One-Step Fabrication of Protonic Ceramic Fuel Cells Using a Convenient Tape Calendering Method
Abstract
1. Introduction
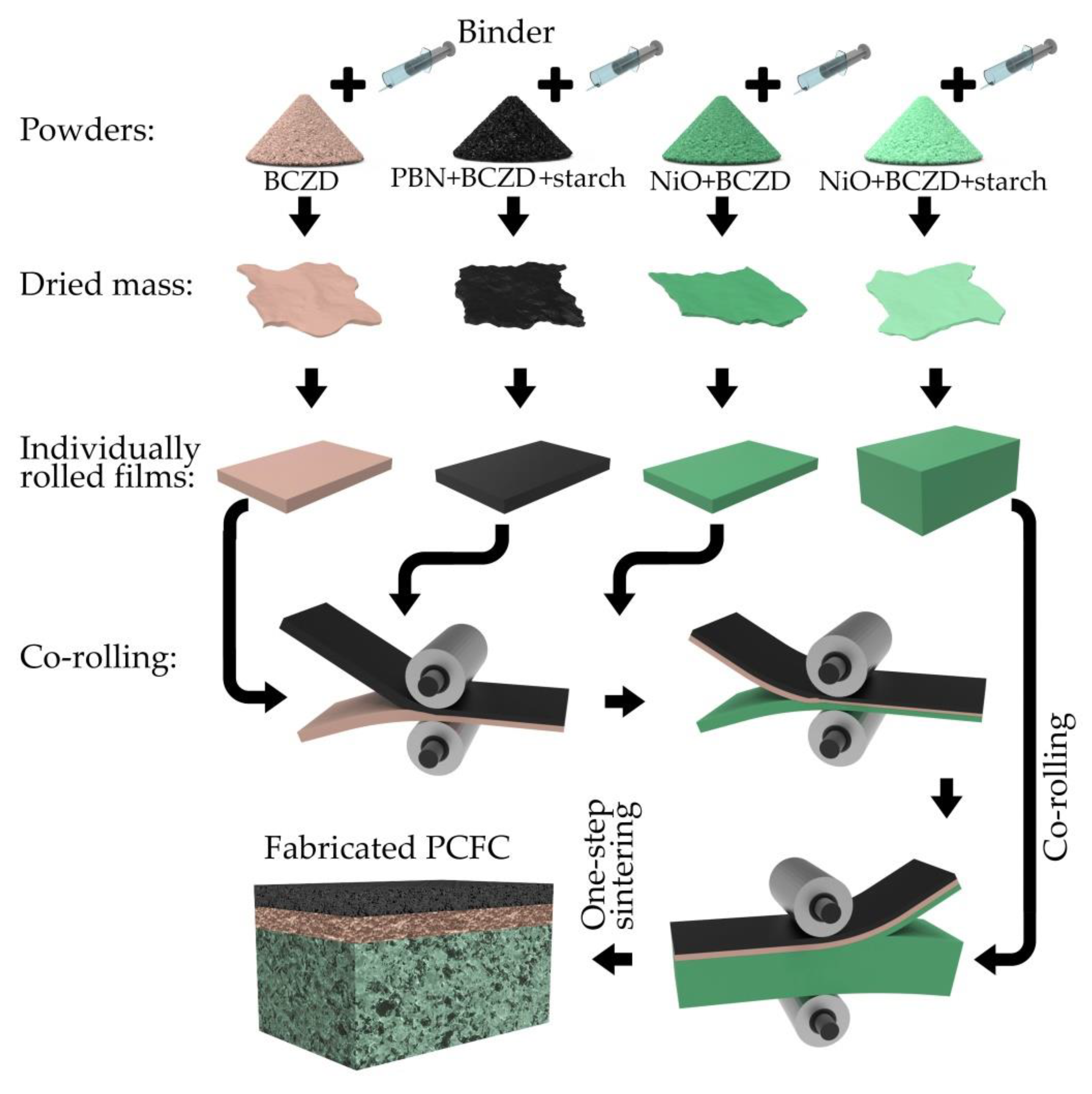
2. Materials and Methods
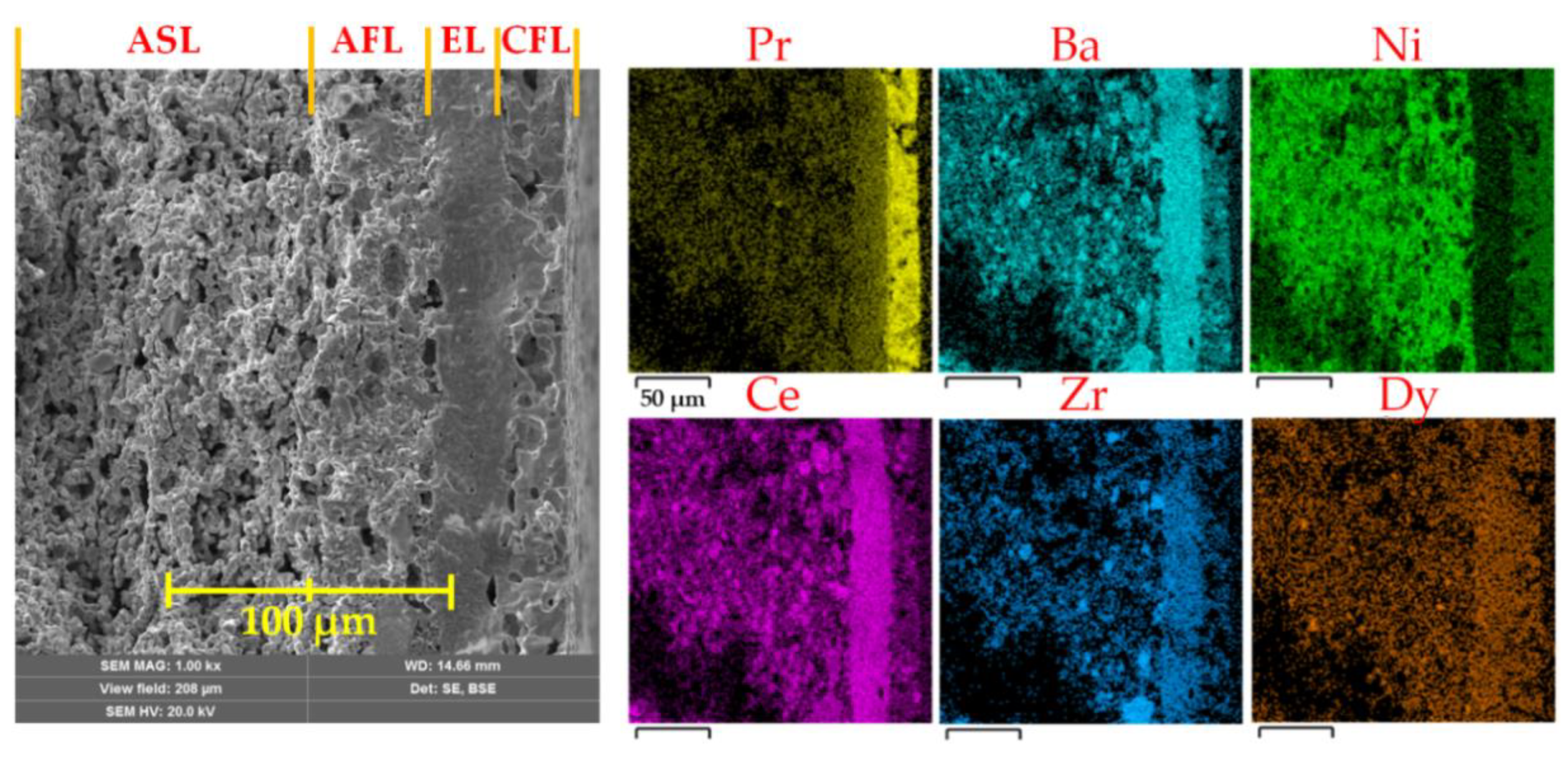
- An anode supporting layer (ASL) was prepared by thorough the mixing of the NiO, BCZD and pore former (starch) powders in a weight ratio of 60:40:20;
- The anode functional layer (AFL) consisted of NiO and BCZD (without starch) powders mixed in a weight ratio of 55:45;
- An electrolyte layer (EL) was formed using the BCZD powder only;
- A cathode functional layer (CFL) was prepared by thorough mixing of the PBN, BCZD and starch in a weight ratio of 70:30:10.
