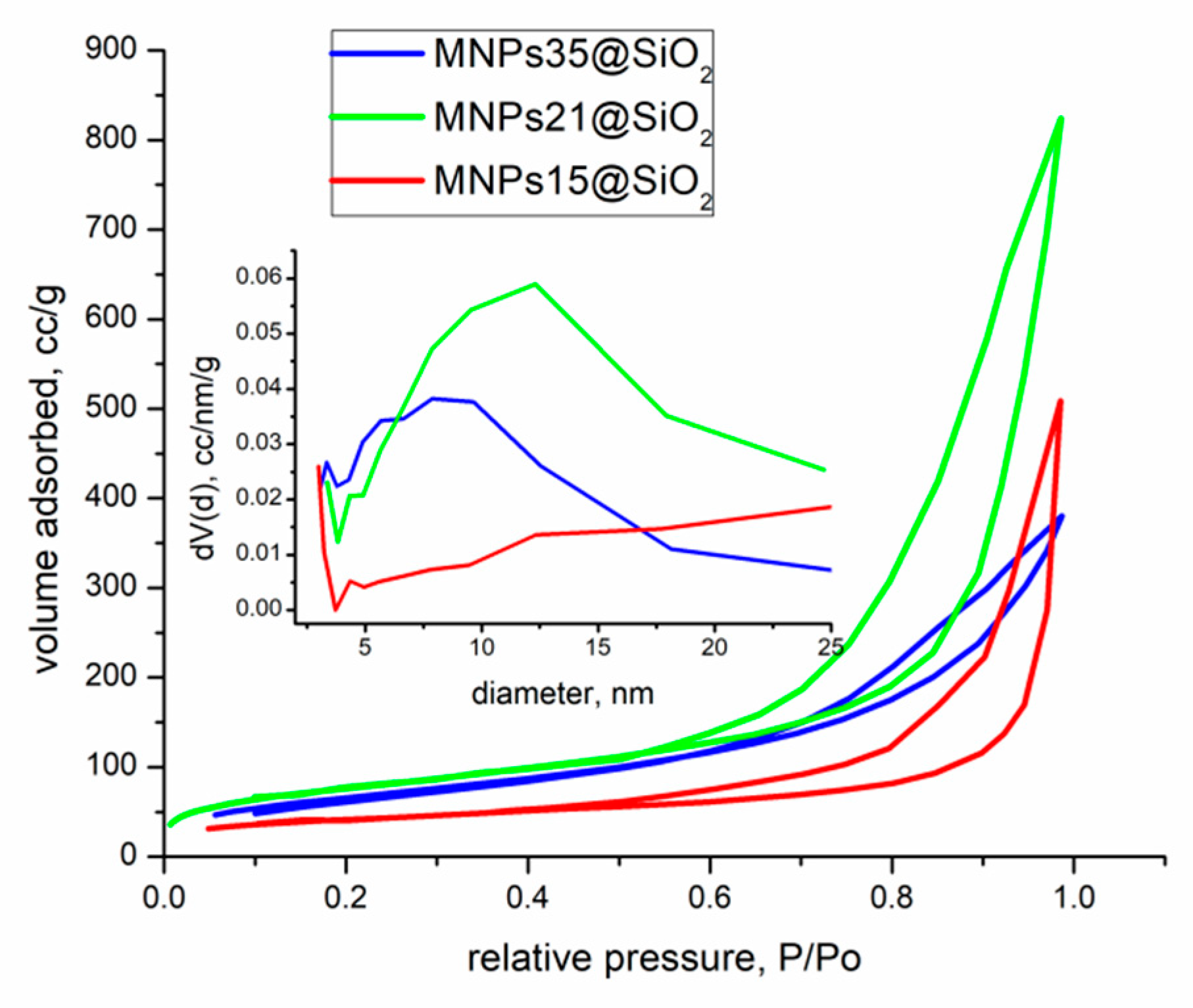
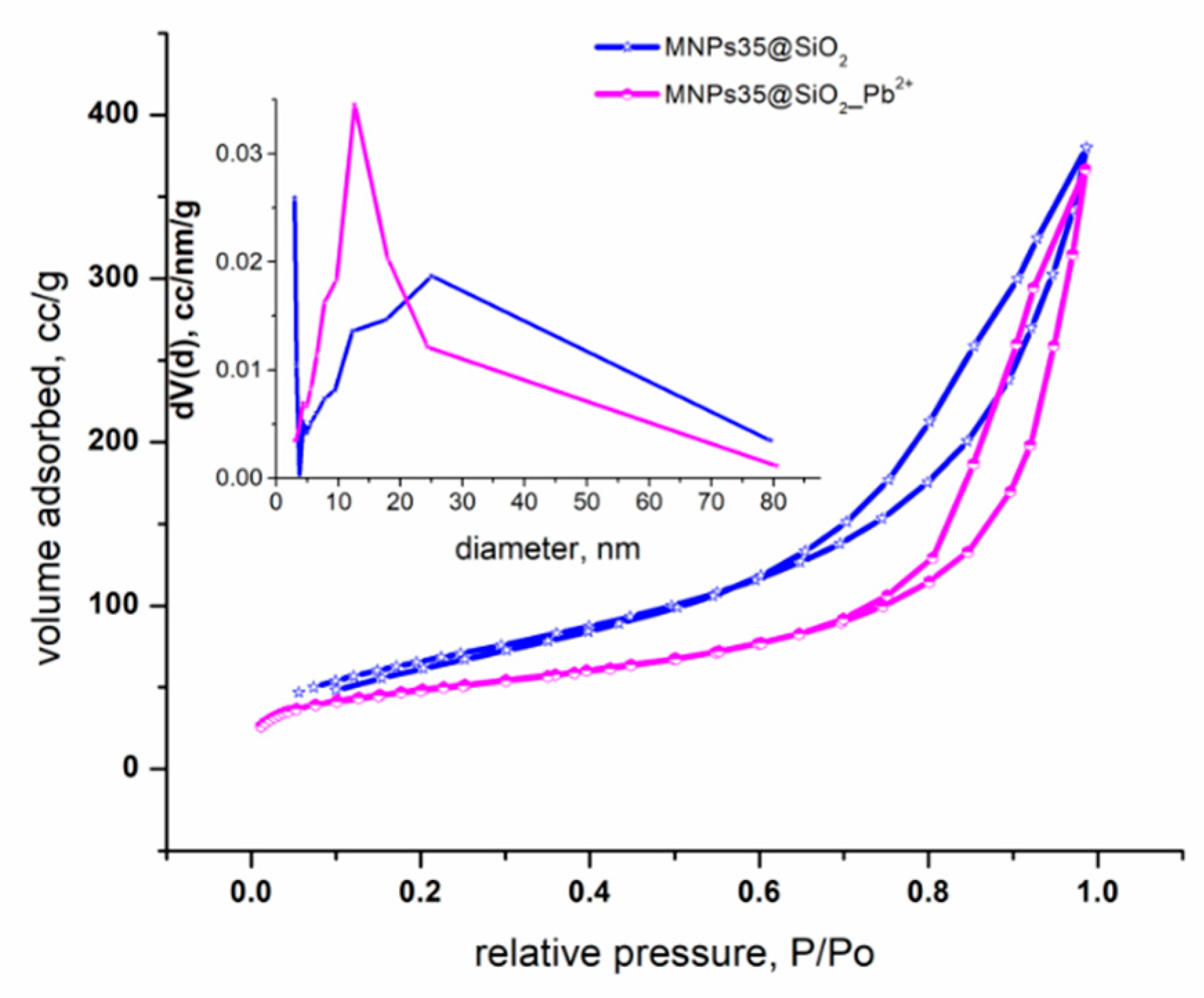
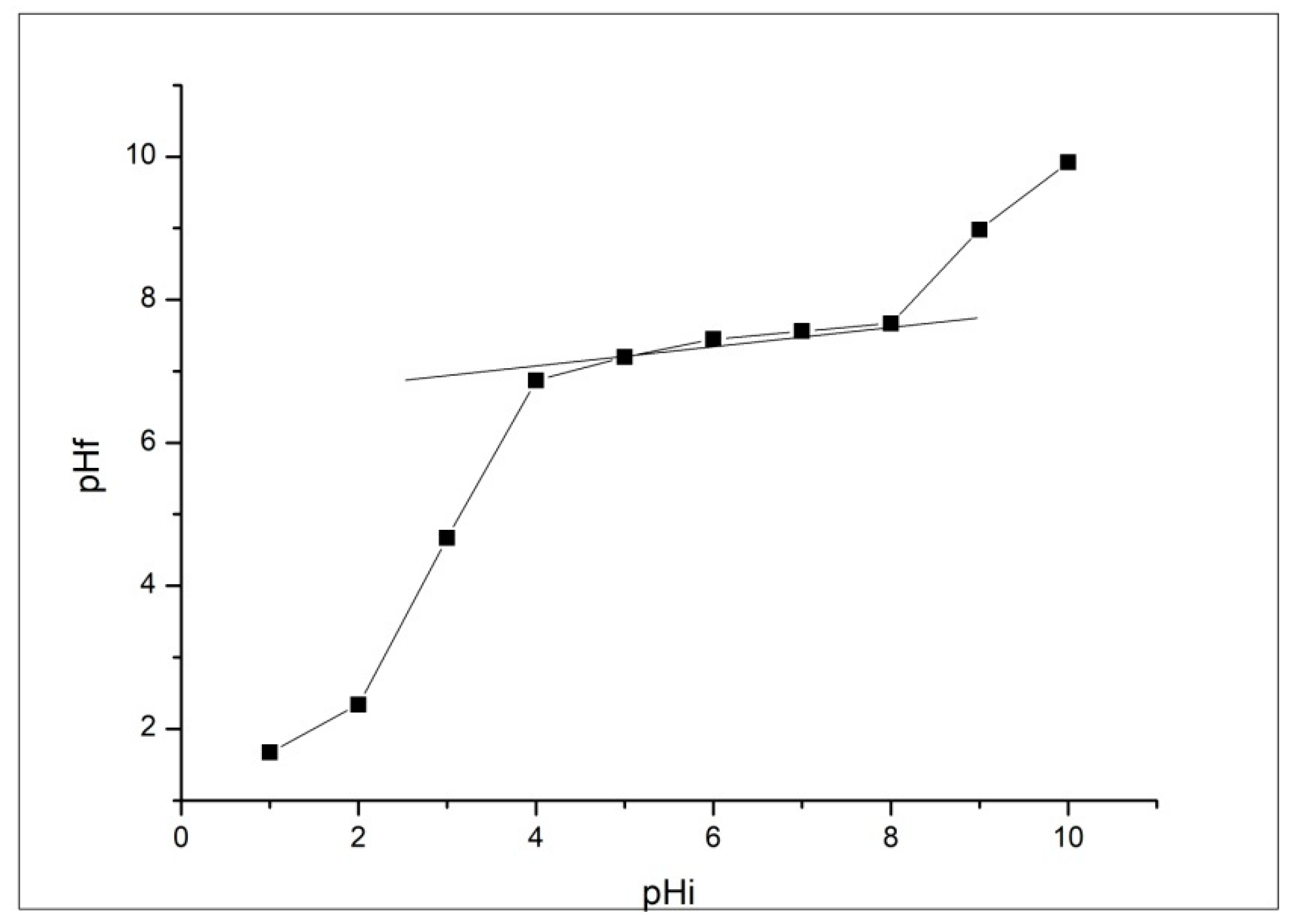
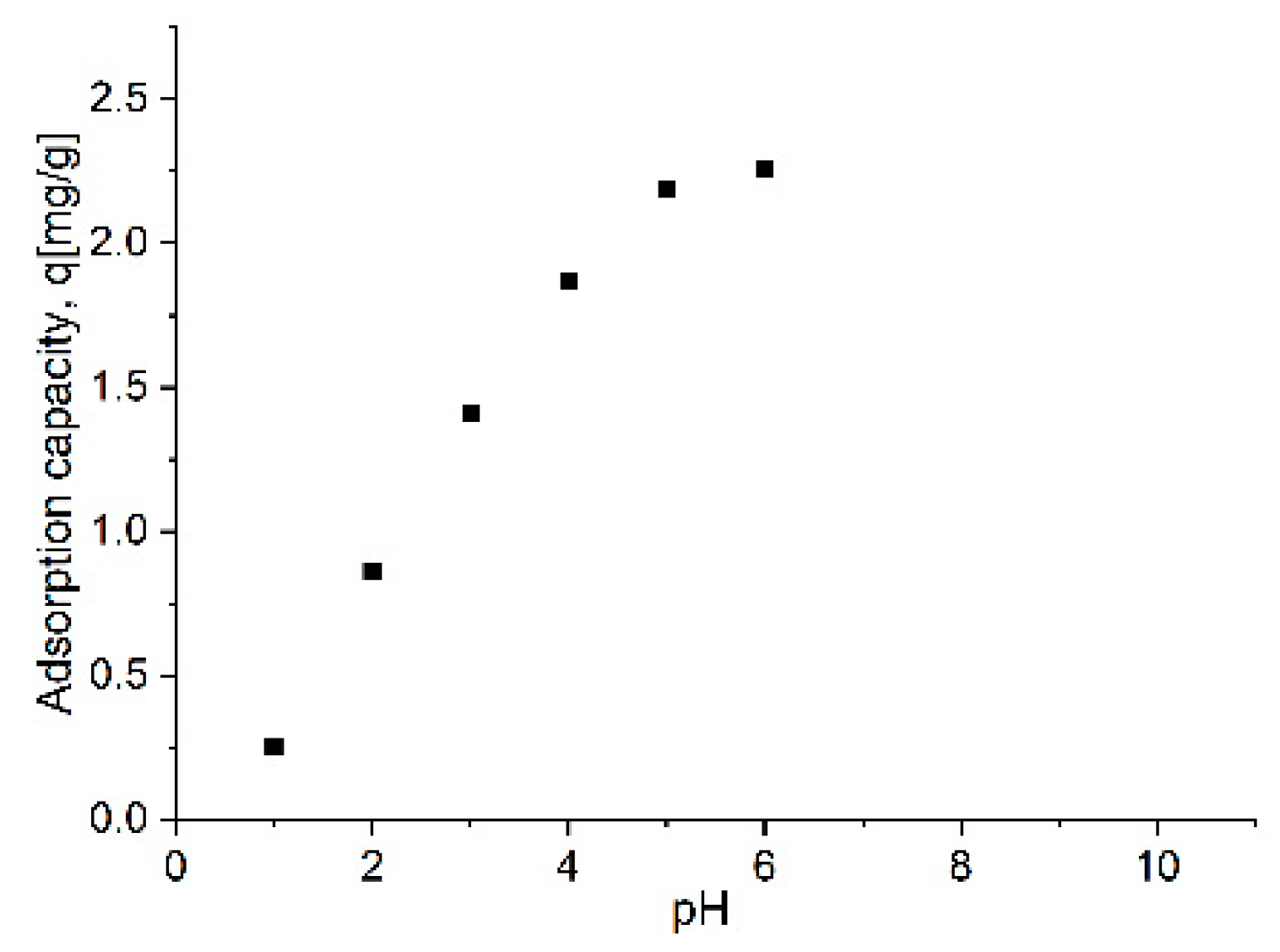
Adsorption Kinetics
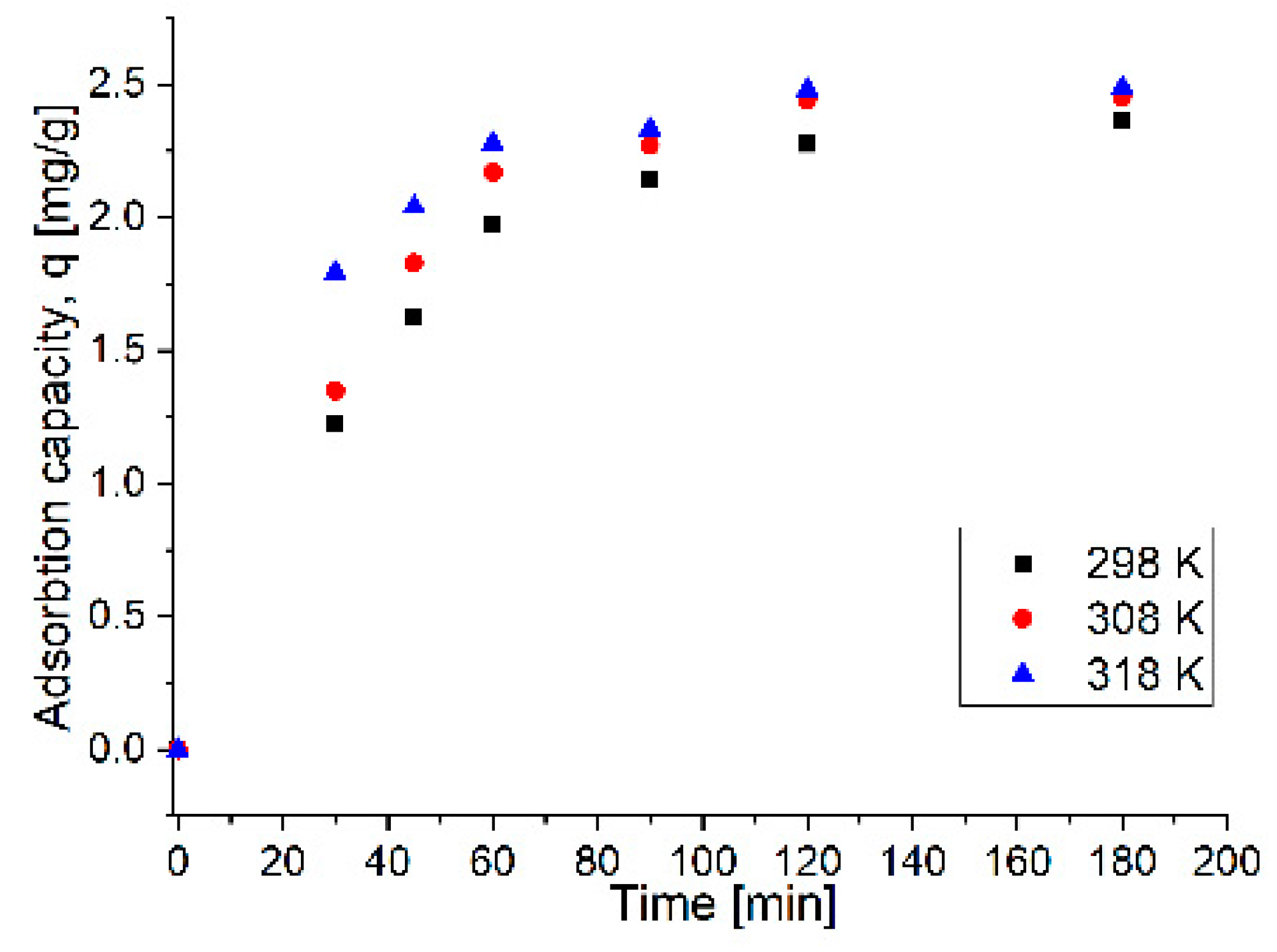
The kinetics of the Pb
2+ adsorption process from aqueous solution using the nanocomposite MNPs35@SiO
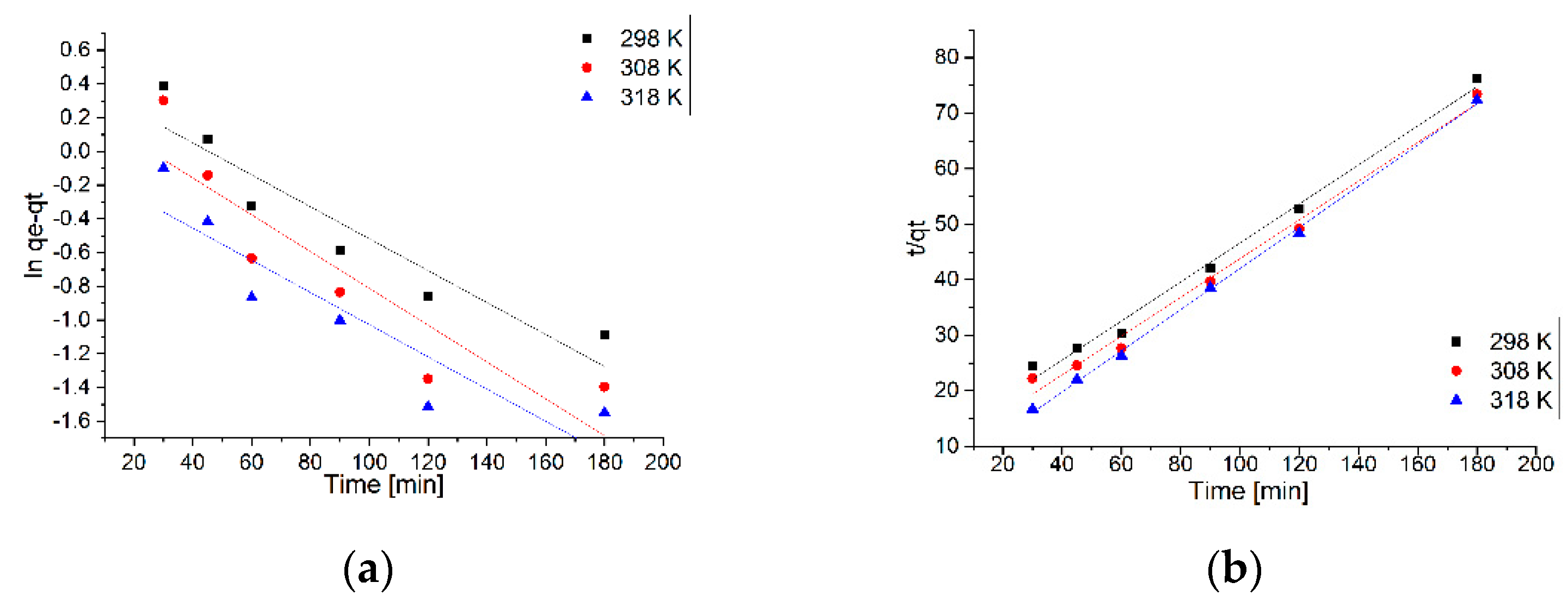
