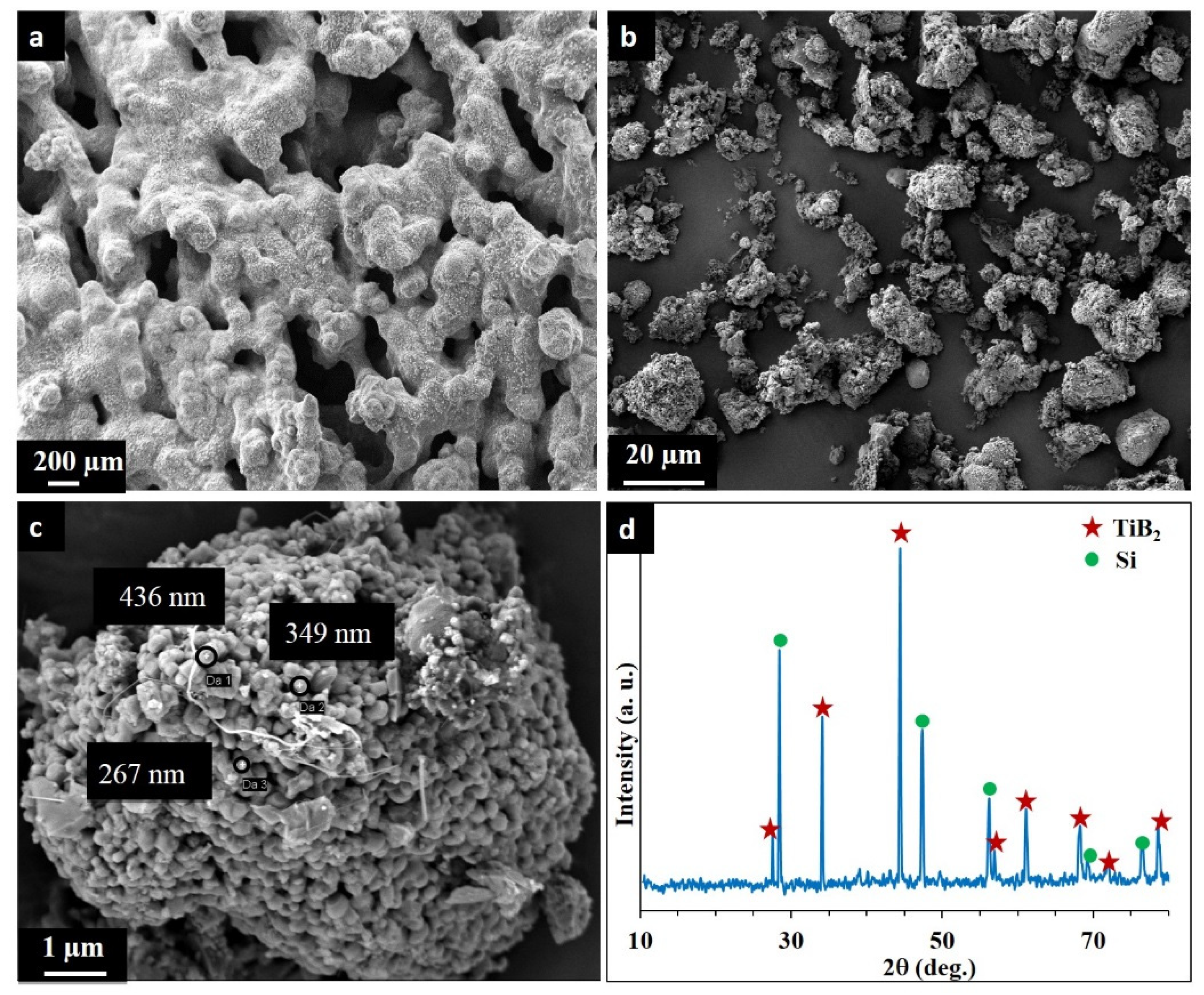
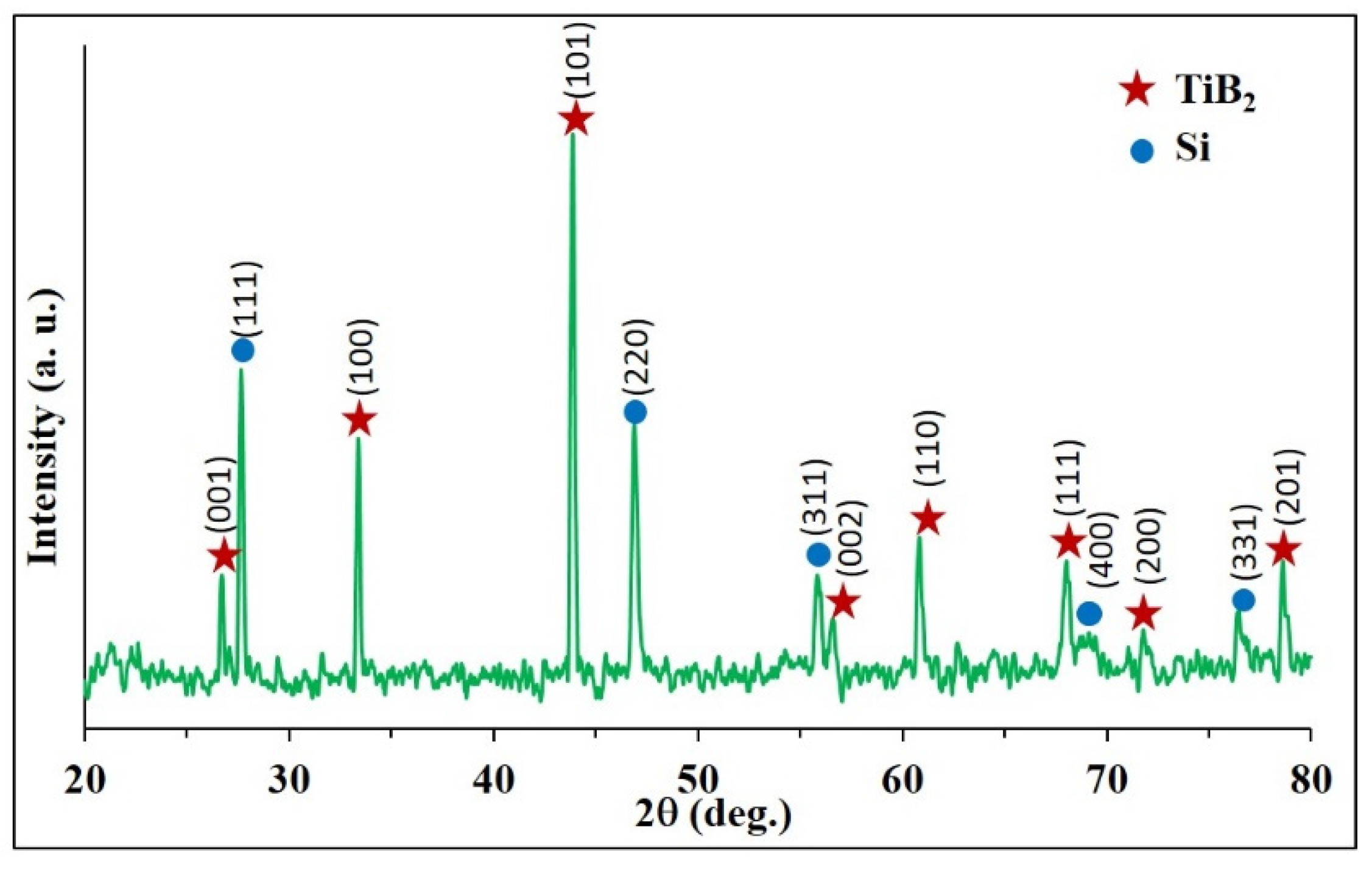
3.1. Combustion Dynthesis in the Ti-B-Si System
3.2. Selective Laser Melting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayna, T.; Striukova, L. From rapid prototyping to home fabrication: How 3D printing is changing business model innovation. Technol. Forecast. Soc. Chang. 2016, 102, 214–224. [Google Scholar] [CrossRef]
- Kietzmann, J.; Pitt, L.; Berthon, P. Disruptions, decisions, and destinations: Enter the age of 3D printing and additive manufacturing. Bus. Horiz. 2015, 58, 209–215. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Aided Design. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Sing, S.L.; Yeong, W.Y.; Wiria, F.E.; Tay, B.Y.; Zhao, Z.; Zhao, L.; Tian, Z.; Yang, S. Direct selective laser sintering and melting of ceramics: A review. Rapid Prototyp. J. 2017, 23, 611–623. [Google Scholar] [CrossRef]
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.M.; Clare, A. Materials for additive manufacturing. CIRP Ann. 2017, 66, 659–681. [Google Scholar] [CrossRef]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Demut, I.F.; Schlier, L.; Schlordt, T.; Greil, P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; Ded, A.; Zhang, W. Additive manufacturing of metallic components–Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Minasyan, T.; Aghayan, M.; Aydinyan, S.; Liu, L.; Hussainova, I. Combustion synthesis of MoSi2 based composite and selective laser sintering thereof. J. Eur. Ceram. Soc. 2018, 38, 3814–3821. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principles and applications. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 1998; Volume 24, pp. 79–226. [Google Scholar] [CrossRef]