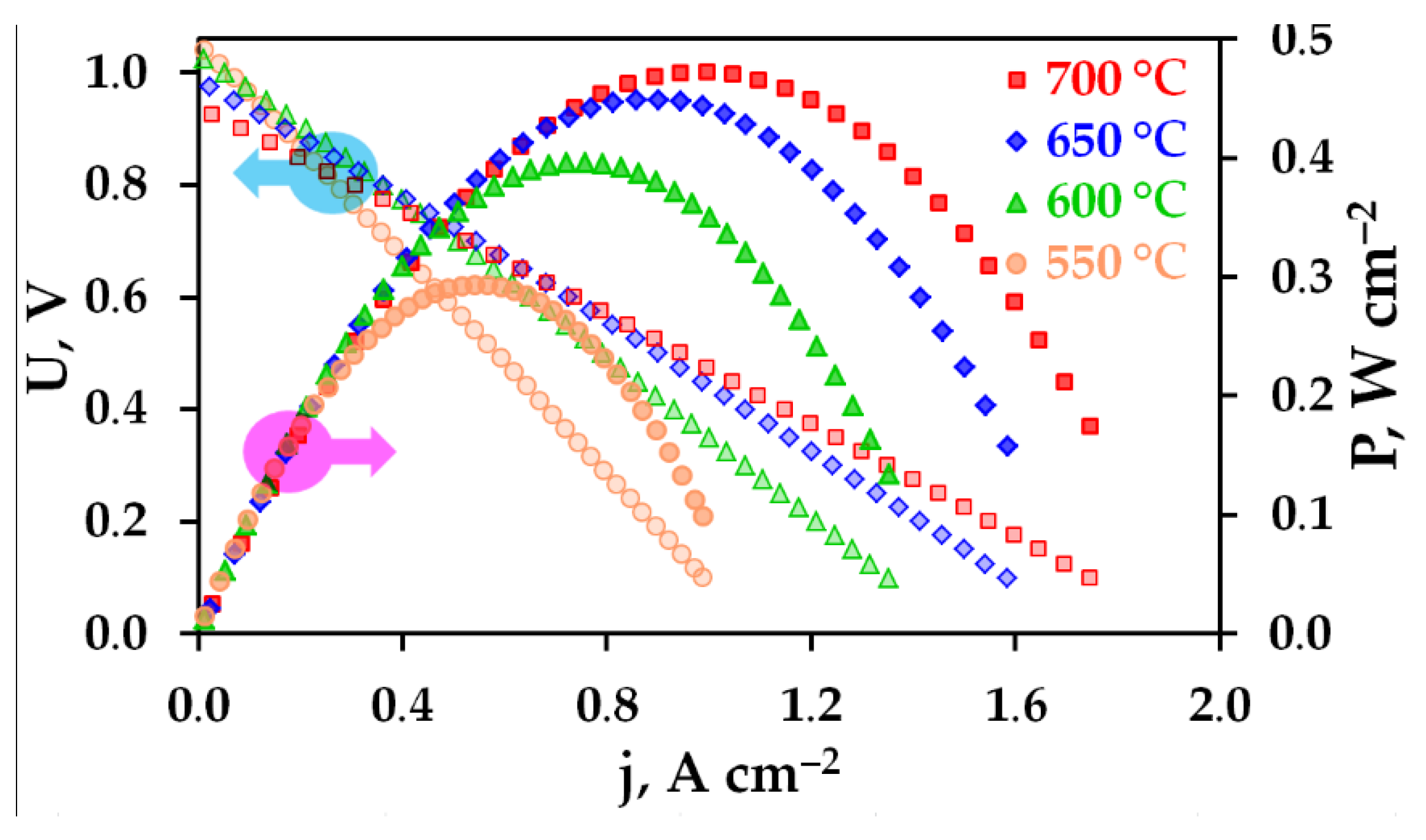
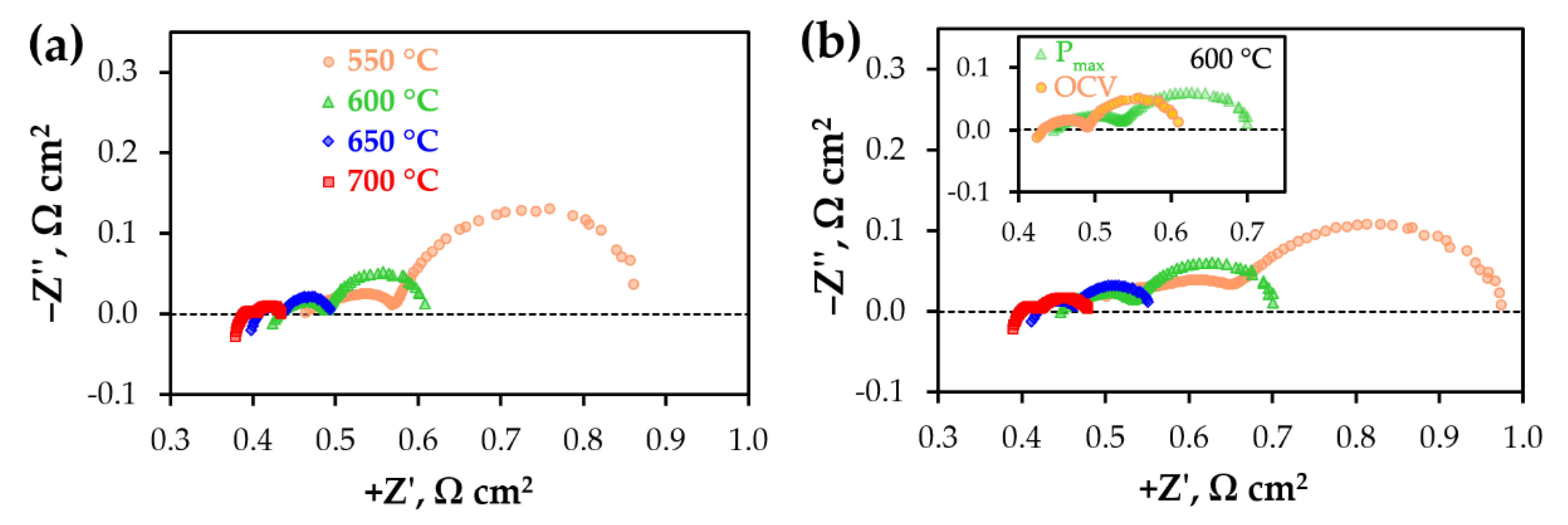
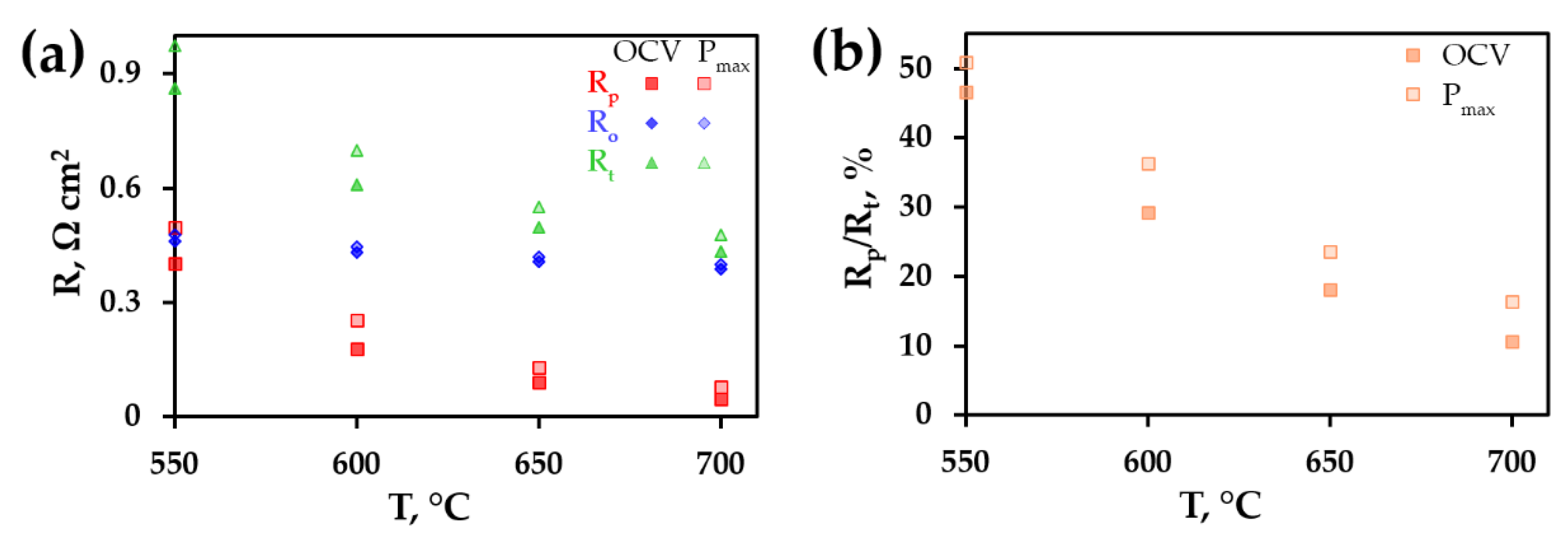
3. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mojaver, P.; Chitsaz, A.; Sadeghi, M.; Khalilarya, S. Comprehensive comparison of SOFCs with proton-conducting electrolyte and oxygen ion-conducting electrolyte: Thermoeconomic analysis and multi-objective optimisation. Energy Convers. Manag. 2020, 205, 112455. [Google Scholar] [CrossRef]
- Dubois, A.; Ricote, S.; Braun, R.J. Benchmarking the expected stack manufacturing cost of next generation, intermediate-temperature protonic ceramic fuel cells with solid oxide fuel cell technology. J. Power Sources 2017, 369, 65–77. [Google Scholar] [CrossRef]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kim, S.; Kwon, O.; Bu, Y.; Kim, G. Proton conducting oxides: A review of materials and applications for renewable energy conversion and storage. Renew. Sustain. Energy Rev. 2019, 109, 606–618. [Google Scholar] [CrossRef]
- Rashid, N.L.R.M.; Samat, A.A.; Jais, A.A.; Somalu, M.R.; Muchtar, A.; Baharuddin, N.A.; Isahak, W.N.R.W. Review on zirconate-cerate-based electrolytes for proton-conducting solid oxide fuel cell. Ceram. Int. 2019, 45, 6605–6615. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, D.E. Understanding hydrogen in perovskites from first principles. Comput. Mater. Sci. 2020, 174, 109461. [Google Scholar] [CrossRef]
- Lei, Z.; Jing, J.; Pang, J.; Hu, R.; Shi, X.; Yang, Z.; Peng, S. Highly conductive proton-conducting electrolyte with a low sintering temperature for electrochemical ammonia synthesis. Int. J. Hydrogen Energy 2020, 45, 8041–8051. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I.; Galisheva, A.; Korona, D. Incorporation and conduction of protons in Ca, Sr, Ba-doped BaLaInO4 with Ruddlesden-Popper structure. Materials 2019, 12, 1668. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Abrantes, J.C.C.; Gomes, E.; Lyskov, N.V.; Konysheva, E.Y.; Chernyak, S.A.; Shcherbakova, L.G. Evolution of oxygen–ion and proton conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), located near pyrochlore–fluorite phase boundary. Materials 2019, 12, 2452. [Google Scholar] [CrossRef]
