Removal of Chromium from Synthetic Wastewater Using Modified Maghemite Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Adsorbent Preparation and Characterization
2.3. Batch Adsorption Experiment and Chromium Analysis
2.4. Experimental Design and Optimization of Parameters
3. Results
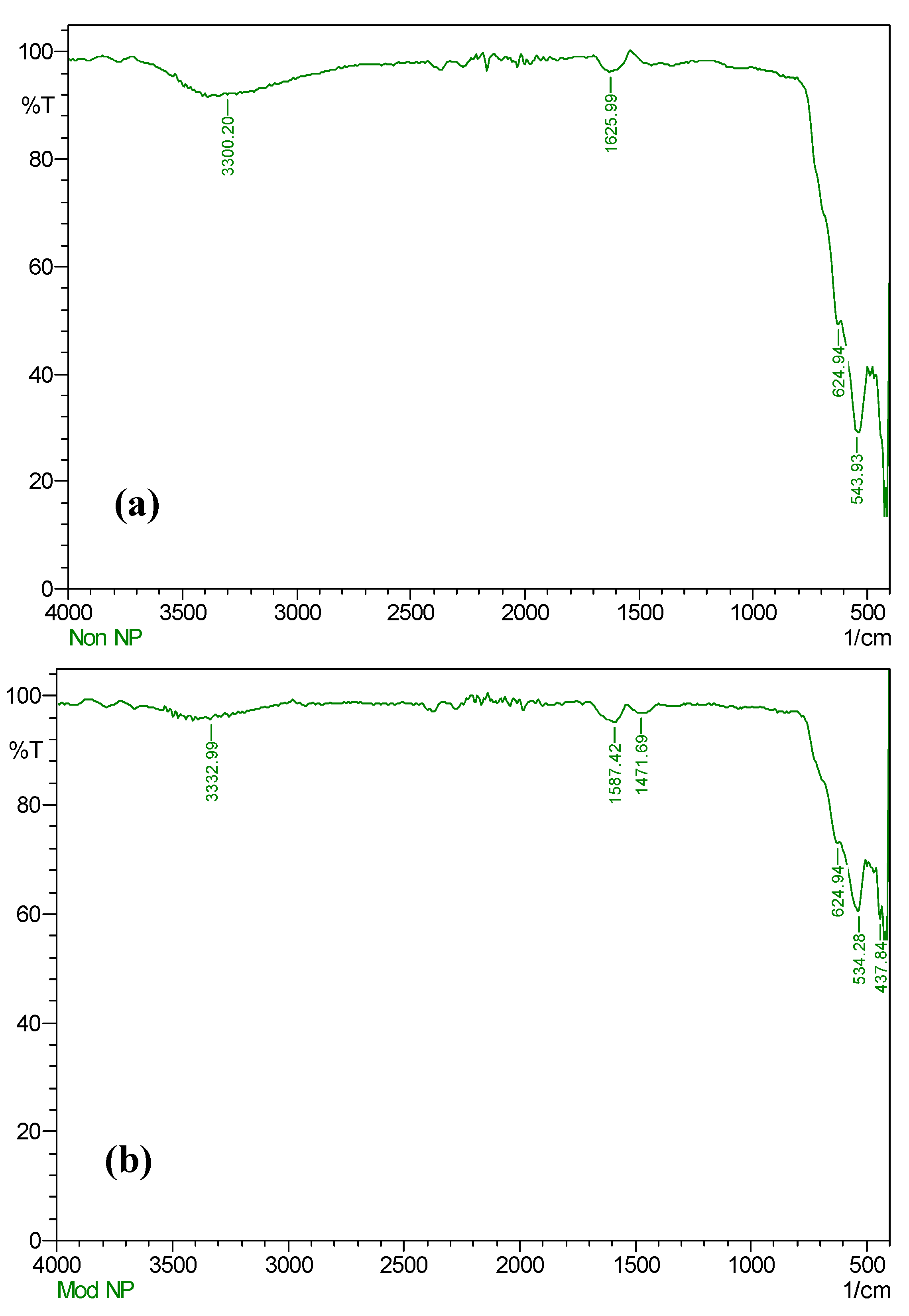
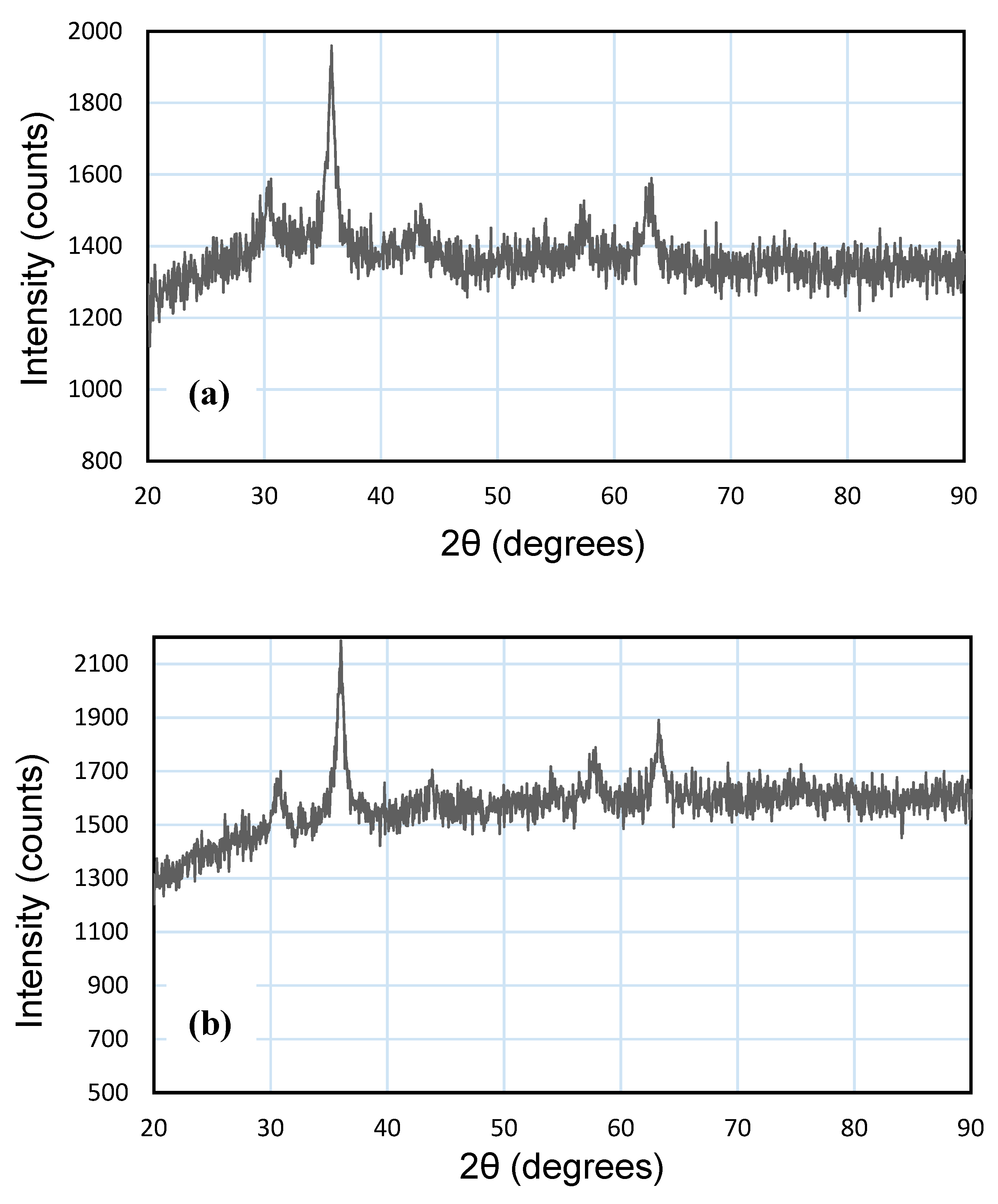
3.1. Characterization of NPs and MNPs
3.2. Development of the Regression Model and Statistical Analysis
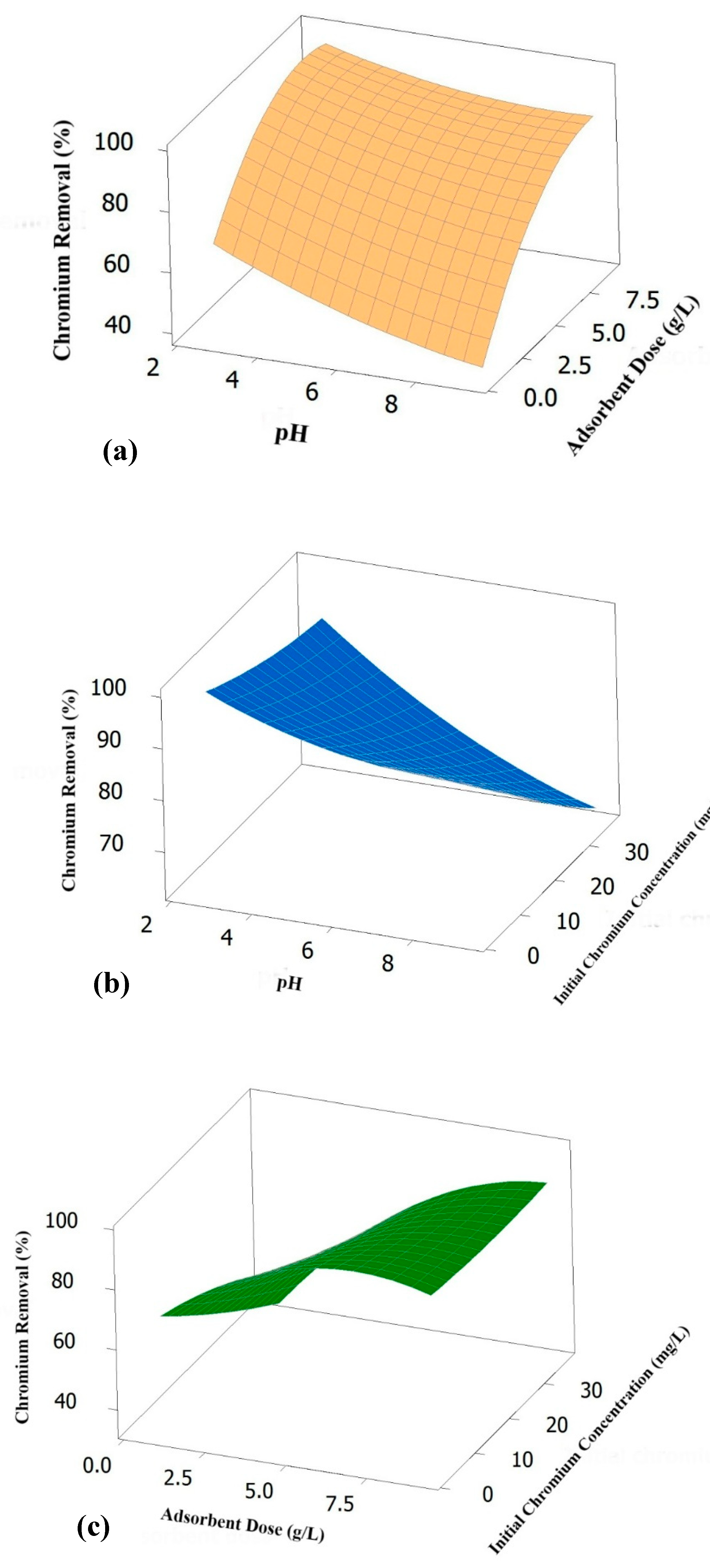
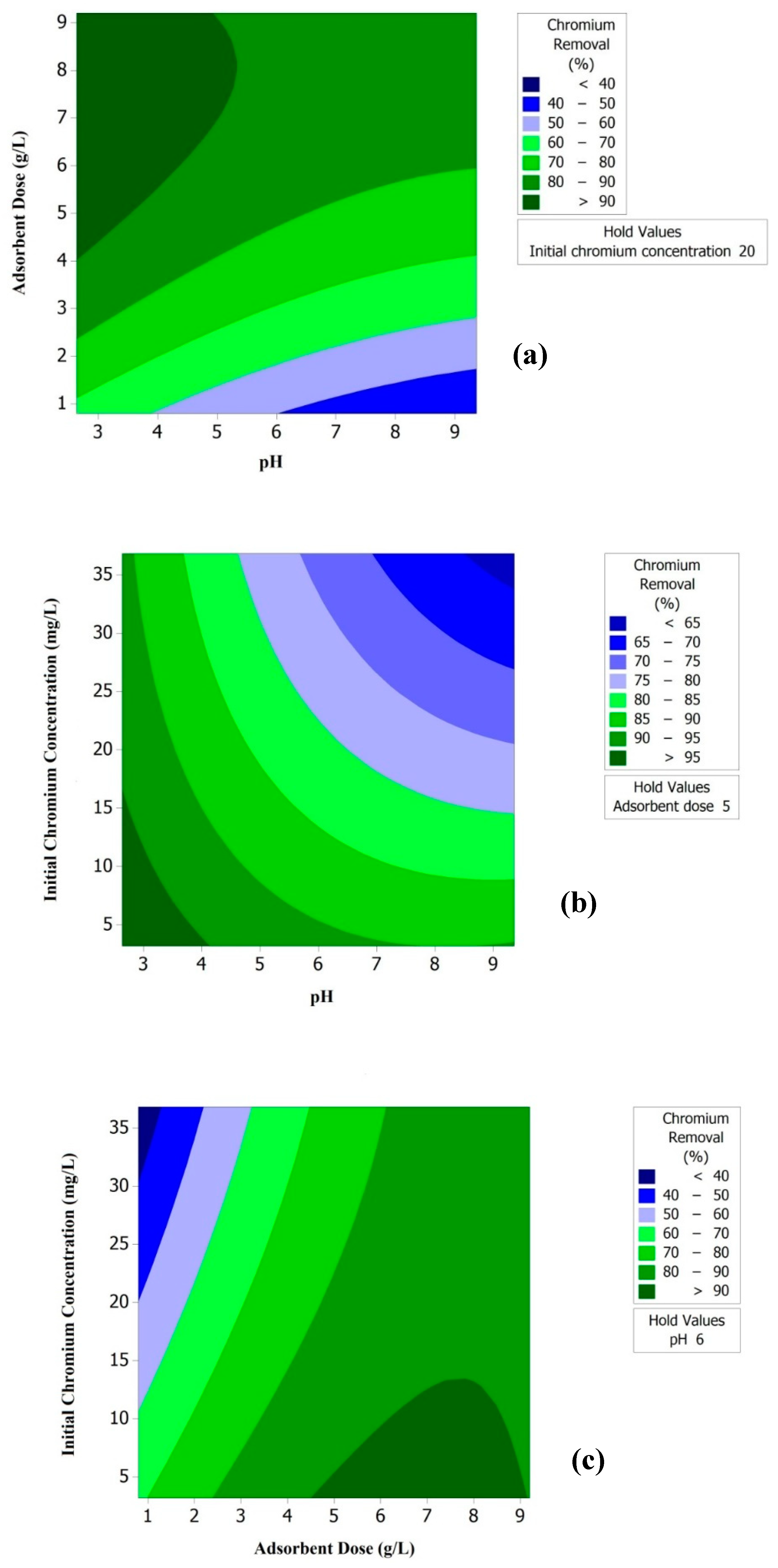
3.3. Effect of pH on % Removal of Chromium
3.4. Effect of Adsorbent Dose on % Removal of Chromium
3.5. Effect of Initial Concentration on % Removal of Chromium
3.6. Practical Implications of the Work and Future Research Prospects
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dupont, L.; Guillon, E. Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environ. Sci. Technol. 2003, 37, 4235–4241. [Google Scholar] [CrossRef]
- WHO Guidelines for Drinking-water Quality. Available online: https://www.who.int/water_sanitation_health/water-quality/guidelines/en/ (accessed on 4 March 2020).