- Bogdanov, S.P.; Sychov, M.M.; Lebedev, L.A.; Mjakin, S.V.; Gravit, M.V. Core-shell powders for additive manufacturing of articles for underground construction. Procedia Eng. 2016, 165, 1579–1586. [Google Scholar] [CrossRef]
- Minasyan, T.; Liu, L.; Holovenko, Y.; Aydinyan, S.; Hussainova, I. Additively manufactured mesostructured MoSi2-Si3N4 ceramic lattice. Ceram. Int. 2019, 45, 9926–9933. [Google Scholar] [CrossRef]
- Telle, R. Analysis of pressureless sintering of titanium diboride ceramics with nickel, cobalt, and tungsten carbide additives. J. Eur. Ceram. Soc. 2019, 39, 2266–2276. [Google Scholar] [CrossRef]
- Basu, B.; Raju, G.B.; Suri, A.K. Processing and properties of monolithic TiB2 based materials. Int. Mater. Rev. 2006, 51, 352–374. [Google Scholar] [CrossRef]
- Liu, L.; Aydinyan, S.; Minasyan, T.; Baronins, J.; Antonov, M.; Kharatyan, S.; Hussainova, I. Spark plasma sintering of combustion synthesized TiB2-Si composite. Ceram. Mod. Technol. 2019, 1. [Google Scholar] [CrossRef]
- Bertrand, P.; Bayle, F.; Combe, C.; Gœuriot, P.; Smurov, I. Ceramic components manufacturing by selective laser sintering. Appl. Surf. Sci. 2007, 254, 989–992. [Google Scholar] [CrossRef]
- Li, X.P.; Ji, G.; Chen, Z.; Addad, A.; Wu, Y.; Wang, H.W.; Vleugels, J.; van Humbeeck, J.; Kruth, J.P. Selective laser melting of nano-TiB2 decorated AlSi10Mg alloy with high fracture strength and ductility. Acta Mater. 2017, 129, 183–193. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium–titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Shishkovsky, I.; Kakovkina, N.; Sherbakov, V. Graded layered titanium composite structures with TiB2 inclusions fabricated by selective laser melting. Compos. Struct. 2017, 169, 90–96. [Google Scholar] [CrossRef]