2 are studied to show the efficiency of the adsorption process. The pseudo-first-order hand the pseudo-second-order kinetic equations were applied to explore the mechanisms of the adsorption processes. The fitting curves of ln(q
e-q
t) and t/q
t versus time are shown in
Figure 10a,b, respectively. The relevant kinetic model parameters for Pb
2+ adsorption are presented in
Table 7.
Kinetic models can identify the type of adsorption mechanism of the system and the potential stages to control the rate, including mass transport processes and chemical reactions [
38]. The pseudo-first-order kinetic models, or the Lagergren model and pseudo-second-order, or the Ho and McKay model [
39], were applied to describe the kinetics of the adsorption of Pb
2+. The kinetic model that describes best the adsorption process can be established based on the values of the resulting constants and the obtained regression coefficient (R
2). Thus, from the data presented in
Table 7, it can be observed that the kinetic model of pseudo-second-order is the model that describe the adsorption process of Pb
2+ ions on MNPs35@SiO
2 nanocomposite, since R
2 is in the range ~0.9919–0.9986, depending on temperature. This correlation is in line with the literature data showing that the adsorption process of Pb
2+ ions is influenced by pH and temperature and that the chemical reactions between the functional groups present on the sorbent and the metal ions are the rate-controlling step throughout the sorption process [
40,
41,
42].