- Bi, L.; Da’as, E.H.; Shafi, S.P. Proton-conducting solid oxide fuel cell (SOFC) with Y-doped BaZrO3 electrolyte. Electrochem. Commun. 2017, 80, 20–23. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Duan, C.; Kee, R.; Zhu, H.; Sullivan, N.; Zhu, L.; Bian, L.; O’Hayre, R. Highly efficient reversible protonic ceramic electrochemical cells for power generation and fuel production. Nat. Energy 2019, 4, 230–240. [Google Scholar] [CrossRef]
- An, H.; Lee, H.W.; Kim, B.K.; Son, J.W.; Yoon, K.J.; Kim, H.; Lee, J.H. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm−2 at 600 °C. Nat. Energy 2018, 3, 870–875. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Tarutina, L.R.; Rudenko, A.O.; Lyagaeva, Y.G.; Medvedev, D.A. Ba(Ce,Zr)O3-based electrodes for protonic ceramic electrochemical cells: Towards highly compatible functionality and triple-conducting behaviour. Russ. Chem. Rev. 2020, 89. in press. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Shu, L.; Sunarso, J.; Hashim, S.S.; Mao, J.; Zhou, W.; Liang, F. Advanced perovskite anodes for solid oxide fuel cells: A review. Int. J. Hydrogen Energy 2019, 44, 31275–31304. [Google Scholar] [CrossRef]
- Medvedev, D. Trends in research and development of protonic ceramic electrolysis cells. Int. J. Hydrogen Energy 2019, 44, 26711–26740. [Google Scholar] [CrossRef]
- Wang, F.; Lyu, Y.; Chu, D.; Jin, Z.; Zhang, G.; Wang, D. The electrolyte materials for SOFCs of low-intermediate temperature. J. Mater. Sci. Technol. 2019, 35, 1551–1562. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Abu Hassan, S.H.; Boon Kar, Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2019, 44, 594–611. [Google Scholar] [CrossRef]
- Carneiro, J.; Nikolla, E. Nanoengineering of solid oxide electrochemical cell technologies: An outlook. Nano Res. 2019, 12, 2081–2092. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, F.; Wang, D.; Jin, Z. Alternative preparation methods of thin films for solid oxide fuel cells. Mater. Technol. 2019, 35, 212–227. [Google Scholar] [CrossRef]
