Coastal Lakes as a Buffer Zone for the Accumulation and Redistribution of Plastic Particles from Continental to Marine Environment: A Case Study of the Dishui Lake in Shanghai, China
Abstract
1. Introduction
2. Methods
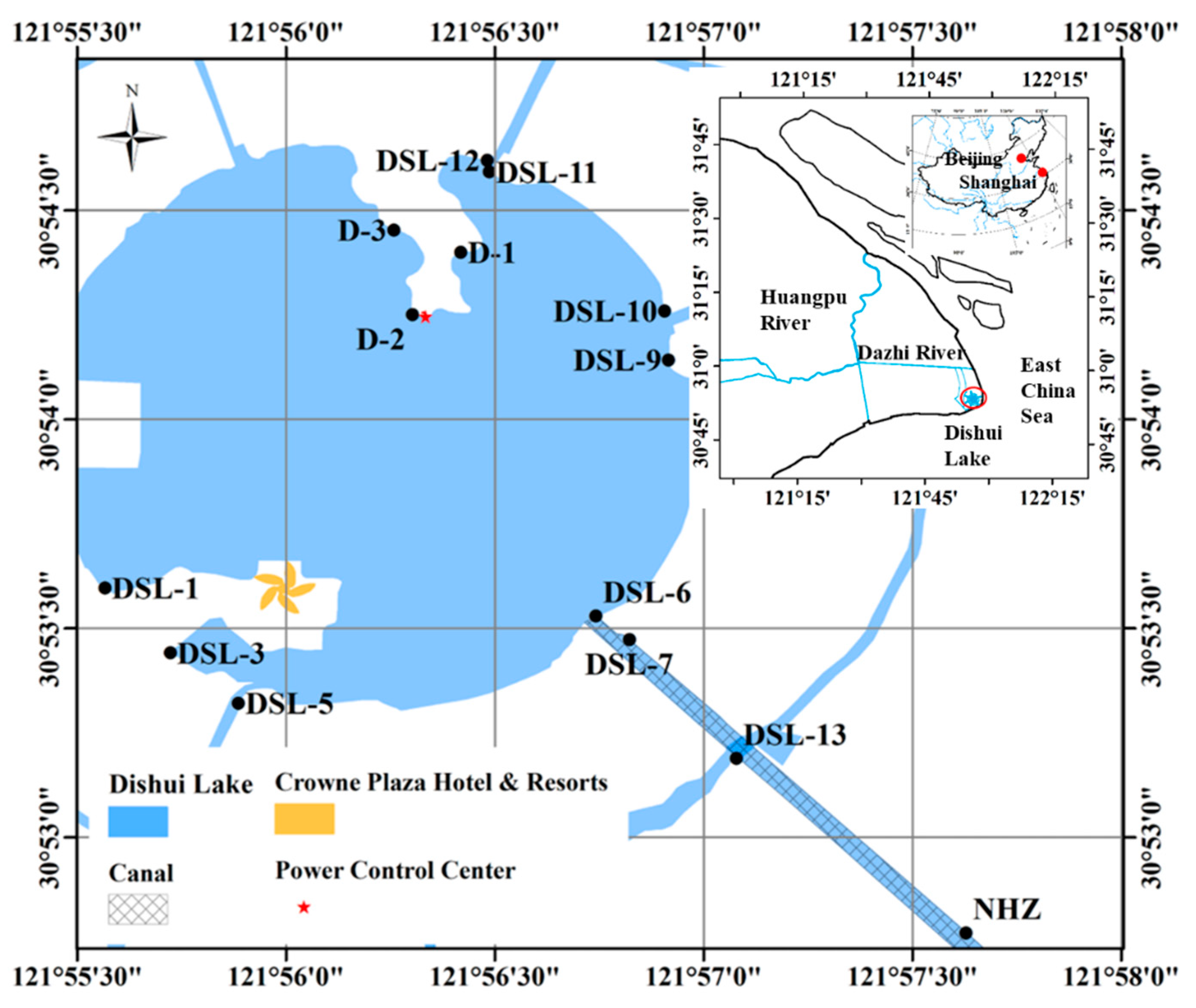
2.1. Sampling Area
2.2. Sampling Sites and Methods
2.3. Density Separation of Plastic Particles
2.4. Identification of Plastics
2.5. Quality Assurance and Control
3. Results
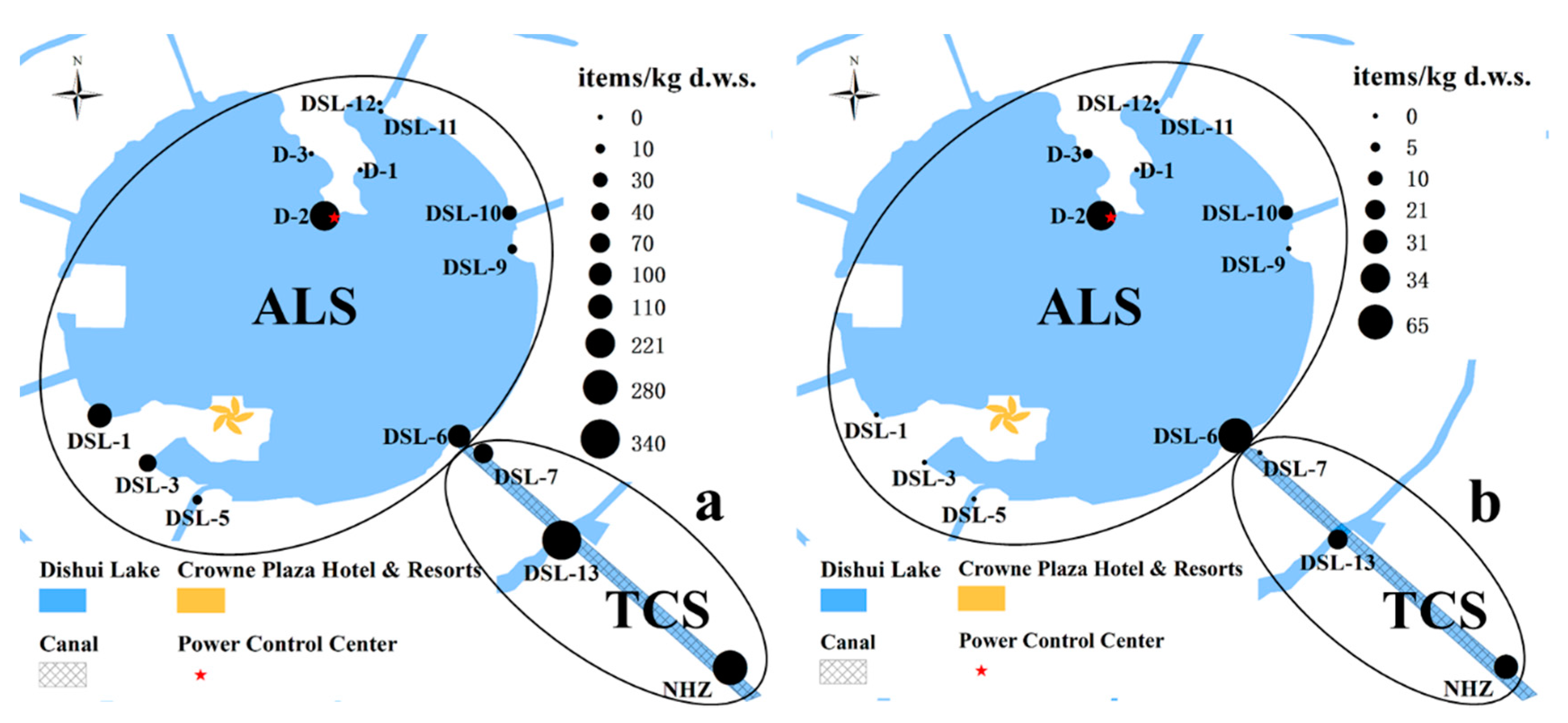
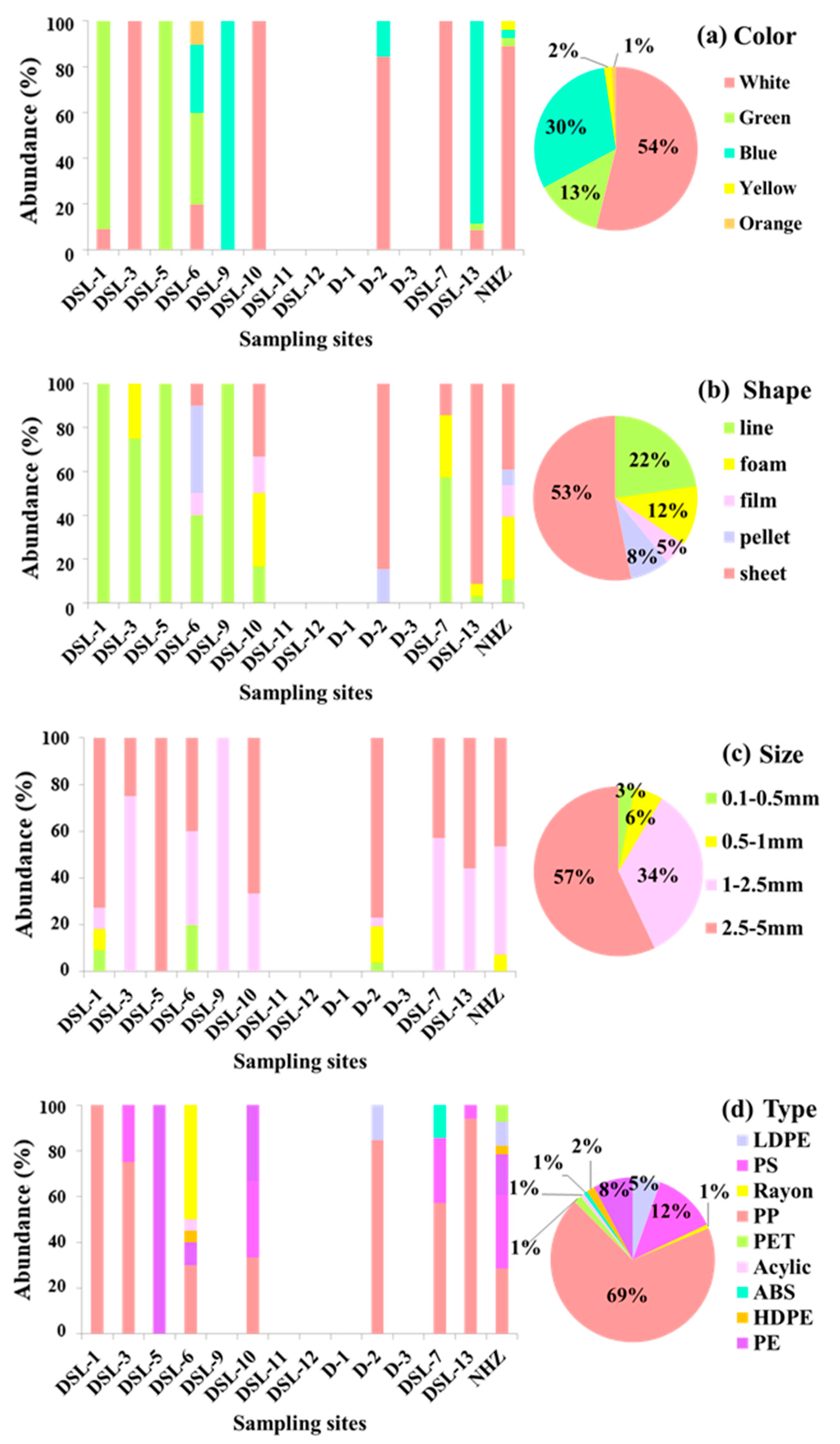
3.1. Composition, Abundance, and Distribution of Microplastics in the Lake Sediment
3.2. Mesoplastics and Macroplastics in Dishui Lake Sediment
4. Discussion
4.1. Sources of Microplastics in Dishui Lake Sediment
4.2. Coastal Lakes as a Buffer Zone between the Continental and Marine Environment
5. Conclusions & Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2017, An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe: Brussels, Belgium, 2017. [Google Scholar]
- Wilcox, C.; Van Sebille, E.; Hardesty, B.D. Threat of plastic pollution to seabirds is global, pervasive, and increasing. PNAS 2015, 112, 11899–11904. [Google Scholar] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar]
- Haward, M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat. Commun. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Isensee, K.; Valdes, L. GSDR 2015 Brief Marine Litter: Microplastics; IOC-UNECSCO: New York, NY, USA, 2015. [Google Scholar]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.D. Lost at sea: Where is all the plastic? Sci. Total Environ. 2004, 304, 838. [Google Scholar]
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects, and Fate of Microplastic Marine Debris, 9–11 September 2008; NOAA, University of Washington Tacoma: Tacoma, WA, USA, 2009. [Google Scholar]