- Álvarez-Ayuso, E.; García-Sánchez, A.; Querol, X. Purification of metal electroplating waste waters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.D.; Wittbrodt, P.R. Processes affecting the remediation of chromium-contaminated sites. Environ. Health Perspect. 1991, 92, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Richard, F.C.; Bourg, A.C.M. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasami, T. Bioaccumulation of chromium from tannery wastewater: An approach for chrome recovery and reuse. Environ. Sci. Technol. 2004, 38, 300–306. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Selective Removal of Heavy Metals from industrial wastewater using maghemite nanoparticle: Performance and mechanisms. J. Environ. Eng. 2006, 132, 709–715. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Asouhidou, D.D. Kinetics of sorptive removal of chromium(VI) from aqueous solutions by calcined Mg–Al–CO3 hydrotalcite. Water Res. 2003, 37, 2875–2882. [Google Scholar] [CrossRef]
- Periasam, K.; Srinivasan, K.; Murugan, P. Studies on chromium(VI) removal by activated ground-nut husk carbon. Indian J. Environ. Health 1991, 33, 433–439. [Google Scholar]
- Singh, V.K.; Tiwari, P.N. Removal and recovery of chromium(VI) from industrial waste water. J. Chem. Technol. Biotechnol. 1997, 69, 376–382. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Lin, S.-H.; Juang, R.-S. Removal of heavy metal ions from aqueous solutions using various low-cost adsorbents. J. Hazard. Mater. 2003, 102, 291–302. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A Review. Water. Air. Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Hayashi, N.; Chen, J.; Seko, N. Nitrogen-containing fabric adsorbents prepared by radiation grafting for removal of chromium from wastewater. Polymers (Basel) 2018, 10, 744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Z.; Hursthouse, A.; Ren, B. Gemini surfactant-modified activated carbon for remediation of hexavalent chromium from Water. Water 2018, 10, 91. [Google Scholar] [CrossRef]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D.; O’Shea, K. Chromium(VI) removal by maghemite nanoparticles. Chem. Eng. J. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Lin, S.; Lu, D.; Liu, Z. Removal of arsenic contaminants with magnetic γ-Fe2O3 nanoparticles. Chem. Eng. J. 2012, 211–212, 46–52. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem. Eng. J. 2012, 211–212, 493–500. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D.; O’Shea, K.E. Cr(VI) adsorption and reduction by humic acid coated on magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef]
- Wang, F.; Yang, W.; Zheng, F.; Sun, Y. Removal of Cr(VI) from simulated and leachate wastewaters by bentonite-supported zero-valent iron nanoparticles. Int. J. Environ. Res. Public Health 2018, 15, 2162. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Uptake of Zn2+ and As3+ from wastewater by adsorption onto imine functionalized magnetic nanoparticles. Water (Switzerland) 2018, 10, 36. [Google Scholar] [CrossRef]
- Ostroumov, S.A. Biological Effects of Surfactants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Rosen, M.J. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 0471478180. [Google Scholar]