- Kalinina, E.G.E.; Pikalova, E.Y. New trends in the development of electrophoretic deposition method in the solid oxide fuel cell technology: Theoretical approaches, experimental solutions and development prospects. Russ. Chem. Rev. 2019, 88, 1179. [Google Scholar] [CrossRef]
- Zhang, L.; Lan, R.; Cowin, P.I.; Tao, S. Fabrication of solid oxide fuel cell based on doped ceria electrolyte by one-step sintering at 800 °C. Solid State Ion. 2011, 203, 47–51. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, S. An intermediate temperature solid oxide fuel cell fabricated by one step co-press-sintering. Int. J. Hydrogen Energy 2011, 36, 14643–14647. [Google Scholar] [CrossRef]
- Wang, B.; Bi, L.; Zhao, X.S. Fabrication of one-step co-fired proton-conducting solid oxide fuel cells with the assistance of microwave sintering. J. Eur. Ceram. Soc. 2018, 38, 5620–5624. [Google Scholar] [CrossRef]
- Sun, W.; Tao, Z.; Shi, Z.; Yan, L.; Zhu, Z.; Liu, W. Fabrication of BaZr0.1Ce0.7Y0.2O3–δ-based proton-conducting solid oxide fuel cells co-fired at 1150 °C. Fuel Cells 2010, 10, 1108–1113. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Medvedev, D.; Pikalova, E.; Plaksin, S.; Brouzgou, A.; Demin, A.; Tsiakaras, P. A detailed analysis of thermal and chemical compatibility of cathode materials suitable for BaCe0.8Y0.2O3−δ and BaZr0.8Y0.2O3−δ proton electrolytes for solid oxide fuel cell application. Int. J. Hydrogen Energy 2017, 42, 1715–1723. [Google Scholar] [CrossRef]
- Tarutin, A.; Lyagaeva, J.; Farlenkov, A.; Plaksin, S.; Vdovin, G.; Demin, A.; Medvedev, D.A. Reversible protonic ceramic cell with symmetrically designed Pr2NiO4+δ-based electrodes: Fabrication and electrochemical features. Materials 2019, 12, 118. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Vdovin, G.; Beresnev, S.; Demin, A.; Tsiakaras, P. A tape calendering method as an effective way for the preparation of proton ceramic fuel cells with enhanced performance. Electrochim. Acta 2016, 210, 681–688. [Google Scholar] [CrossRef]
- Pikalova, E.; Medvedev, D. Effect of anode gas mixture humidification on the electrochemical performance of the BaCeO3-based protonic ceramic fuel cell. Int. J. Hydrogen Energy 2016, 41, 4016–4025. [Google Scholar] [CrossRef]
