A United Sign Coherence Factor Beamformer for Coherent Plane-Wave Compounding with Improved Contrast
Abstract
1. Introduction
2. Methods
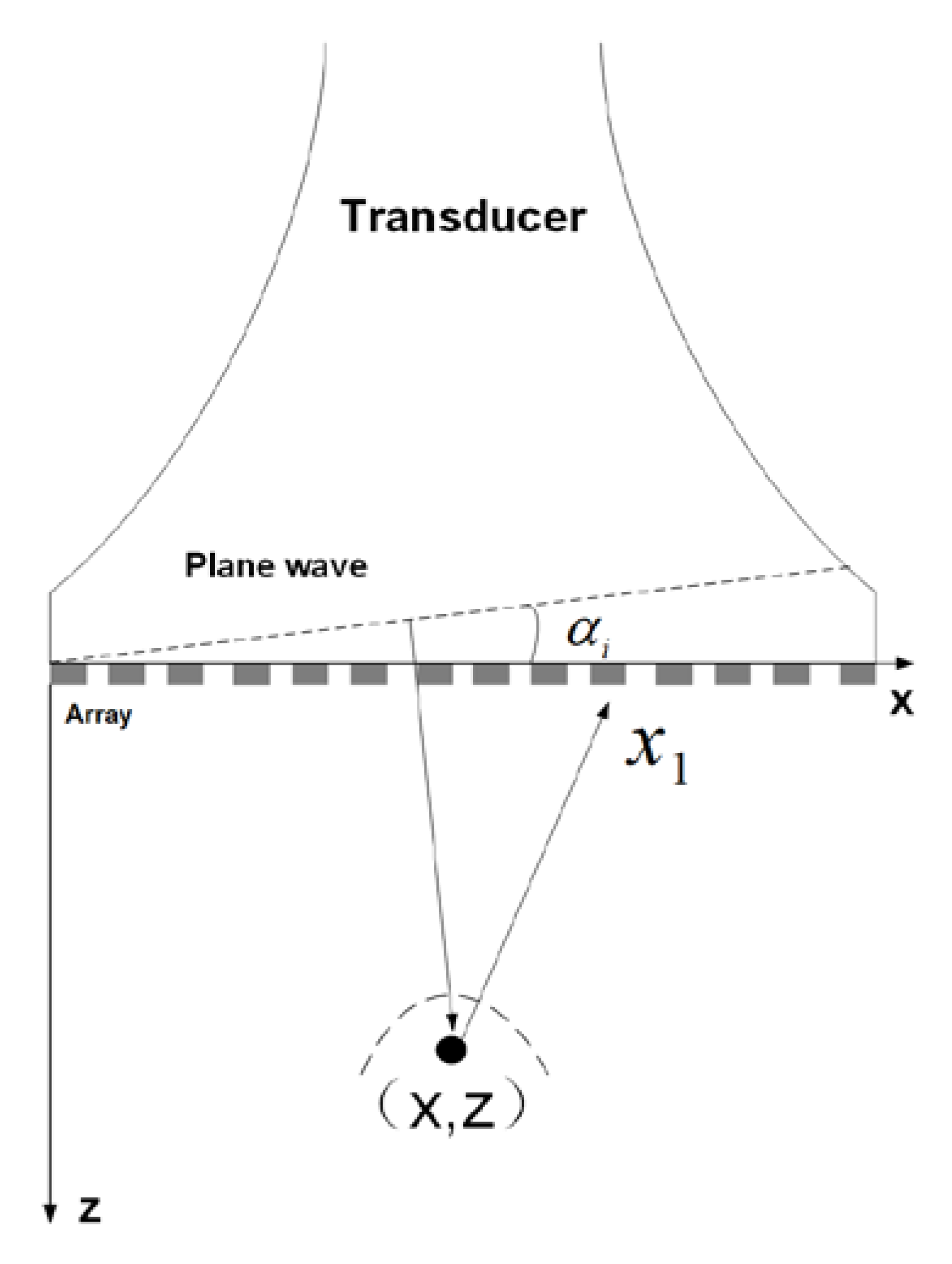
2.1. Proposed Coherent Plane-Wave Compounding
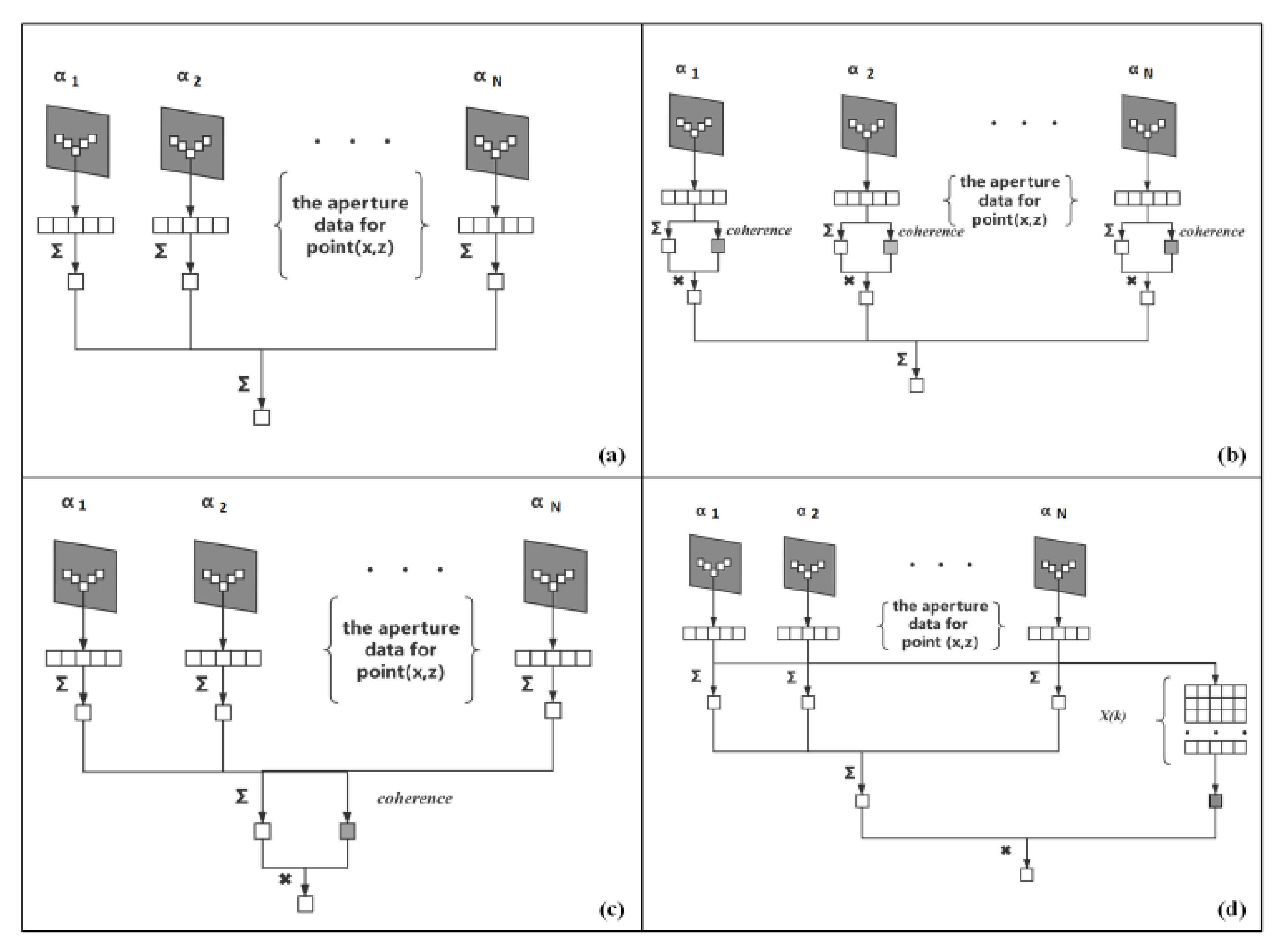
2.2. Proposed Spatial and Angular SCF Beamformer
2.3. United CF-based Beamforming Framework
2.4. United SCF Beamformer
2.5. Implementation Summary of Algorithm
3. Acquisition Parameters and Evaluation Metrics
3.1. Acquisition Parameters
3.2. Evaluation Metrics
4. Imaging Data and Results
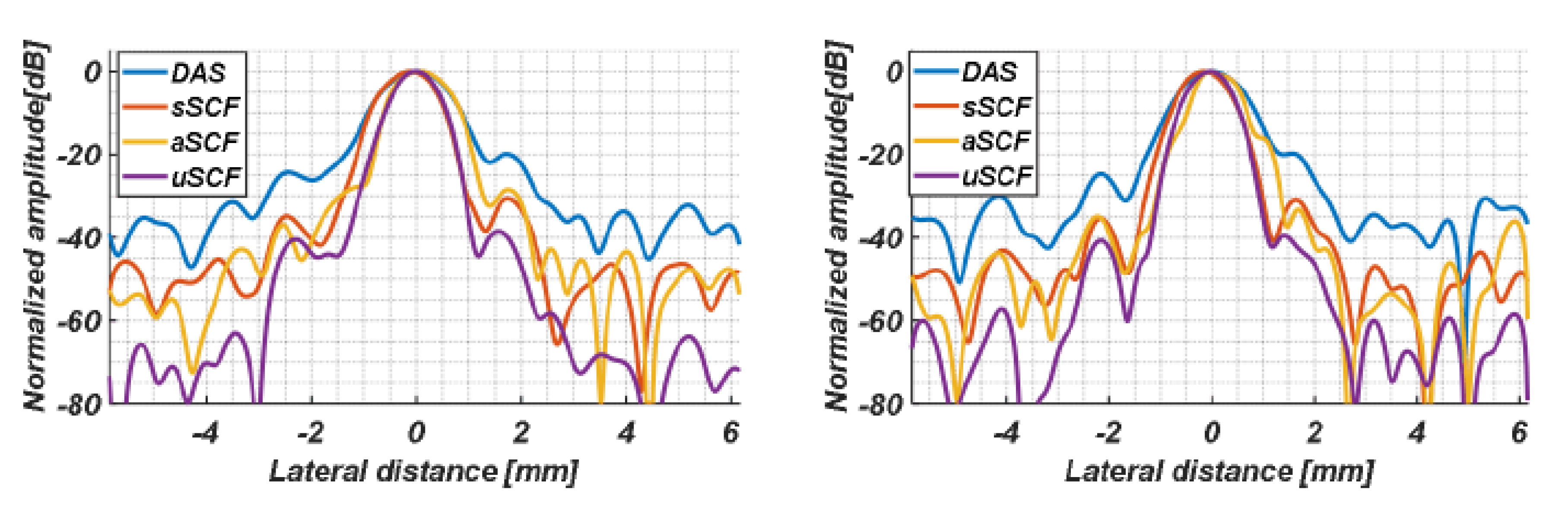
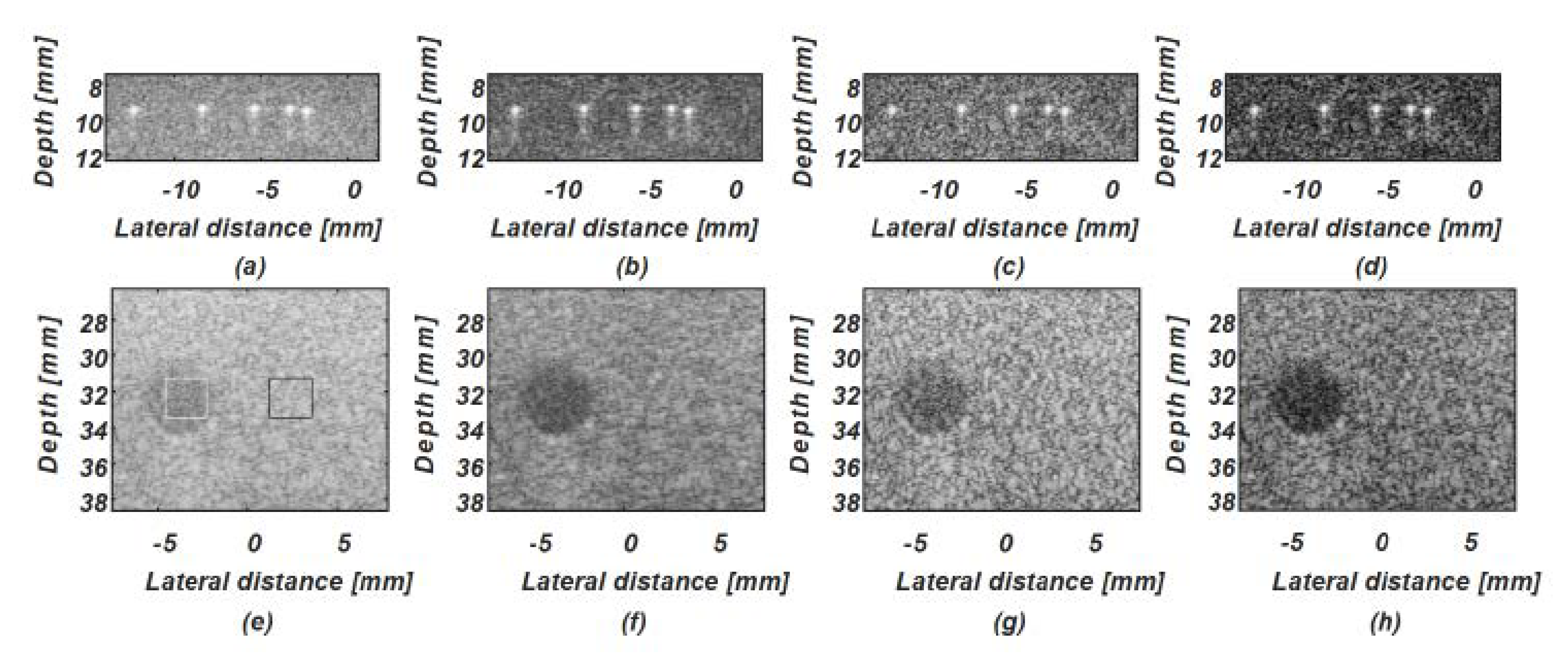
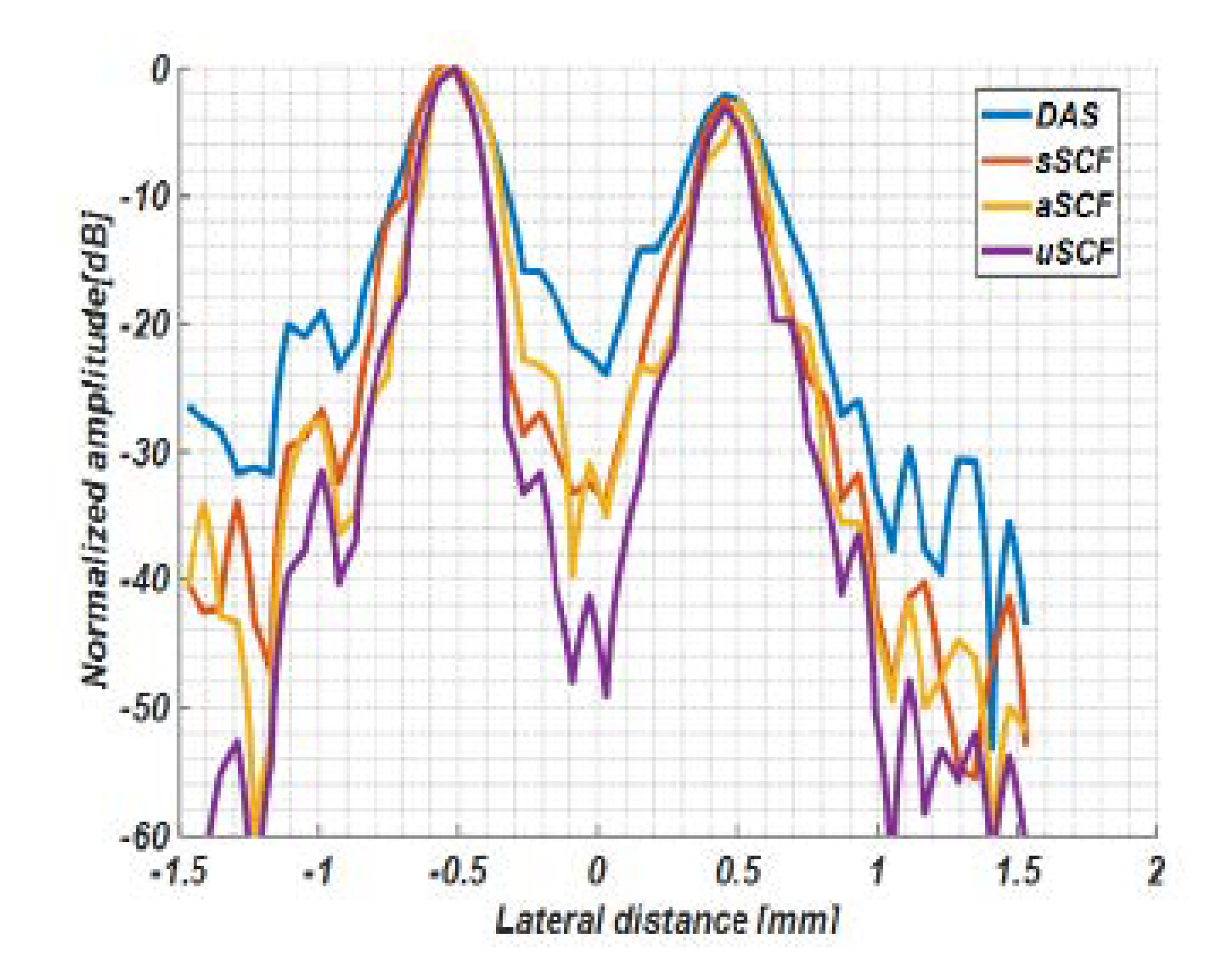
4.1. Simulation Study
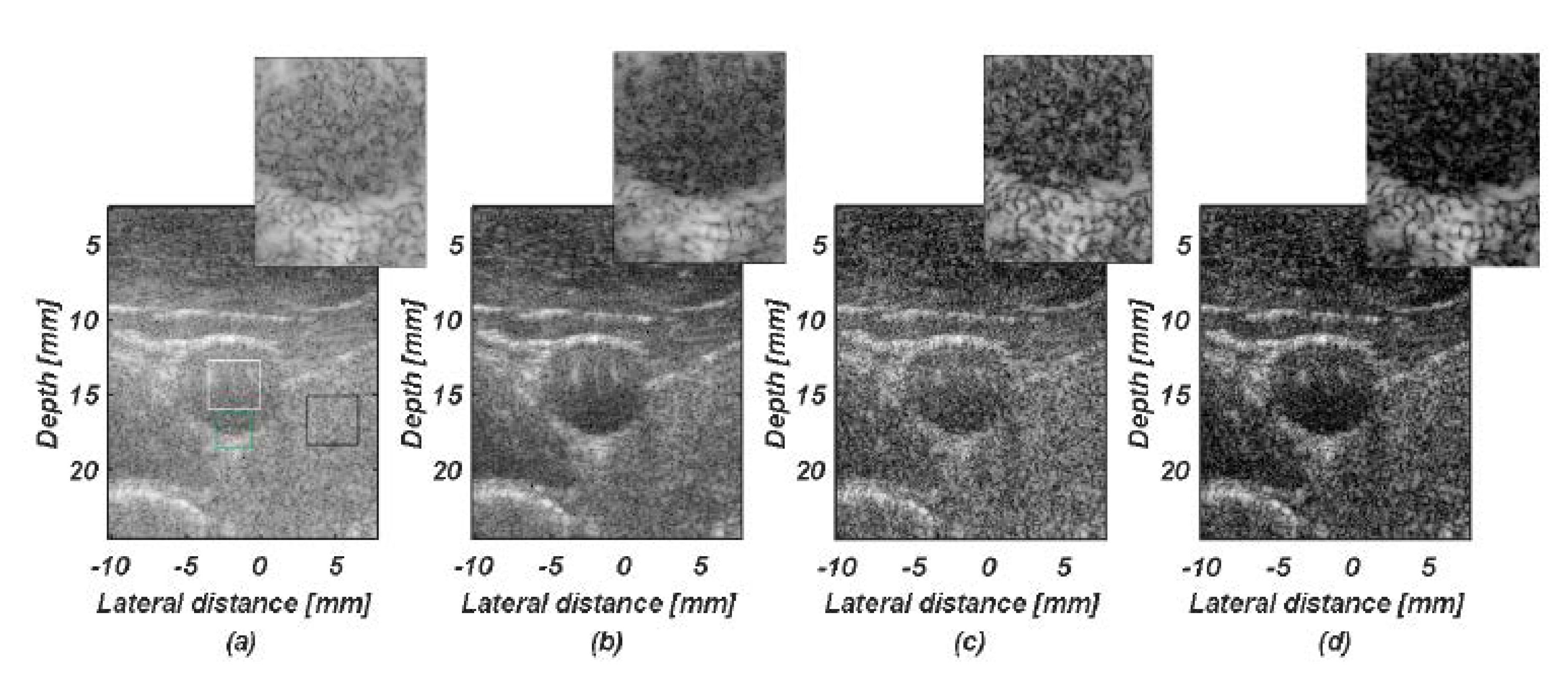
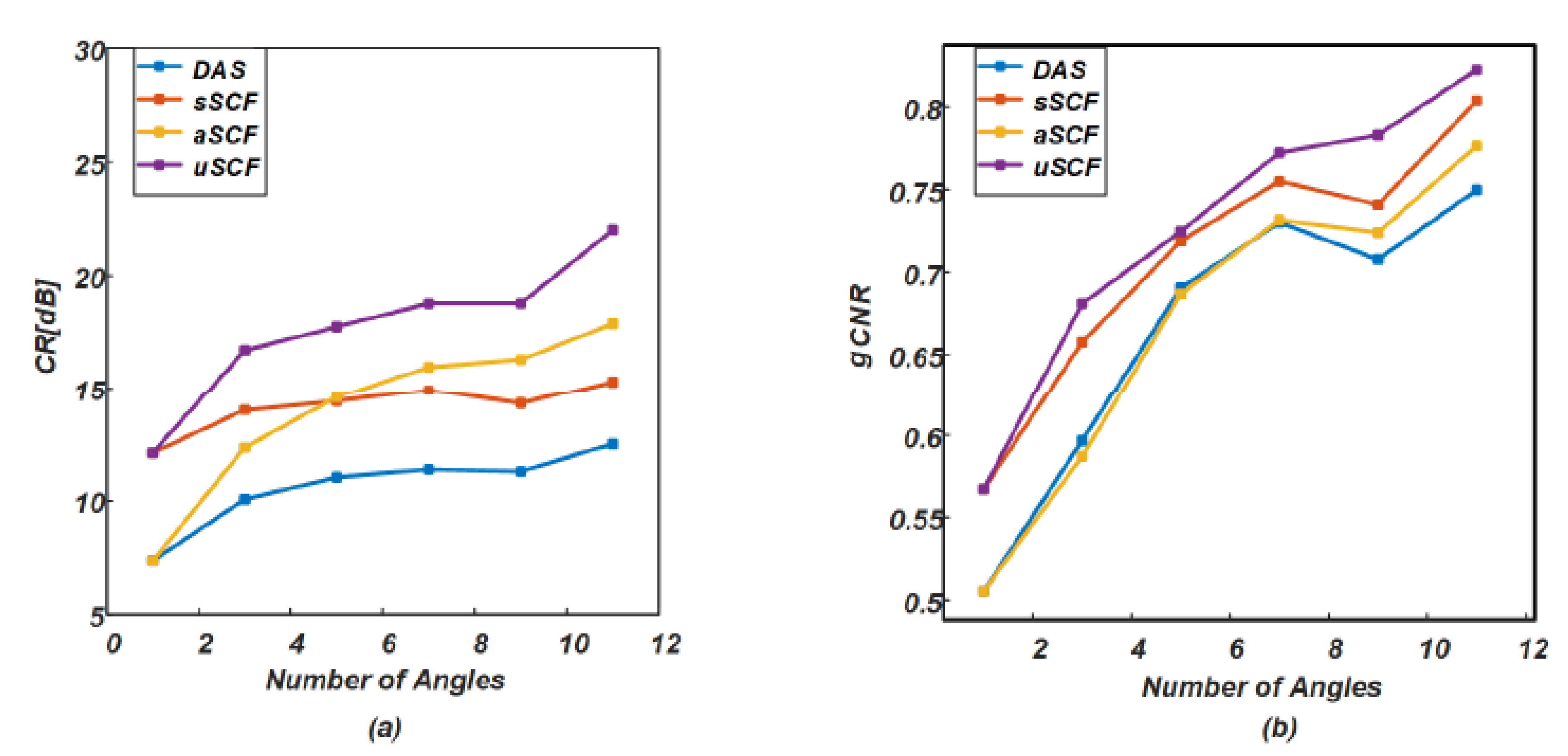
4.2. Phantom Study
4.3. In Vivo Study
5. Discussion
5.1. Comparision of SCF-Based Beamformers Performance
5.2. Computational Complexity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Nesbitt, S.A.; Thanh, N.T.K.; Pankhurst, Q.A. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar]