- Gao, X.; Chorover, J. Adsorption of sodium dodecyl sulfate (SDS) at ZnSe and α-Fe2O3 surfaces: Combining infrared spectroscopy and batch uptake studies. J. Colloid Interface Sci. 2010, 348, 167–176. [Google Scholar] [CrossRef]
- Khatoon, N.; Khan, A.H.; Pathak, V.; Agnihotri, N.; Rehman, M. Removal of hexavalent chromium from synthetic waste water using synthetic nano zero valent iron (nZVI) as adsorbent. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 6140–6149. [Google Scholar]
- Bagheban Shahri, F.; Niazi, A. Synthesis of modified maghemite nanoparticles and its application for removal of acridine orange from aqueous solutions by using Box-Behnken design. J. Magn. Magn. Mater. 2015, 396, 318–326. [Google Scholar] [CrossRef]
- Han, M.J.; Behera, S.K.; Park, H.-S. Anaerobic co-digestion of food waste leachate and piggery wastewater for methane production: Statistical optimization of key process parameters. J. Chem. Technol. Biotechnol. 2012, 87, 1541–1550. [Google Scholar] [CrossRef]
- Lkhagvadulam, B.; Tsagaantsetseg, B.; Tergel, D.; Chuluunkhuyag, S. Removal of chromium from a tannery wastewater by using a maghemite nanoparticles. Int. J. Environ. Sci. Dev. 2017, 8, 696–702. [Google Scholar] [CrossRef]
- Predescu, A.; Nicolae, A. Adsorption of Zn, Cu and Cd from waste waters by means of maghemite nanoparticles. UPB Bul. Stiint. Ser. B Chem. Mater. Sci. 2012, 74, 255–264. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Gallo-Cordova, A.; Lemus, J.; Palomares, F.J.; Morales, M.P.; Mazarío, E. Superparamagnetic nanosorbent for water purification: Assessment of the adsorptive removal of lead and methyl orange from aqueous solutions. Sci. Total Environ. 2020, 711, 134644. [Google Scholar] [CrossRef]
- Pérez-Candela, M.; Martín-Martínez, J.; Torregrosa-Maciá, R. Chromium(VI) removal with activated carbons. Water Res. 1995, 29, 2174–2180. [Google Scholar] [CrossRef]
- Babel, S.; Opiso, E.M. Removal of Cr from synthetic wastewater by sorption into volcanic ash soil. Int. J. Environ. Sci. Technol. 2007, 4, 99–107. [Google Scholar] [CrossRef]
- Kan, C.-C.; Ibe, A.H.; Rivera, K.K.P.; Arazo, R.O.; de Luna, M.D.G. Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sustain. Environ. Res. 2017, 27, 163–171. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Nie, H.-L.; Branford-White, C.; He, Z.-Y.; Zhu, L.-M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J. Colloid Interface Sci. 2009, 330, 29–37. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Seraj, S.; Mirzayi, B. Catecholamine coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem. Eng. J. 2012, 181–182, 323–333. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Donia, D.T.; Carbone, M. Fate of the nanoparticles in environmental cycles. Int. J. Environ. Sci. Technol. 2019, 16, 583–600. [Google Scholar] [CrossRef]
- Liu, Y.; Tourbin, M.; Lachaize, S.; Guiraud, P. Nanoparticles in wastewaters: Hazards, fate and remediation. Powder Technol. 2014, 255, 149–156. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.; Chen, T. Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.; Chen, G. Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep. Purif. Technol. 2007, 58, 76–82. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Fan, M.; Yang, D.; Zhu, R.; Ge, F.; Zhu, J.; He, H. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef]