- Danilov, N.; Pikalova, E.; Lyagaeva, J.; Antonov, B.; Medvedev, D.; Demin, A.; Tsiakaras, P. Grain and grain boundary transport in BaCe0.5Zr0.3Ln0.2O3−δ (Ln–Y or lanthanide) electrolytes attractive for protonic ceramic fuel cells application. J. Power Sources 2017, 366, 161–168. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Wang, X.; Bi, L. Sintering aids for proton-conducting oxides—A double-edged sword: A mini review. Electrochem. Commun. 2020, 112, 106672. [Google Scholar] [CrossRef]
- Loureiro, F.J.; Nasani, N.; Reddy, G.S.; Munirathnam, N.R.; Fagg, D.P. A review on sintering technology of proton conducting BaCeO3-BaZrO3 perovskite oxide materials for protonic ceramic fuel cells. J. Power Sources 2019, 438, 226991. [Google Scholar] [CrossRef]
- Danilov, N.A.; Lyagaeva, J.G.; Medvedev, D.A.; Demin, A.K.; Tsiakaras, P. Transport properties of highly dense proton-conducting BaCe0.8–xZrxDy0.2O3–δ materials in low-and high-temperature ranges. Electrochim. Acta 2018, 284, 551–559. [Google Scholar] [CrossRef]
- Danilov, N.; Lyagaeva, J.; Vdovin, G.; Medvedev, D. Multifactor performance analysis of reversible solid oxide cells based on proton-conducting electrolytes. Appl. Energy 2019, 237, 924–934. [Google Scholar] [CrossRef]
- Huan, D.; Shi, N.; Zhang, L.; Tan, W.; Xie, Y.; Wang, W.; Lu, Y. New, efficient, and reliable air electrode material for proton-conducting reversible solid oxide cells. ACS Appl. Mater. Interfaces 2018, 10, 1761–1770. [Google Scholar] [CrossRef]
- He, F.; Song, D.; Peng, R.; Meng, G.; Yang, S. Electrode performance and analysis of reversible solid oxide fuel cells with proton conducting electrolyte of BaCe0.5Zr0.3Y0.2O3−δ. J. Power Sources 2010, 195, 3359–3364. [Google Scholar] [CrossRef]
- Danilov, N.; Lyagaeva, J.; Vdovin, G.; Medvedev, D.; Demin, A.; Tsiakaras, P. Electrochemical approach for analysing electrolyte transport properties and their effect on protonic ceramic fuel cell performance. ACS Appl. Mater. Interfaces 2017, 9, 26874–26884. [Google Scholar] [CrossRef]
- Putilov, L.P.; Demin, A.K.; Tsidilkovski, V.I.; Tsiakaras, P. Theoretical modeling of the gas humidification effect on the characteristics of proton ceramic fuel cells. Appl. Energy 2019, 242, 1448–1459. [Google Scholar] [CrossRef]
