Allothermal Gasification of Peat and Lignite by a Focused Light Flow
Abstract
1. Introduction
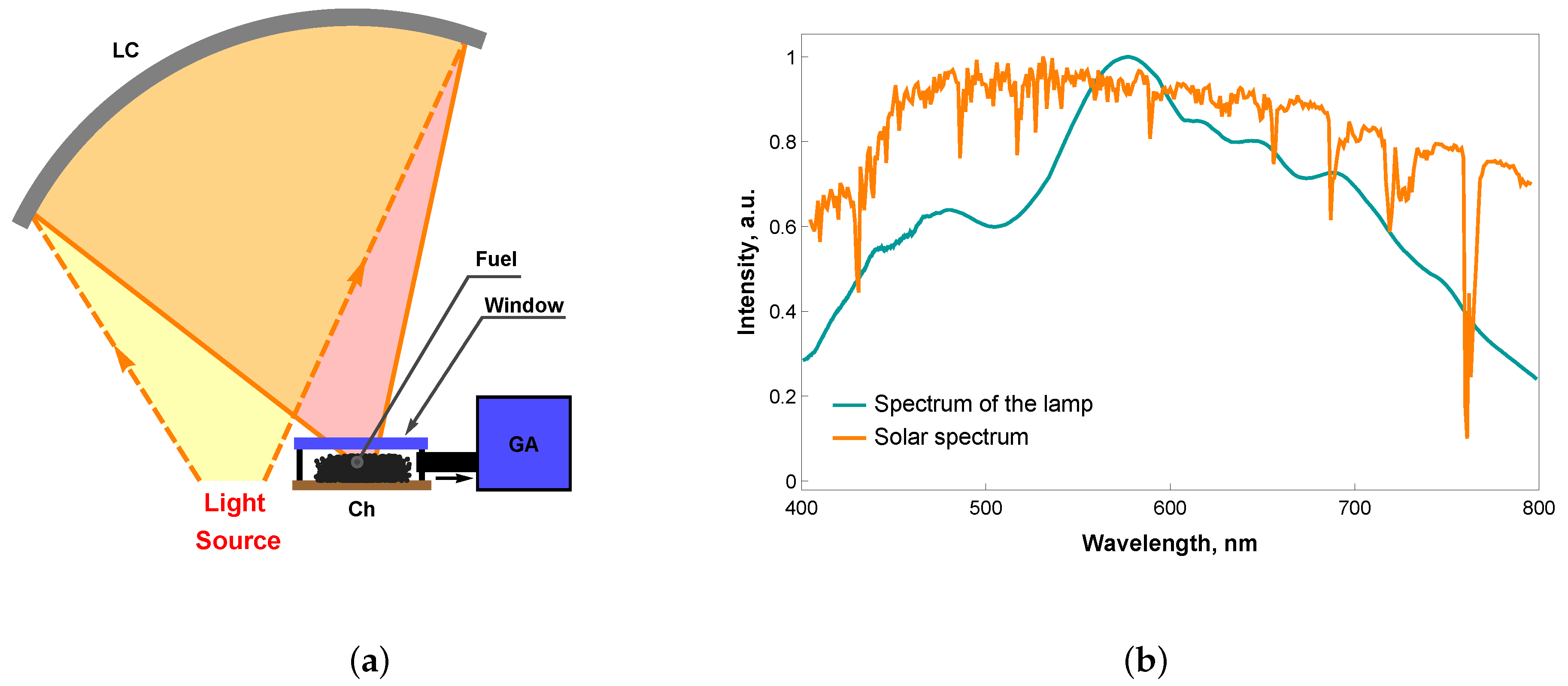
2. Materials and Methods
3. Results and Discussion
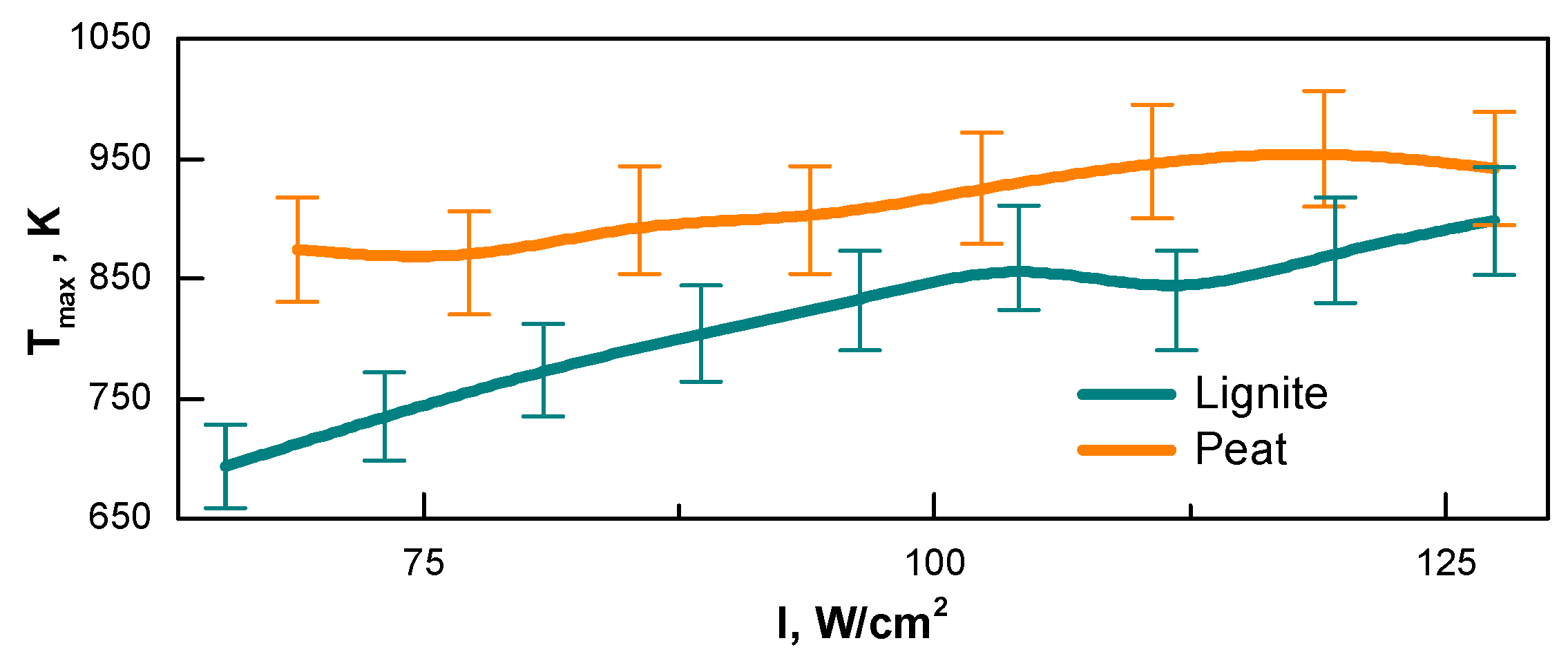
3.1. Fuel Surface Temperature under the Effect of Focused Light Flow
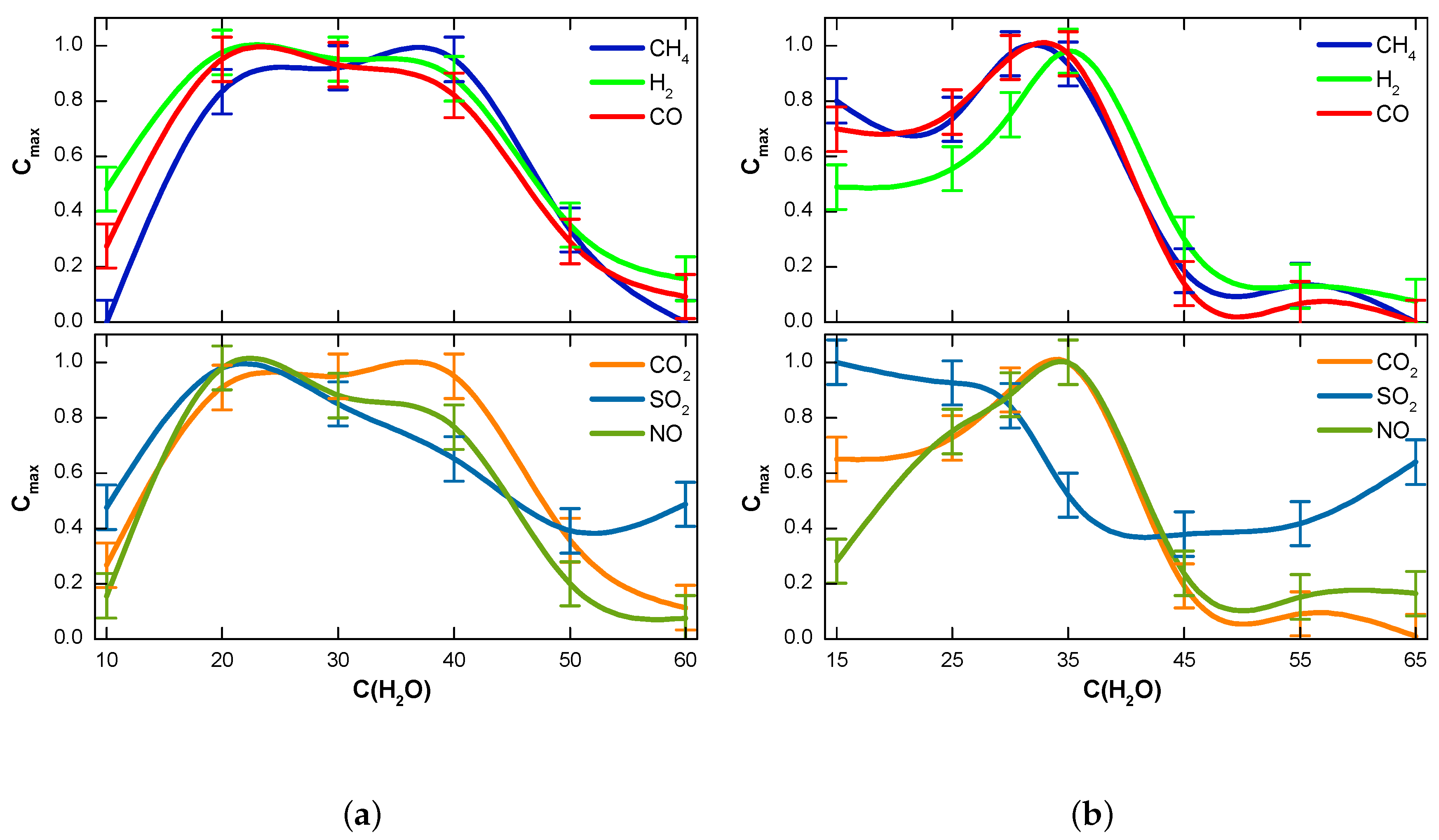
3.2. Influence of Fuel Moisture on the Chemical Composition of Syngas
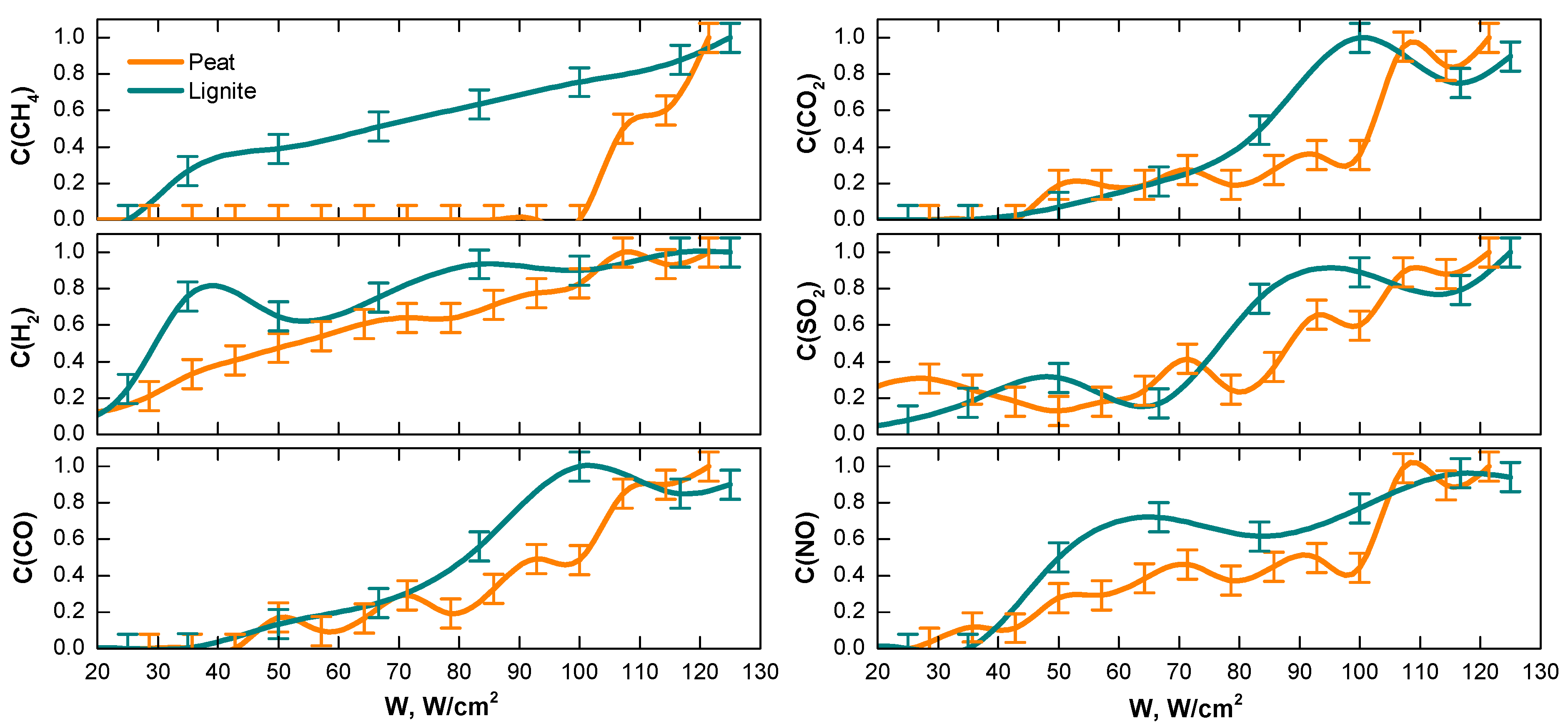
3.3. Fuel Gasification at Various Light Flow Intensities
- -
- Very low inertia of the undergoing processes that started and stopped within seconds.
- -
- Easy adjustment of operating parameters for a chosen type of fuel.
- -
- High efficiency of using solar light (the absorption coefficient of water-fuel mixtures was above 60% for a wide range of fossil fuels).
- -
- Convenient scaling that allowed the creation of table-sized scientific setups, as well as big solar concentrators.
4. Conclusions
- The required light flow intensities were an order of magnitude lower than for the conversion of bituminous coals and their waste.
- The effect of the initial moisture content of the fuel was compared with the influence of the light flow intensity. The intensity threshold for peat conversion was shown to be (∼100 W/cm2); above this value, the process accelerated. The fuel moisture content above 50 wt.% and 45 wt.% for peat and lignite, respectively, caused gasification to stop nearly at such an intensity within the reasonable time term.
