Abstract
Laser additive manufacturing with mixed powders of boron carbide and aluminum alloy is investigated. Parameters such as laser power, scan speed, scan pattern, and hatching space are systematically evaluated and optimized to obtain the desired density and porosity. These results show that the AM part contains 20 wt% boron carbide and 80 wt% aluminum alloy, which are well mixed and synthesized during the melting process. Its mechanical properties are close to those of aluminum. A thin-wall structure based two dimensional and three dimensional radial collimators were fabricated with well-controlled geometry for neutron scattering measurement.
1. Introduction
Using neutron scattering measurement, materials at the atomic level can be probed, which is essential to develop innovations like faster computers, tougher armor, and effective medicines. Radial collimators are used when a neutron scattering instrument employs a large area of detectors to cover a wide range of scattering angles [1,2]. A radial collimator consists of a series of absorbing septa radially oriented from a fixed point. These collimators will often oscillate several degrees at the sample position. Conventional neutron collimators use thin foils, typically made from mylar, aluminum (Al), or stainless steel, coated in an absorbing material, such as gadolinium (Ga, melting temperature 1312 °C, thermal conductivity 10.6 W/m.K) or enriched boron (B, melting temperature 2076 °C, thermal conductivity 27.4 W/m.K), to absorb divergent incident neutrons, or those scattered from unwanted sources. The foils are held together and tensioned to ensure uniformity, using bolted frames. The unit for gripping and tensioning the foils limits how closely they may be placed together, their angular separation, their proximity to the sample, and therefore the efficiency of the collimator. Modelling software, in combination with ray tracing simulations, shows that there are designs that can greatly improve the performance of the collimator but are not possible to implement using conventional assembly or machining techniques.
Additive manufacturing (AM), especially laser-based AM, becomes a powerful tool to replace conventional methods such as casting and hot spray, due to its cost effectiveness and capability of making complex structures and compositions [3,4,5,6,7,8,9]. Specifically, AM shows significant advantages in parts fabrication with mixed powders, metals, and/or ceramics, to achieve desired performance [7,8,9]. However, direct thin-wall (2D and 3D) fabrication for radial collimators for neutron scattering instrument is still a challenging field [1,2], especially for multimaterials such as a mixture of boron carbide (B4C) and Al alloy (AlSi10Mg). This is mainly due to a limited understanding of composition control, laser melting control, melted structure formation, and phase transition.
In this paper, research is extended from the fabrication of functional ceramic components to directly make radial collimators for neutron scattering measurement without binders and post process. To the best of our knowledge, this is the first publication demonstrating additive manufacturing using mixed powders of B4C and Al alloy.
2. Materials and Methods
Boron carbide (melting temperature 2763 °C, thermal conductivity 29 W/m.K, density 2.52 g/cm3, and thermal expansion coefficient 3 × 10−6/K) is a very common material used for radial collimator, because it is relatively cheap, and safe in terms of handling. However, its brittleness makes it hard to use in fabrication of thin-wall radial collimators with 3D printing technology. It is found that by adding Al (melting temperature 660 °C, thermal conductivity 237 W/m.K, density 2.70 g/cm3, and thermal expansion coefficient 23 × 10−6/K) to B4C, the strength and thermal handling capability can be improved significantly and the brittleness can be reduced without increasing in weight. In this paper, B4C powder (American Elements, Los Angeles, CA) and Al alloy (AlSi10Mg) powder (CNPC, China) are used to make radial collimator parts. These powders are readily available standard commercial products. The mixing ratio of 20% B4C and 80% AlSi10Mg is selected to make thin walls matching the performance of neutron absorption (B [10] concentration) by using the conventional method, whose Myler septa are coated with Boron [2].