- Jamieson, A.J.; Malkocs, T.; Piertney, S.B.; Fujii, T.; Zhang, Z. Bioaccumulation of persistent organic pollutants in the deepest ocean fauna. Nat. Ecol. Evol. 2017, 1, 51. [Google Scholar]
- Wang, N.; Shen, S.; Sun, W.; Ding, P.; Zhu, S.; Yi, W.; Yu, Z.; Sha, Z.; Mi, M.; He, L.; et al. Penetration of Bomb 14C Into the Deepest Ocean Trench. Geophysical Research Letters. Geophys. Res. Lett. 2019, 46, 5413–5419. [Google Scholar] [CrossRef]
- Razanajatovo, R.M.; Ding, J.; Zhang, S.; Jiang, H.; Zou, H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018, 136, 516–523. [Google Scholar]
- Wang, W.F.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar]
- Tang, G.W.; Liu, M.Y.; Zhou, Q.; He, H.X.; Chen, K.; Zhang, H.B.; Hu, J.H.; Huang, Q.H.; Luo, Y.M.; Ke, H.W.; et al. Microplastics and polycyclic aromatic hydrocarbons (PAHs) in Xiamen coastal areas: Implications for anthropogenic impacts. Sci. Total Environ. 2018, 634, 811–820. [Google Scholar]
- Vedolin, M.C.; Teophilo, C.Y.S.; Turra, A.; Figueira, R.C.L. Spatial variability in the concentrations of metals in beached microplastics. Mar. Pollut. Bull. 2018, 129, 487–493. [Google Scholar]
- Hüffer, T.; Hofmann, T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016, 214, 194–201. [Google Scholar]
- Latini, G.; De Felice, C.; Verrotti, A. Plasticizers, infant nutrition and reproductive health. Reprod. Toxicol. 2004, 19, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Verrotti, A.; De Felice, C. Di-2-ethylhexyl phthalate and endocrine disruption: A review. Curr. Drug Targets Immune Endocr. Metab. Disord. 2004, 4, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Shim, W.J.; Han, G.M.; Rani, M.; Song, Y.K.; Hong, S.H. Widespread detection of a brominated flame retardant, hexabromocyclododecane, in expanded polystyrene marine debris and microplastics from South Korea and the Asia-Pacific coastal region. Environ. Pollut. 