Adsorption Isotherm Models
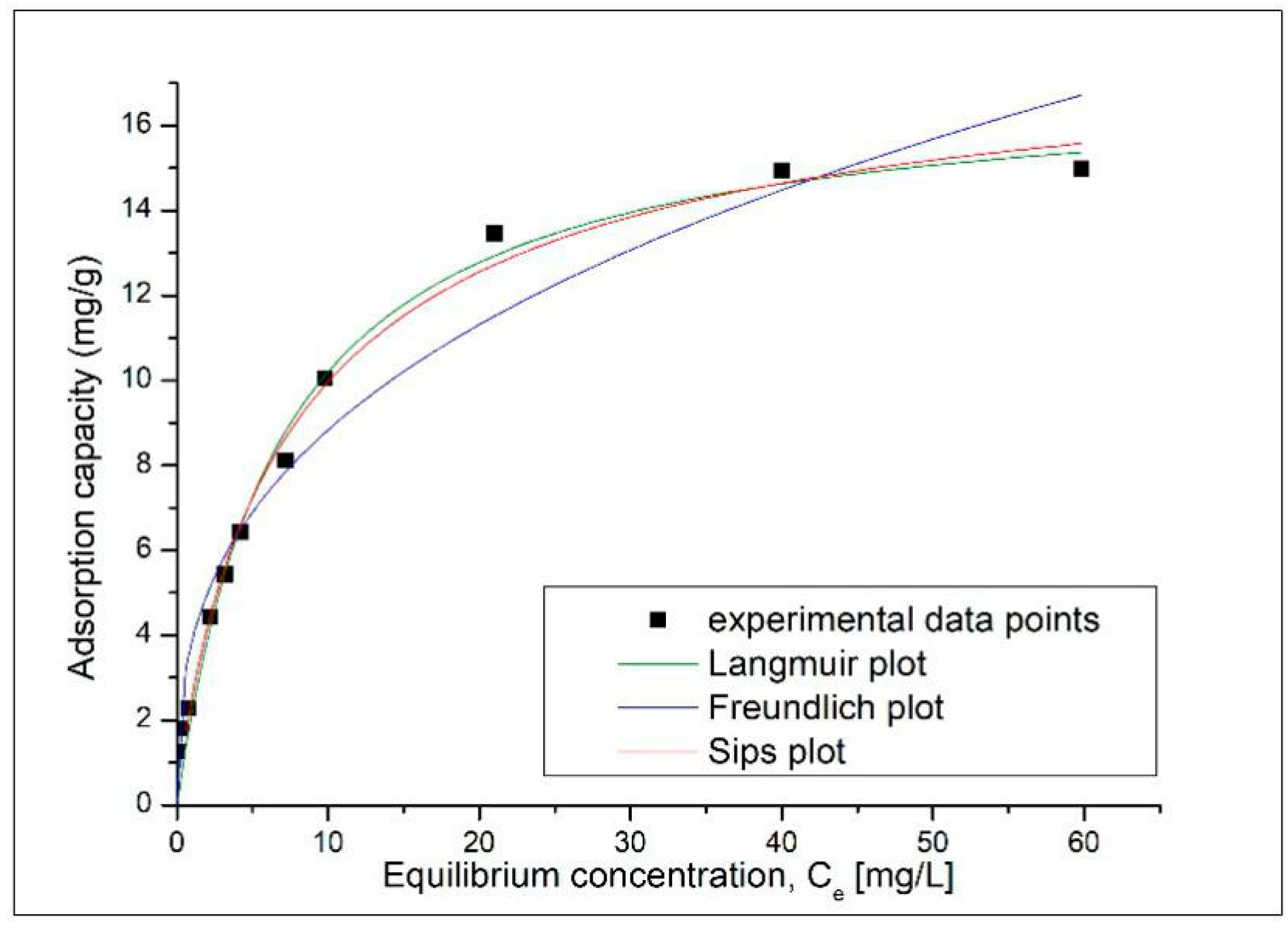
To determine the behavior of Pb
2+ ions on the surface of the adsorbent material during the adsorption process, the experimental data obtained was processed according to the Langmuir, Freundlich and Sips equilibrium isotherms, which describe well the adsorption processes. The adsorption isotherms of Pb
2+ on the magnetic nanocomposite MNPs35@SiO
2 are shown in
Figure 11, and the resulting parameters are shown in
Table 8.