Microscopic images and size characterization are given in Figure 1 and Figure 2 for B4C powder and AlSi10Mg powder, respectively. The size of the B4C particles is generally in the range of 10 to 45 µm. However, the shapes are irregular, which is not ideal for AM process. In reality, we have no other choice since this is the best quality we could find from all suppliers. AlSi10Mg particle size is finer than B4C, since 80% of the particles are measured within 10 to 30 µm. Moreover, it has spherical shape, which has better flowability. Practically, different powder sizes can help make dense components [10].
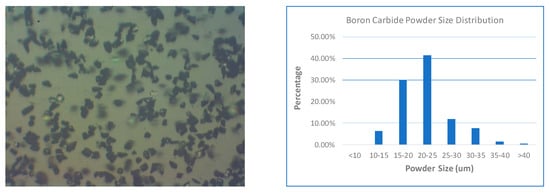
Figure 1.
Characterization of boron carbide (B4C) powder.
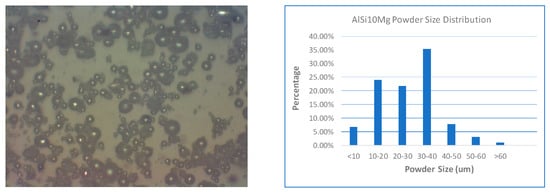
Figure 2.
Characterization of Al alloy (AlSi10Mg) powder.
80 wt% of AlSi10Mg alloy powder was mixed with 20 wt% B4C powder before the AM process, by using a jar mill machine to run 24 h. A series of cubic samples were printed to measure the density and surface roughness. Powder bed fusion (PBF) system made by PolarOnyx was used to do the AM process. This system was modified to fit for low-mass ceramic 3D printing and filled with Argon gas. The process parameters are listed as follows: laser average power: 100, 150, and 200 W on targets; laser type: fiber laser; laser operation mode: pulse repetition rate of 200 MHz, pulse width of 1 ns; laser wavelength: 1070 nm; scan speed: 100 mm/s to 500 mm/s; patterning type: lines; hatching order: bidirectional; hatching space: 50 to 150 μm; and powder layer thickness: 50 μm. Fiber laser can provide stable operation and reasonable absorption, which is essential to the AM process.
All of the measurement data on roughness and density are summarized in Figure 3. It is noted here that some of the data are not complete due to the fact that those samples are damaged during polishing process. However, the rest of the data are sufficient enough to make a conclusion on process optimization. Over 99% relative density (image-J is used) and acceptable roughness (<50 μm, top surface) were obtained when the average power on target was greater than 150 W. Further study on uniformity was carried out by fixing 150 W average power and scan speed of 100 and 150 mm/s. The hatching space varies from 130 to 150 μm (or 0.13 mm to 0.15mm) at a step of 10 μm. Figure 4 shows that all of the samples have good uniformity of relative density (<2% variation, from 97–99%).
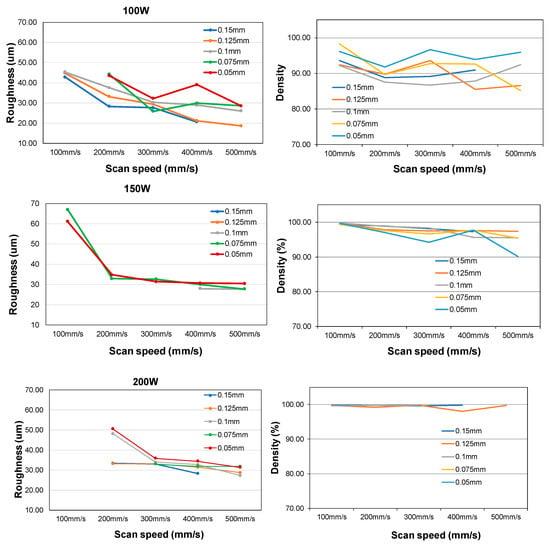
Figure 3.
Top surface roughness (left column) and density measurement (right column) of mixed B4C (20 wt%) and AlSi10Mg (80 wt%).

Figure 4.