- Wu, P.C.; Shy, S.S. Cell performance, impedance, and various resistances measurements of an anode-supported button cell using a new pressurised solid oxide fuel cell rig at 1–5 atm and 750–850 °C. J. Power Sources 2017, 362, 105–114. [Google Scholar] [CrossRef]
- Wu, P.C.; Jheng, H.S.; Shy, S.S. Electrochemical impedance measurement and analysis of anodic concentration polarisation for pressurised solid oxide fuel cells. J. Electrochem. Soc. 2014, 161, F513–F517. [Google Scholar] [CrossRef]
- Dai, H.; Da’as, E.H.; Shafi, S.P.; Wang, H.; Bi, L. Tailoring cathode composite boosts the performance of proton-conducting SOFCs fabricated by a one-step co-firing method. J. Eur. Ceram. Soc. 2018, 38, 2903–2908. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Fang, S.; Zhu, Z.; Yan, L.; Liu, W. Evaluation of BaZr0.1Ce0.7Y0.2O3−δ-based proton-conducting solid oxide fuel cells fabricated by a one-step co-firing process. Electrochim. Acta 2011, 56, 1447–1454. [Google Scholar] [CrossRef]
- Hou, J.; Miao, L.; Hui, J.; Bi, L.; Liu, W.; Irvine, J.T. A novel in situ diffusion strategy to fabricate high performance cathodes for low temperature proton-conducting solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 10411–10420. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Xu, C.; Sun, W.; Qiao, J.; Rooney, D.W.; Sun, K. Tuning the defects of the triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3−δ perovskite toward enhanced cathode activity of protonic ceramic fuel cells. J. Mater. Chem. A 2019, 7, 18365–18372. [Google Scholar] [CrossRef]
- Choi, S.M.; An, H.; Yoon, K.J.; Kim, B.K.; Lee, H.W.; Son, J.W.; Lee, J.H. Electrochemical analysis of high-performance protonic ceramic fuel cells based on a columnar-structured thin electrolyte. Appl. Energy 2019, 233, 29–36. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Zhang, S.; Yang, Y. Cerium and gadolinium co-doped perovskite oxide for cathode. Int. J. Hydrogen Energy 2019, 44, 27921–27929. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Li, L.; Wang, Z.; Liu, M.; Rainwater, B.H.; Bai, Y. Electrochemical properties of micro-tubular intermediate temperature solid oxide fuel cell with novel asymmetric structure based on BaZr0.1Ce0.7Y0.1Yb0.1O3−δ proton conducting electrolyte. Int. J. Hydrogen Energy 2019, 44, 16887–16897. [Google Scholar] [CrossRef]