- Bercoff, J.; Montaldo, G.; Loupas, T.; Savery, D.; Mézière, F.; Fink, M.; Tanter, M. Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Couture, O.; Fink, M.; Tanter, M. Ultrasound contrast plane wave imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Tanter, M.; Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 102–119. [Google Scholar] [CrossRef]
- Bercoff, J. Ultrafast ultrasound imaging. In Ultrasound Imaging-Medical Applications; Minin, I.V., Minin, O.V., Eds.; InTech: New York, NY, USA, 2011; pp. 3–24. [Google Scholar]
- Hverven, S.M.; Rindal, O.M.H.; Rodriguez-Molares, A.; Austeng, A. The influence of speckle statistics on contrast metrics in ultrasound imaging. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017. [Google Scholar]
- Capon, J. High-resolution frequency-wavenumber spectrum analysis. Proc. IEEE 1969, 57, 1408–1418. [Google Scholar] [CrossRef]
- Asl, B.M.; Mahloojifar, A. Eigenspace-based minimum variance beamforming applied to medical ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 2381–2390. [Google Scholar] [CrossRef]
- Gasse, M.; Millioz, F.; Roux, E.; Garcia, D.; Liebgott, H.; Friboulet, D. High-quality plane wave compounding using convolutional neural networks. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1637–1639. [Google Scholar] [CrossRef]
- Schiffner, M.F.; Schmitz, G. Pulse-echo ultrasound imaging combining compressed sensing and the fast multipole method. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014. [Google Scholar]
- Mallart, R.; Fink, M. The van Cittert–Zernike theorem in pulse echo measurements. J. Acoust. Soc. Am. 1991, 90, 2718–2727. [Google Scholar] [CrossRef]
- Mallart, R.; Fink, M. Adaptive focusing in scattering media through sound-speed inhomogeneities: The van Cittert Zernike approach and focusing criterion. J. Acoust. Soc. Am. 1994, 96, 3721–3732. [Google Scholar] [CrossRef]