Uniformity test for samples using different parameters. All samples on the top use scan speed 100 mm/s, whereas on the bottom uses scan speed 150 mm/s. From the bottom to the top rows, the hatching space is 0.13, 0.14, 0.15, 0.16, and 0.17 mm, respectively.
3. Results and Discussion
Hardness, Young’s modulus, SEM, and energy dispersive x-ray spectroscopy (EDX) were tested for a sample printed with 150 W power and 150 mm/s scan speed. Figure 5 shows the load versus displacement for a cube sample. Nanovea PB1000 Mechanical Tester is used for the hardness test. It appears that the hardness (110 MPa) and Young’s modulus (11.7 GPa) of mixed B4C/Al are close to those of Al. This is mainly due to a large portion of Al powder in the sample. An important feature is that the synthesized part is less brittle and has better elastic property than B4C.
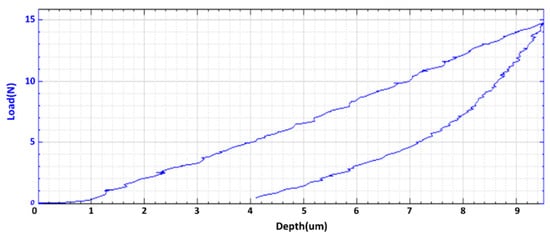
Figure 5.
Load curve.
Figure 6 shows the SEM and EDX results of a polished cubic sample with 99% density. EDX element analysis over an area of 1 mm x 1 mm indicates that Boron increases by 7 wt% and Al decreases by 30 wt% after the AM process. This has been verified with different testing areas and samples. One reasonable explanation is that a significant portion of Al was evaporated during the process due to its low melting temperature (660 °C) and boiling temperature (2740 °C). Its boiling temperature is close to the melting point of B4C (2763 °C). However, this will need to be further studied and confirmed in on-going research activities.
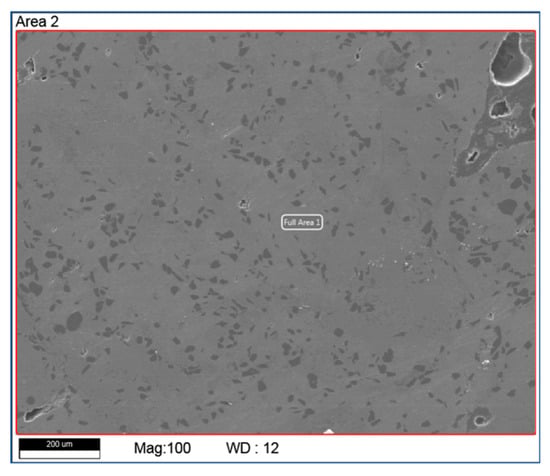
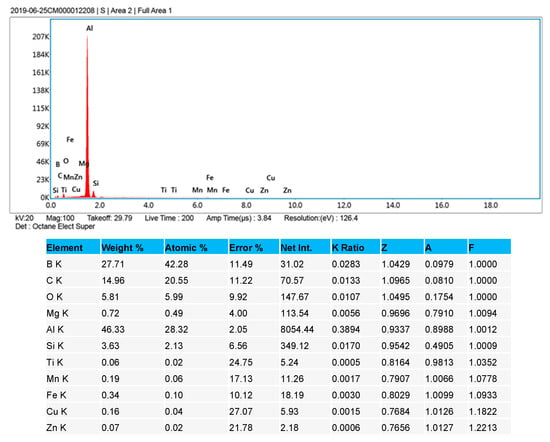
Figure 6.
SEM and EDX test results of a cubic sample with mixed B4C (20 wt%) and AlSi10Mg (80 wt%).