- Xia, M.; Liu, A.; Hou, Z.; Li, N.; Chen, Z.; Ding, H. Microstructure growth behavior and its evolution mechanism during laser additive manufacture of in-situ reinforced (TiB+TiC)/Ti composite. J. Alloy. Compd. 2017, 728, 436–444. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Sani, E.; Meucci, M.; Mercatelli, L.; Jafrancesco, D.; Sans, J.L.; Silvestroni, L.; Sciti, D. Optical properties of boride ultrahigh-temperature ceramics for solar thermal absorbers. J. Photonics Energy 2014, 4, 045599. [Google Scholar] [CrossRef]
- Riley, D.P.; Oliver, C.P.; Kisi, E.H. In-situ neutron diffraction of titanium silicide, Ti5Si3, during self-propagating high-temperature synthesis (SHS). Intermetallics 2006, 14, 33–38. [Google Scholar] [CrossRef]
- Li, H.P. The numerical simulation of the heterogeneous composition effect on the combustion synthesis of TiB2 compound. Acta Mater. 2003, 51, 3213–3224. [Google Scholar] [CrossRef]
- Li, H.P. A numerical investigation of the ignition power on micropyretic synthesis of TiB2. Model. Simul. Mater. Sci. Eng. 2005, 13, 1331. [Google Scholar] [CrossRef]
- Kennedy, S.K.; Dalley, A.M.; Kotyk, G.J. Additive Manufacturing: Assessing Metal Powder Quality through Characterizing Feedstock and Contaminants. J. Mater. Eng. Perform. 2019, 28, 728–740. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Inter. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]


| No | Precursor | Particle Size, μm | Producer | Purity, % |