- The dynamics of the sample weight changes showed an essential contribution of partial combustion to the fuel conversion. The processes were not purely allothermal, but the observed smoldering of the fuel was not self-sustaining. It stopped in some seconds after the pumping light flow was turned off.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FWHM | Full width at half maximum |
| HPD | Heat penetration depth |
| LWIR | Long-wavelength infrared (8–15 μm) |
| TIS | Thermal imaging system |
References
- BP Statistical Review of World Energy, 68th ed.; BP: London, UK, 2019; Available online: bp.com/statisticalreview (accessed on 8 April 2020).
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. Sci. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Toporov, D.; Abraham, R. Gasification of low-rank coal in the High-Temperature Winkler (HTW) process. J. S. Afr. Inst. Min. Metall. 2015, 115, 589–597. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas: An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828. [Google Scholar] [CrossRef]
- Tchapda, A.H.; Pisupati, S.V. A review of thermal co-conversion of coal and biomass/waste. Energies 2014, 7, 1098–1148. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Proc. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Beller, M.; Cornils, B.; Frohning, C.D.; Kohlpaintner, C.W. Progress in hydroformylation and carbonylation. J. Mol. Catal. A Chem. 1995, 104, 17–85. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Cheng, G.; Niu, Z.; Zhang, C.; Zhang, X.; Li, X. Extraction of humic acid from lignite by KOH-hydrothermal method. Appl. Sci. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; De Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chyou, Y.-P.; Wang, T. Simulation of coal gasification in a low-temperature, high-pressure entrained-bed reactor with a volatiles condensation and re-evaporation model. Appl. Sci. 2019, 9, 510. [Google Scholar] [CrossRef]
- Amelin, I.I.; Salgansky, E.A.; Volkova, N.N.; Zholudev, A.F.; Alekseev, A.P.; Polianchik, E.V.; Manelis, G.B. Region of the stationary filtration combustion wave in the charge with a low carbon content. Russ. Chem. Bull. Int. Ed. 2011, 60, 1150–1157. [Google Scholar] [CrossRef]
- Salganskaya, M.V.; Glazov, S.V.; Salganskii, E.A.; Kislov, V.M.; Zholudev, A.F.; Manelis, G.B. Filtration combustion of humid fuels. Russ. J. Phys. Chem. B 2008, 2, 71–76. [Google Scholar]
- Sobek, S.; Werle, S. Comparative Review of Artificial Light Sources for Solar-Thermal Biomass Conversion Research Applications. Ecol. Chem. Eng. S 2019, 26, 443–453. [Google Scholar] [CrossRef]
- Gregg, D.W.; Taylor, R.W.; Campbell, J.H.; Taylor, J.R.; Cotton, A. Solar gasification of coal, activated carbon, coke and coal and biomass mixtures. Sol. Energy 1980, 25, 353–364. [Google Scholar] [CrossRef]