Mapping of element test using EDX was done with the same testing area shown in Figure 6 to identify the mixing quality of B4C and Al. As shown in Figure 7, Boron element is well distributed in the Al structure. Again, it shows that Boron appears to have a larger percentage after the AM process. More interestingly, the X-ray powder diffraction data, shown in Figure 8, clearly demonstrates newly formed crystal phases of aluminum carbide (Al4C3) and aluminum diboride (AlB2) during the melting and solidification process for the mixed compound powders (i.e., 20 wt% B4C and 80 wt% AlSi10Mg). This provides strong evidence that AM can not only melt powders to form 3D structures but also can carry on in situ synthesis to form new compounds to change their properties (such as mechanical, electrical, etc.) for specific applications. As a note, from the phase diagram of Al and B4C, aluminum carbide (Al4C3) forms at a temperature over 1400 °C and aluminum diboride (AlB2) at a temperature over 600 °C. Detailed study of AM synthesis is beyond the scope of this paper and will be present in a separate publication.
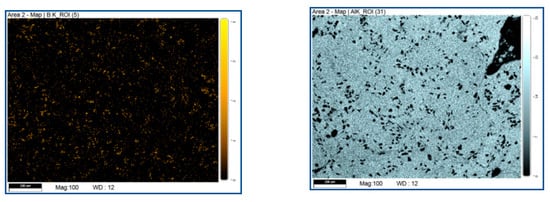
Figure 7.
EDX mapping test of boron (left) and Al (right). Boron is well distributed in the Al structure.
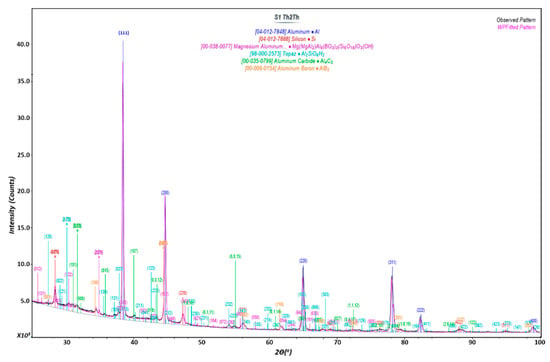
Figure 8.
XRD test of mixed B4C and AlSi10Mg sample.
Two types of collimators were made with the same parameters for hardness test (150 W power and 150 mm/s scan speed). One is the honeycomb type (3D), of which geometrical parameters were taken from reference [2]; the results are shown in Figure 9. In control of the geometry and tolerance (10 μm level), very consistent results have been achieved. Another type is the thin-wall collimator (2D). A small version of the optimized thin-wall type of collimator was printed and is given in Figure 10. It is under evaluation at Oak Ridge National Labs (ORNL). Part of the sample was cut and tested with EDX and XRD. It is consistent with the data from cubic samples (Figure 8) in terms of synthesis of Al4C3 and AlB2. Especially, we observed that Boron concentration has been increased significantly and Al has been decreased due to evaporation during the collimator printing.
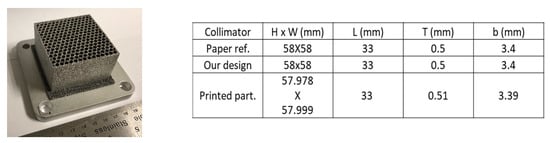
Figure 9.
3D collimator structures (honeycomb).
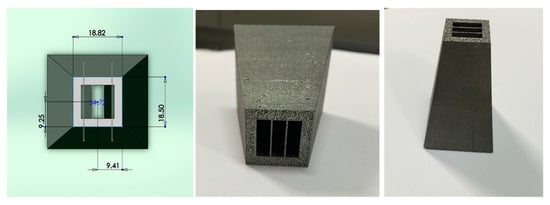
Figure 10.
A small version of the optimized collimator design.
4. Conclusions
For the first time, 3D printing using PBF system was demonstrated to manufacture radial collimators using mixed B4C (20 wt%) and AlSi10Mg (80 wt%) powders. The processed samples were examined and characterized by optical microscope, SEM, EDX, and XRD. It is found that Boron element is well distributed in Al structure, and the synthesized parts demonstrated excellent mechanical strength and brittleness improvement. We observed a significant portion of Al was lost during the AM process, for which further investigation is on-going to understand the mechanism.