- Liu, D.-L.; Waag, R.C. About the application of the van Cittert-Zernike theorem in ultrasonic imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 590–601. [Google Scholar]
- Liu, D.L.; Waag, R.C. Correction of ultrasonic wavefront distortion using backpropagation and a reference waveform method for time-shift compensation. J. Acoust. Soc. Am. 1994, 96, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Lediju, M.A.; Trahey, G.E.; Byram, B.C.; Dahl, J.J. Short-lag spatial coherence of backscattered echoes: Imaging characteristics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Li, M.-L. Adaptive imaging using the generalized coherence factor. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 128–141. [Google Scholar] [PubMed]
- Camacho, J.; Parrilla, M.; Fritsch, C. Phase coherence imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Bottenus, N.; Üstüner, K.F. Acoustic reciprocity of spatial coherence in ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Dahl, J.J. Angular coherence in ultrasound imaging: Theory and applications. J. Acoust. Soc. Am. 2017, 141, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Walther, A. Radiometry and coherence. JOSA 1968, 58, 1256–1259. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Peng, H.; Zhang, C. Coherent plane-wave compounding based on normalized autocorrelation factor. IEEE Access 2018, 6, 36927–36938. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Zeng, X.; Yu, J.; Yiu, B.Y.; Alfred, C.H. Plane wave compounding based on a joint transmitting-receiving adaptive beamformer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1440–1452. [Google Scholar] [CrossRef]
- Viberg, M. Introduction to Array Processing, in Academic Press Library in Signal Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 463–502. [Google Scholar]
- Jensen, J.A.; Svendsen, N.B. Calculation of pressure fields from arbitrarily shaped, apodized, and excited ultrasound transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 262–267. [Google Scholar] [CrossRef]