According to the correlation coefficient R
2 the Langmuir isotherm describes best the adsorption process of Pb
2+ on the MNPs35@SiO
2 nanocomposite. Moreover, q
L = 17.1 mg g
−1, is much closer to the experimental value, i.e., q
m, exp = 14.9 mg g
−1. According to the literature, the fact that the adsorption process is better modelled according to the Langmuir model shows that the adsorption process is positively influenced by the increase in the concentration of metal ions [
14] and that the adsorption process of Pb
2+ ions on the adsorbent occurs only in a monolayer, all surface sites are energetically identical housing a single Pb
2+ ion, and the ability of a Pb
2+ ion to adsorb on the surface is independent of occupying adjacent sites [
43]. At the same time, the adsorption mechanism is controlled by chemosorption processes as a result of strong chelation between Pb
2+ ions and OH
- groups and/or free electron pairs present on the surface of the adsorbent material [
44]. By following the value of the coefficient n
s <2, we can confirm that the adsorption process is likely to occur by moving the metal ions from the aqueous phase to the surface of the nanocomposite.
Adsorption Thermodynamics
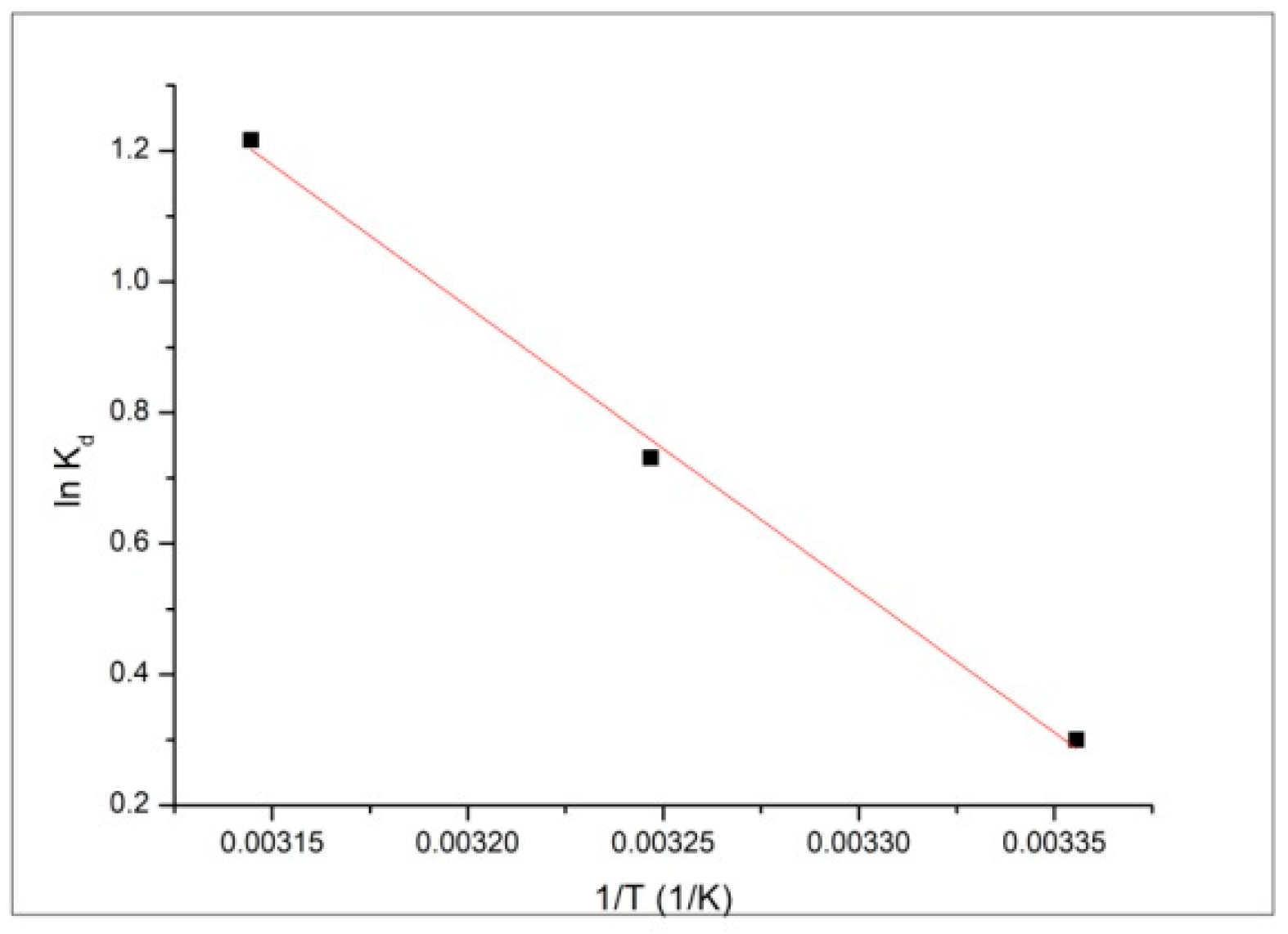
Usually, adsorption processes are significantly influenced by temperature. Therefore, to investigate the feasibility and the physiochemical characteristics, the effect of temperature (in the range of 298–318 K) on the adsorption of Pb
2+ ions on the magnetic nanocomposite adsorbent MNPs35@SiO
2 was followed. The thermodynamic parameters were calculated from the slope and the intercept, by using linear plot of lnK
d function of 1/T, presented in
Figure 12. Thermodynamic parameters: Gibbs free energy (ΔG°), free enthalpy (ΔH°), free entropy (ΔS°) and regression coefficient R
2 values are listed in
Table 7.