- Adegoke, H.I.; AmooAdekola, F.; Fatoki, O.S.; Ximba, B.J. Adsorption of Cr(VI) on synthetic hematite (α-Fe2O3) nanoparticles of different morphologies. Korean J. Chem. Eng. 2014, 31, 142–154. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and chromium removal by mixed magnetite–maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
| Variables | Coded Values | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| pH (X1) | 2.6 | 4 | 6 | 8 | 9.4 |
| Adsorbent dose (X2, g/L) | 0.8 | 2.5 | 5 | 7.5 | 9.2 |
| Initial chromium concentration (X3, mg/L) | 3.2 | 10 | 20 | 30 | 36.8 |
| Run | Actual Level of Factors | Coded Level of Factors | Chromium RE (%) | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 (g/L) | X3 (mg/L) | X1 | X2 | X3 | Experimental | Predicted | |
| 1 | 4 | 2.5 | 10 | −1 | −1 | −1 | 80.54 | 84.38 |
| 2 | 8 | 2.5 | 10 | 1 | −1 | −1 | 69.79 | 69.87 |
| 3 | 4 | 7.5 | 10 | −1 | 1 | −1 | 92.72 | 94.54 |
| 4 | 8 | 7.5 | 10 | 1 | 1 | −1 | 90.36 | 89.56 |
| 5 | 4 | 2.5 | 30 | −1 | −1 | 1 | 67.46 | 67.44 |
| 6 | 8 | 2.5 | 30 | 1 | −1 | 1 | 52.35 | 49.72 |
| 7 | 4 | 7.5 | 30 | −1 | 1 | 1 | 93.82 | 92.92 |
| 8 | 8 | 7.5 | 30 | 1 | 1 | 1 | 82.2 | 77.54 |
| 9 | 2.64 | 5 | 20 | −1.682 | 0 | 0 | 95.48 | 93.94 |
| 10 | 9.36 | 5 | 20 | 1.682 | 0 | 0 | 73.95 | 74.85 |
| 11 | 6 | 0.795 | 20 | 0 | −1.682 | 0 | 49.58 | 49.69 |
| 12 | 6 | 9.204 | 20 | 0 | 1.682 | 0 | 88.34 | 87.69 |
| 13 | 6 | 5 | 3.182 | 0 | 0 | −1.682 | 92.81 | 91.28 |
| 14 | 6 | 5 | 36.82 | 0 | 0 | 1.682 | 72.15 | 72.98 |
| 15 | 6 | 5 | 20 | 0 | 0 | 0 | 80.85 | 80.99 |
| 16 | 6 | 5 | 20 | 0 | 0 | 0 | 80.89 | 80.99 |
| 17 | 6 | 5 | 20 | 0 | 0 | 0 | 80.84 | 80.99 |
| 18 | 6 | 5 | 20 | 0 | 0 | 0 | 80.87 | 80.99 |
| 19 | 6 | 5 | 20 | 0 | 0 | 0 | 83.49 | 80.99 |
| 20 | 6 | 5 | 20 | 0 | 0 | 0 | 81.02 | 80.99 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | P Value | Remarks |
|---|---|---|---|---|---|---|
| Regression | 2976.04 | 9 | 330.67 | 144.94 | 0 | Significant |
| Linear | 2546.38 | 3 | 848.79 | 372.05 | 0 | Significant |
| X1 | 423.48 | 1 | 423.48 | 185.63 | 0 | Significant |
| X2 | 1739.86 | 1 | 1739.86 | 762.64 | 0 | Significant |
| X3 | 383.03 | 1 | 383.03 | 167.9 | 0 | Significant |
| Square | 320.03 | 3 | 106.68 | 46.76 | 0 | Significant |
| X12 | 36.28 | 1 | 21.38 | 9.37 | 0.012 | |
| X22 | 281.11 | 1 | 272.98 | 119.66 | 0 | Significant |
| X32 | 2.64 | 1 | 2.64 | 1.16 | 0.308 | Insignificant |
| Interaction | 109.63 | 3 | 36.54 | 16.02 | 0 | Significant |
| X1× X2 | 17.64 | 1 | 17.64 | 7.73 | 0.019 | Significant |
| X1× X3 | 23.19 | 1 | 23.19 | 10.16 | 0.01 | |
| X2× X3 | 68.8 | 1 | 68.8 | 30.16 | 0 | |
| Residual Error | 22.81 | 10 | 2.28 | _ | _ | |
| Lack of fit | 17.18 | 5 | 3.44 | 3.05 | 0.123 | Insignificant |
| Pure Error | 5.64 | 5 | 1.13 | _ | _ | |
| Total | 2998.85 | 19 |
| Model Term Coefficient | t -Value | P-Value |
|---|---|---|
| Constant | 132.014 | 0 |
| X1 | −13.624 | 0 |
| X2 | 27.616 | 0 |
| X3 | −12.957 | 0 |
| X12 | 3.061 | 0.012 |
| X22 | −10.939 | 0 |