- Jensen, J.A. Field: A program for simulating ultrasound systems. Med. Biol. Eng. Comput. 1996, 34, 351–353. [Google Scholar]
- Üstüner, K.F.; Holley, G.L. Ultrasound imaging system performance assessment. In Proceedings of the AAPM Annual Meeting, San Diega, CA, USA, 10–14 August 2003. [Google Scholar]
- Rodriguez-Molares, A.; Rindal, O.M.H.; D’hooge, J.; Måsøy, S.E.; Austeng, A.; Torp, H. The generalized contrast-to-noise ratio. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018. [Google Scholar]
- Nguyen, N.Q.; Prager, R.W. A spatial coherence approach to minimum variance beamforming for plane-wave compounding. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2018, 65, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Denarie, B.; Tangen, T.A.; Ekroll, I.K.; Rolim, N.; Torp, H.; Bjåstad, T.; Lovstakken, L. Coherent plane wave compounding for very high frame rate ultrasonography of rapidly moving targets. IEEE Trans. Med Imaging 2013, 32, 1265–1276. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Value |
|---|---|
| Pitch | 0.3 mm |
| Element width | 0.27 mm |
| Number of elements | 128 |
| Transmit frequency | 7.81 M [Hz] |
| Bandwidth | 4.8M~10.8 M [Hz] |
| Sampling frequency | 30 M [Hz] |
| F-number | 1.75 |
| Frame rate | 50 Hz |
| Beamformer | FWHM (mm) | 25 mm CR (dB) | 25 mm gCNR | 35 mm CR (dB) | 35 mm gCNR |
|---|---|---|---|---|---|
| 0.1397 ± 0.0025 | 9.12 | 0.63 | 11.45 | 0.75 | |
| 0.1105 ± 0.0045 | 13.88 | 0.70 | 15.70 | 0.79 | |
| 0.1068 ± 0.0173 | 15.15 | 0.70 | 17.85 | 0.78 | |
| 0.0966 ± 0.0030 | 20.77 | 0.74 | 22.48 | 0.82 |
| Beamformer | FWHM (mm) (phantom) | CR (dB) (phantom) | gCNR (phantom) | CR (dB) (in vivo) | gCNR (in vivo) |
|---|---|---|---|---|---|
| 0.3192 ± 0.0150 | 11.50 | 0.39 | 3.57 | 0.58 | |
| 0.2304 ± 0.0090 | 16.98 | 0.54 | 7.73 | 0.67 | |
| 0.2484 ± 0.0180 | 16.68 | 0.53 | 5.75 | 0.62 | |
| 0.2028 ± 0.0090 | 22.17 | 0.61 | 10.59 | 0.70 |
| Beamformer | Addition Operations | Multiplication Operations |
|---|---|---|
| DAS | MN | 0 |
| 2MN | N | |
| MN+N | 1 | |
| 2MN | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Jiao, Y.; Jiang, T.; Xu, Y.; Cui, Y. A United Sign Coherence Factor Beamformer for Coherent Plane-Wave Compounding with Improved Contrast. Appl. Sci. 2020, 10, 2250. https://doi.org/10.3390/app10072250
Yang C, Jiao Y, Jiang T, Xu Y, Cui Y. A United Sign Coherence Factor Beamformer for Coherent Plane-Wave Compounding with Improved Contrast. Applied Sciences. 2020; 10(7):2250. https://doi.org/10.3390/app10072250
Chicago/Turabian StyleYang, Chen, Yang Jiao, Tingyi Jiang, Yiwen Xu, and Yaoyao Cui. 2020. "A United Sign Coherence Factor Beamformer for Coherent Plane-Wave Compounding with Improved Contrast" Applied Sciences 10, no. 7: 2250. https://doi.org/10.3390/app10072250
APA StyleYang, C., Jiao, Y., Jiang, T., Xu, Y., & Cui, Y. (2020). A United Sign Coherence Factor Beamformer for Coherent Plane-Wave Compounding with Improved Contrast. Applied Sciences, 10(7), 2250. https://doi.org/10.3390/app10072250