The positive enthalpy value ΔH°, 36.1 kJ·mol
−1, showed that the adsorption process is endothermic in nature. When the adsorbent material is dispersed in solution, the intermolecular hydrogen from the water molecules interacts with oxygen-containing groups from the adsorbent surface, partially converting them into hydrogen bonds. This tends to occupy the active sites of the adsorbent and consequently decrease the adsorption of Pb
2+ ions [
40]. The adsorption process in the solid–liquid system is a combination of two processes: (i) the desorption of the solvent (water) molecules adsorbed on the surface of the adsorbent, and (ii) the adsorption of the adsorbate species. In consequence, the energy required for the adsorption process is the energy consumed to displace the water molecules from the surface by the Pb
2+ ions [
41]. At the same time, the adsorption of Pb
2+ ions involve electrostatic interactions and also possible complexation, that is consistent with the adsorption abilities at high temperature.
The value of the Gibbs free energy, ΔG°, −0.7 kJ·mol−1, at 298 K, calculated from the experimental data, is negative, indicating that the adsorption of metal ions on the magnetic nanocomposite is a natural spontaneous process, and becomes more negative with the increase in temperature, −0.7, −1.9 and −3.1 kJ·mol−1 for 298 K, 308 K and 318 K, respectively, suggesting that the adsorption of Pb2+ ions increases with the temperature.
This can be attributed to effective surface growth contact between the adsorbent material and the metal ions. Also, this could be possible because the mobility of the Pb
2+ ions in the solution increases with temperature and that the affinity of the metal ions on the magnetic nanocomposite is higher at high temperatures. The positive value of ΔS°, 123.4 J·(mol∙K)
−1 reflects an increase in randomness during the process of Pb
2+ ions adsorption on magnetic nanocomposite, and the existence of the affinity between the adsorbent and Pb
2+ ions [
41,
42]. At the same time, the adsorption of Pb
2+ ions involve electrostatic interactions and also possible complexation, which is consistent with the adsorption abilities at high temperature. The value of the Gibbs free energy, ΔG°, calculated from the experimental data, is negative, indicating that the adsorption of metal ions on the magnetic nanocomposite is a natural spontaneous process, and becomes more negative with the increase in temperature, suggesting that the adsorption of Pb
2+ ions increases with the temperature. This can be attributed to effective surface growth contact between the adsorbent material and the metal ions. Also, this could be possible because the mobility of the Pb
2+ ions in the solution increases with temperature and that the affinity of the metal ions on the magnetic nanocomposite is higher at high temperatures. The positive value of ΔS° reflects an increase in randomness during the process of Pb
2+ ions adsorption on magnetic nanocomposite, and the existence of the affinity between the adsorbent and Pb
2+ ions [
45,
46].
It is very difficult to compare the results with various data reported in the literature since the differences of lead adsorption capacities depend not only on the structure and morphology of the adsorbent such as surface area, porosity, presence of functional groups, but also on the adsorption process variables like pH, adsorbent dose, metal ion concentration, temperature, contact time etc. The complexity derives from the non-uniformity of the adsorption process variables employed and the different measuring units for adsorption capacity or parameters that were used to express the results. Roughly, functionalization of the silica shell is significant when the initial metal concentration is as low as 0.01–0.2 mg/mL [
13,
47,
48] or when small quantities of adsorbent (0.4–1 mg/mL) [
11,
12,
14,
15,
16] and reduced contact times (30 min) [
12] should be used.
Finally, the selection of most suitable adsorbents should depend on a number of factors including cost-effectiveness, availability, and reusability. The results showed that our nanocomposite material exhibit Pb2+ adsorption properties comparable with some of the functionalized ones, an increase of the adsorption properties being obtained mainly for porous silica nanocomposites and for materials with hybrid coating which follows multilayer adsorption. Functionalization of the silica considerably reduces the contact time and the quantity of adsorbent used. However, the physico-chemical stability and economic issues regarding the adsorbent cost should be taken into account.