2017, 231, 785–794. [Google Scholar] [CrossRef]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Gutow, L.; Eckerlebe, A.; Giménez, L.; Saborowski, R. Experimental evaluation of seaweeds as a vector for microplastics into marine food webs. Environ. Sci. Technol. 2015, 50, 915–923. [Google Scholar] [CrossRef]
- Ory, N.C.; Gallardo, C.; Lenz, M.; Thiel, M. Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environ. Pollut. 2018, 240, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.F.; Li, J.X.; Sun, C.J.; He, C.F.; Jiang, F.H.; Gao, F.L.; Zheng, L. Separation and Identification of Microplastics in Digestive System of Bivalves. Chin. J. Anal. Chem. 2018, 46, 690–697. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zheng, R.; Zhang, Y.; Hong, F.; Mu, J.; Chen, M.; Song, P.; Lin, L.; Lin, H.; Le, F.; et al. Microplastic contamination in benthic organisms from the Arctic and sub-Arctic regions. Chemosphere 2018, 209, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Peng, X.; Chen, M.; Chen, S.; Dasgupta, S.; Xu, H.; Ta, K.; Du, M.; Li, J.; Guo, Z.; Bai, S. Microplastics contaminate the deepest part of the world’s ocean. Geochem. Perspect. Let. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Frank, J.K.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Best, J. Anthropogenic stresses on the world’s big rivers. Nat. Geosci. 2019, 12, 7–21. [Google Scholar] [CrossRef]
- Frei, S.; Piehl, S.; Gilfedder, B.S.; Löder, M.G.J.; Krutzke, J.; Wilhelm, L.; Laforsch, C. Occurence of microplastics in the hyporheic zone of rivers. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W.; Farley, H.; Amato, S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [PubMed]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [PubMed]
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2015, 5, 17988. [Google Scholar] [PubMed]