Moreover, newly formed crystal phases such as aluminum carbide (Al4C3) and aluminum diboride (AlB2) are observed during the melting and solidification process. By control of melt pool temperature and composition, different level of synthesis can be achieved to match specific application. This opens a new frontier for 3D printing to synthesize new compounds and explore new properties. We believe there will be a great potential for the aerospace and defense, automobile, semiconductor, and energy industries.
Author Contributions
S.B. did design, experiment and analysis, and manuscript writing; H.J.L. did experiment and data process; J.L. did design, analysis and manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Department of Energy (DOE) Small Business Innovation Program (SBIR) DE-SC0019721.
Acknowledgments
Special thanks to the DOE program manager Pappannan Thiyagarajan for his valuable guidance and support. We also acknowledge our appreciation to Mathew Stone (Oak Ridge National Labs) for his thoughtful discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Copley, J.R.D.; Cook, J.C. An Analysis of the Effectiveness of Oscillating Radial Collimators in Neutron Scattering Applications. Nucl. Instrum. Methods Phys. Res. Sect. A 1994, 345, 313–323. [Google Scholar] [CrossRef]
- Stone, M.B.; Niedziela, J.L.; Loguillo, M.J.; Overbay, M.A.; Abernathy, D.L. A Radial Collimator for a Time-of-Flight Neutron Spectrometer. Rev. Sci. Instrum. 2014, 85, 085101. [Google Scholar] [CrossRef] [PubMed]
- Abe, F.; Osakada, K.; Shiomi, M.; Uematsu, K.; Matsumoto, M. The manufacturing of hard tools from metallic powders by selective laser melting. J. Mater. Process. Technol. 2001, 111, 210–213. [Google Scholar] [CrossRef]
- Bai, S.; Liu, J. Femtosecond laser additive manufacturing of Multi-layer YSZ and Ni-YSZ. Appl. Sci. 2020, 10, 979. [Google Scholar] [CrossRef]
- Liu, J.; Bai, S. Femtosecond laser additive manufacturing of YSZ. Appl. Phys. A 2017, 123, 293. [Google Scholar] [CrossRef]
- Lu, R.; Miller, D.J.; Frane, W.L.; Chandrasekaran, S.; Landingham, R.L.; Worsley, M.A.; Kuntz, J.D. Negative Additive Manufacturing of Complex Shaped Boron Carbides. J. Vis. Exp. 2018, 139, e58438. [Google Scholar] [CrossRef] [PubMed]
- Brueckner, F.; Riede, M.; Müller, M.; Marquardt, F.; Willner, R.; Seidel, A.; Lopéz, E.; Leyens, C.; Beyer, E. Enhanced manufacturing possibilities using multi-materials in laser metal deposition. J. Laser Appl. 2018, 30, 032308. [Google Scholar] [CrossRef]
- Brueckner, F.; Riede, M.; Mueller, M.; Marquardt, F.; Knoll, M.; Willner, R.; Seidel, A.; Lopéz, E.; Leyens, C.; Beyer, E. Fabrication of metallic multi-material components using Laser Metal Deposition. In Proceedings of the Solid Free Fabrication Symposium, Austin, TX, USA, 7–9 August 2017; pp. 2530–2538. [Google Scholar]
- Volpp, J. Impact of process parameters on particle distribution and wear resistance during laser deep alloying processes. In Proceedings of the Laser Advanced Materials Processing (LAMP), Fukuoka, Japan, 26–29 May 2015. [Google Scholar]
- King, W.E.; Anderson, A.T.; Ferencz, R.M.; Hodge, N.E.; Kamath, C.; Khairallah, S.A.; Rubenchik, A.M. Laser powder bed fusion additive manufacturing of metals; physics, computational, and materials challenges. Appl. Phys. Rev. 2015, 2, 041304. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).