- Miao, L.; Hou, J.; Gong, Z.; Jin, Z.; Liu, W. A high-performance cobalt-free Ruddlesden-Popper phase cathode La1.2Sr0.8Ni0.6Fe0.4O4+δ for low temperature proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 7531–7537. [Google Scholar] [CrossRef]
- Shared Access Centre “Composition of Compounds” of Institute of High-Temperature Electrochemistry. Available online: http://www.ihte.uran.ru/?page_id=3142 (accessed on 3 April 2020).
| A Number of TS | Fabrication Method/Technique | Rp (in Ω cm2) at OCV | Rt (in Ω cm2) at OCV | Pmax, mW cm−2 | Ref. |
|---|---|---|---|---|---|
| 1 | TC | 0.18 | 0.61 | 397 | This work |
| 1 | DP/P | 0.20 2 | 0.45 2 | 449 2 | [26] |
| 1 | DP/P | 0.19 | 1.06 | 169 | [27] |
| 1 | DP/P | 0.30 | 0.88 | 300 | [43] |
| 1 | DP/P | 0.28 | 0.80 | 276 | [44] |
| 2 | DP/P | 0.19 | 0.48 | 566 | [45] |
| 3 | DP/DC/SP | 0.49 | 0.56 | 669 | [46] |
| 2 | DP/SP | 0.10 | 0.22 | 960 | [47] |
| 2 | DP/SP | 0.12 | 0.50 | 505 | [48] |
| 2 | PI/SP | 0.26 | 0.57 | 700 | [49] |
| 2 | DP/P | 0.40 | 0.69 | 421 | [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarutin, A.; Danilov, N.; Lyagaeva, J.; Medvedev, D. One-Step Fabrication of Protonic Ceramic Fuel Cells Using a Convenient Tape Calendering Method. Appl. Sci. 2020, 10, 2481. https://doi.org/10.3390/app10072481
Tarutin A, Danilov N, Lyagaeva J, Medvedev D. One-Step Fabrication of Protonic Ceramic Fuel Cells Using a Convenient Tape Calendering Method. Applied Sciences. 2020; 10(7):2481. https://doi.org/10.3390/app10072481
Chicago/Turabian StyleTarutin, Artem, Nikolay Danilov, Julia Lyagaeva, and Dmitry Medvedev. 2020. "One-Step Fabrication of Protonic Ceramic Fuel Cells Using a Convenient Tape Calendering Method" Applied Sciences 10, no. 7: 2481. https://doi.org/10.3390/app10072481
APA StyleTarutin, A., Danilov, N., Lyagaeva, J., & Medvedev, D. (2020). One-Step Fabrication of Protonic Ceramic Fuel Cells Using a Convenient Tape Calendering Method. Applied Sciences, 10(7), 2481. https://doi.org/10.3390/app10072481