|---|---|---|---|---|
| 1 | Ti | 20 | Alfa Aesar | >99 |
| 2 | B | <1 | amorphous powder (Sigma-Aldrich) | >95 |
| 3 | Si | 20 | Sigma-Aldrich | >98 |
| No. | Laser Current(mA) | Volumetric Energy Density (J∙mm−3) |
|---|---|---|
| Cylinder 1 | 1400 | 280 |
| Cylinder 2 | 1800 | 360 |
| Cylinder 3 | 2200 | 440 |
| Powder Size, µm | PSD, µm | Hall Flow Rate (FRH), s/50 g |
|---|---|---|
| µ < 20 | - | - |
| 20 < µ< 45 | Dv10 – 5.48 Dv50 – 21.2 Dv90 – 41.4 | 29.75 ± 0.31 |
| 45 < µ < 90 | Dv10 – 12.3 Dv50 – 57.56 Dv90 – 85.4 | 24.68 ± 0.34 |
| No. | Geom. Density (g∙cm−3) | Relative Density (%) | Vickers Hardness (GPa) |
|---|---|---|---|
| Cylinder 1 | 2.77 | 87 | - |
| Cylinder 2 | 2.86 | 90 | 4.4 ± 1 |
| Cylinder 3 | 3.05 | 97 | 7.6 ± 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Aydinyan, S.; Minasyan, T.; Hussainova, I. SHS Produced TiB2-Si Powders for Selective Laser Melting of Ceramic-Based Composite. Appl. Sci. 2020, 10, 3283. https://doi.org/10.3390/app10093283
Liu L, Aydinyan S, Minasyan T, Hussainova I. SHS Produced TiB2-Si Powders for Selective Laser Melting of Ceramic-Based Composite. Applied Sciences. 2020; 10(9):3283. https://doi.org/10.3390/app10093283
Chicago/Turabian StyleLiu, Le, Sofiya Aydinyan, Tatevik Minasyan, and Irina Hussainova. 2020. "SHS Produced TiB2-Si Powders for Selective Laser Melting of Ceramic-Based Composite" Applied Sciences 10, no. 9: 3283. https://doi.org/10.3390/app10093283
APA StyleLiu, L., Aydinyan, S., Minasyan, T., & Hussainova, I. (2020). SHS Produced TiB2-Si Powders for Selective Laser Melting of Ceramic-Based Composite. Applied Sciences, 10(9), 3283. https://doi.org/10.3390/app10093283