| X32 | 1.075 | 0.308 |
| X1 × X2 | 2.781 | 0.019 |
| X1 × X3 | −3.188 | 0.01 |
| X2× X3 | 5.491 | 0 |
| Nanosorbent | Experimental Condition | Adsorption Capacity (mg/g) | Adsorption Mechanism | References |
|---|---|---|---|---|
| Maghemite | pH = 2.5, concentration = 5-200 mg/L, contact time = 15 min, temperature = 25 °C | 19.2 | Electrostatic attraction and ion exchange. | [37] |
| Maghemite | pH = 2.5, concentration = 50 mg/L, temperature = 25 °C, contact time = 10 min, pHZPC = 6.3 | 17.0 | Electrostatic attraction and ion exchange. | [7] |
| Maghemite | pH = 4.0, concentration = 0.3 g/L, contact time = 120 min, pHZPC = 6.6 | 1.62 | Adsorption was controlled by surface sorption and intraparticle diffusion, followed by redox reaction. | [15] |
| Montmorillonite- supported magnetite | pH = 2.0-2.6, concentration = 0.5 g/L, contact time = 120 min | 15.3 | Adsorption was a physico-chemical process, including an electrostatic attraction followed by a redox reaction. | [41] |
| δ-FeOOH-coated maghemite | pH = 2.5, concentration range = 10-200 mg/L, contact time = 30 min, adsorbent dose = 0.1 g | 25.8 | Cr(VI) adsorption onto the δ-FeOOH-coated γ-Fe2O3 is mainly controlled by outer-sphere complexation. | [42] |
| Diatomite-supported magnetite | pH = 2.0-2.5, concentration range = 50 mg/L, contact time = 120 min, temperature = 25 °C | 11.4 | Adsorption was a physico-chemical process, which included an electrostatic attraction followed by a redox process. | [43] |
| Hematite | pH = 3.0, concentration range = 1-100 mg/L, contact time = 240 min, temperature = 25 °C, pHZPC = 9.35 | 200 | Adsorption of Cr (VI) onto hematite nanoparticles was by inner-sphere surface complexation. | [44] |
| Mixed magnetite and maghemite | pH = 2.0, concentration range = 0.5-4 mg/L, contact time = 24 h, adsorbent dose = 0.4 g/L | 2.4 | Electrostatic attraction between chromium and magnetite–maghemite mixture. | [45] |
| Modified maghemite | pH = 2.6, concentration = 20 mg/L, contact time = 80 min | 4.0 | Electrostatic attraction and ion exchange. | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, S.K.; Sahni, S.; Tiwari, G.; Rai, A.; Mahanty, B.; Vinati, A.; Rene, E.R.; Pugazhendhi, A. Removal of Chromium from Synthetic Wastewater Using Modified Maghemite Nanoparticles. Appl. Sci. 2020, 10, 3181. https://doi.org/10.3390/app10093181
Behera SK, Sahni S, Tiwari G, Rai A, Mahanty B, Vinati A, Rene ER, Pugazhendhi A. Removal of Chromium from Synthetic Wastewater Using Modified Maghemite Nanoparticles. Applied Sciences. 2020; 10(9):3181. https://doi.org/10.3390/app10093181
Chicago/Turabian StyleBehera, Shishir Kumar, Srijan Sahni, Gunjan Tiwari, Aditi Rai, Biswanath Mahanty, Ayi Vinati, Eldon R. Rene, and Arivalagan Pugazhendhi. 2020. "Removal of Chromium from Synthetic Wastewater Using Modified Maghemite Nanoparticles" Applied Sciences 10, no. 9: 3181. https://doi.org/10.3390/app10093181
APA StyleBehera, S. K., Sahni, S., Tiwari, G., Rai, A., Mahanty, B., Vinati, A., Rene, E. R., & Pugazhendhi, A. (2020). Removal of Chromium from Synthetic Wastewater Using Modified Maghemite Nanoparticles. Applied Sciences, 10(9), 3181. https://doi.org/10.3390/app10093181