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L.; et al. Microplastic pollution in the rivers of the Tibet Plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar]
- Lv, Y.P.; Mo, Z.L.; Zhang, C.; Deng, J. Study on the Method of Sponge City Special Planning for Watershed Scale: A Case Study on Shanghai Lingang Pilot Area. URP 2019, 2, 18–24. [Google Scholar]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar]
- Dehaut, A.; Cassone, A.L.; Frere, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviere, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar]
- Peng, G.; Xu, P.; Zhu, B.; Bai, M.; Li, D. Microplastics in freshwater river sediments in Shanghai, China: A case study of risk assessment in mega-cities. Environ. Pollut. 2018, 234, 448–456. [Google Scholar] [PubMed]
- Tan, X.; Yu, X.; Cai, L.; Wang, J.; Peng, J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere 2019, 221, 834–840. [Google Scholar]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [PubMed]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and spatial distribution of microplastics in river shore sediments of the Rhine-Main area in Germany. Environ. Sci. Technol. 2015, 40, 6070–6076. [Google Scholar]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar]
- Xiong, X.; Wu, C.; Elser, J.J.; Mei, Z.; Hao, Y. Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River—From inland to the sea. Sci. Total. Environ. 2019, 659, 66–73. [Google Scholar]
- Wang, W.; Ndungu, A.W.; Li, Z.; Wang, J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci. Total Environ. 2017, 575, 1369–1374. [Google Scholar]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibers from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [PubMed]
- Ter Halle, A.; Ladirat, L.; Martignac, M.; Mingotaud, A.F.; Boyron, O.; Perez, E. To what extent are microplastics from the open ocean weathered? Environ. Pollut. 2017, 227, 167–174. [Google Scholar] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [PubMed]