- Batenin, V.M.; Zalkind, V.I.; Kovbasyuk, V.I.; Rogov, B.V. Power Plants with a Biotechnological Fuel Cycle. Ther. Eng. 1997, 44, 291–295. [Google Scholar]
- Pal, D.B.; Chand, R.; Upadhyay, S.N.; Mishra, P.K. Performance of water gas shift reaction catalysts: A review. Renew. Sustain. Energy Rev. 2018, 93, 549–565. [Google Scholar] [CrossRef]
- Barati, M.; Esfahani, S.; Utigard, T.A. Energy recovery from high temperature slags. Energy 2011, 36, 5440–5449. [Google Scholar] [CrossRef]
- Pramanik, S.; Ravikrishna, R.V. A review of concentrated solar power hybrid technologies. Appl. Ther. Eng. 2017, 127, 602–637. [Google Scholar] [CrossRef]
- Egorov, R.I.; Strizhak, P.A. The light-induced gasification of waste-derived fuel. Fuel 2017, 197, 28–30. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Egorov, R.I.; Strizhak, P.A. Light-induced gasification of the coal-processing waste: Possible products and regimes. Fuel 2018, 212, 347–352. [Google Scholar] [CrossRef]
- Egorov, R.I.; Zaitsev, A.S.; Li, H.; Gao, X.; Strizhak, P.A. Intensity dependent features of the light-induced gasification of the waste-derived coal-water compositions. Renew. Energy 2020, 146, 1667–1675. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 7th ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ortiz, M.; Gonzales, N.; Guzman, C.; Paiva, E.; Bello, E. Thermal Conductivity of Mixtures. Ther. Cond. 1985, 18, 213–223. [Google Scholar]

| Fuel | C, % | H, % | N, % | S, % | O, % |
|---|---|---|---|---|---|
| Lignite | 74.52 | 3.08 | 0.73 | 0.85 | 20.8 |
| Peat | 52.06 | 6.31 | 3.58 | 0.2 | 37.85 |
| Fuel | CH4, ppm | H2, ppm | CO, ppm | CO2, ppm | SO2, ppm | NO, ppm |
|---|---|---|---|---|---|---|
| Peat | 142 | 1410 | 6090 | 9530 | 43 | 53 |
| Lignite | 356 | 2613 | 15,170 | 22,610 | 33 | 26 |
| Fuel | CH4, ppm | H2, ppm | CO, ppm | CO2, ppm | SO2, ppm | NO, ppm |
|---|---|---|---|---|---|---|
| Peat | 95 | 1314 | 4411 | 5600 | 25 | 39 |
| Lignite | 105 | 761 | 4240 | 5100 | 10 | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaitsev, A.S.; Taburchinov, R.I.; Ozerova, I.P.; Pereira, A.O., Jr.; Egorov, R.I. Allothermal Gasification of Peat and Lignite by a Focused Light Flow. Appl. Sci. 2020, 10, 2640. https://doi.org/10.3390/app10082640
Zaitsev AS, Taburchinov RI, Ozerova IP, Pereira AO Jr., Egorov RI. Allothermal Gasification of Peat and Lignite by a Focused Light Flow. Applied Sciences. 2020; 10(8):2640. https://doi.org/10.3390/app10082640
Chicago/Turabian StyleZaitsev, Alexandr S., Roman I. Taburchinov, Irina P. Ozerova, Amaro O. Pereira, Jr., and Roman I. Egorov. 2020. "Allothermal Gasification of Peat and Lignite by a Focused Light Flow" Applied Sciences 10, no. 8: 2640. https://doi.org/10.3390/app10082640
APA StyleZaitsev, A. S., Taburchinov, R. I., Ozerova, I. P., Pereira, A. O., Jr., & Egorov, R. I. (2020). Allothermal Gasification of Peat and Lignite by a Focused Light Flow. Applied Sciences, 10(8), 2640. https://doi.org/10.3390/app10082640