- Peng, G.; Zhu, B.; Yang, D.; Su, L.; Shi, H.; Li, D. Microplastics in sediments of the changjiang estuary, china. Environ. Pollut. 2017, 225, 283–290. [Google Scholar]
- Kühn, S.; van Oyen, A.; Booth, A.M.; Meijboom, A.; van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar]
- Isobe, A.; Uchida, K.; Tokai, T.; Iwasaki, S. East Asian seas: A hot spot of pelagic microplastics. Mar. Pollut. Bull. 2015, 101, 618–623. [Google Scholar]
- Siegfried, M.; Koelmans, A.A.; Besseling, E.; Kroeze, C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017, 127, 249–257. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Fang, J. Coastal Lakes as a Buffer Zone for the Accumulation and Redistribution of Plastic Particles from Continental to Marine Environment: A Case Study of the Dishui Lake in Shanghai, China. Appl. Sci. 2020, 10, 1974. https://doi.org/10.3390/app10061974
Liu Y, Fang J. Coastal Lakes as a Buffer Zone for the Accumulation and Redistribution of Plastic Particles from Continental to Marine Environment: A Case Study of the Dishui Lake in Shanghai, China. Applied Sciences. 2020; 10(6):1974. https://doi.org/10.3390/app10061974
Chicago/Turabian StyleLiu, Yan, and Jiasong Fang. 2020. "Coastal Lakes as a Buffer Zone for the Accumulation and Redistribution of Plastic Particles from Continental to Marine Environment: A Case Study of the Dishui Lake in Shanghai, China" Applied Sciences 10, no. 6: 1974. https://doi.org/10.3390/app10061974
APA StyleLiu, Y., & Fang, J. (2020). Coastal Lakes as a Buffer Zone for the Accumulation and Redistribution of Plastic Particles from Continental to Marine Environment: A Case Study of the Dishui Lake in Shanghai, China. Applied Sciences, 10(6), 1974. https://doi.org/10.3390/app10061974



