4-Hexylresorcinol Exhibits Different Characteristics to Estrogen
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures and 4HR and Xenoestrogen Treatment
2.2. MTT Assay
2.3. Western Blotting for ERK1/2, p-ERK1/2, ERα, and ERβ
2.4. Animals and Experimental Design
2.5. Immunohistochemical Determination and Western Blot Analysis in Pituitary Gland Samples
2.6. Statistical Analysis
3. Results
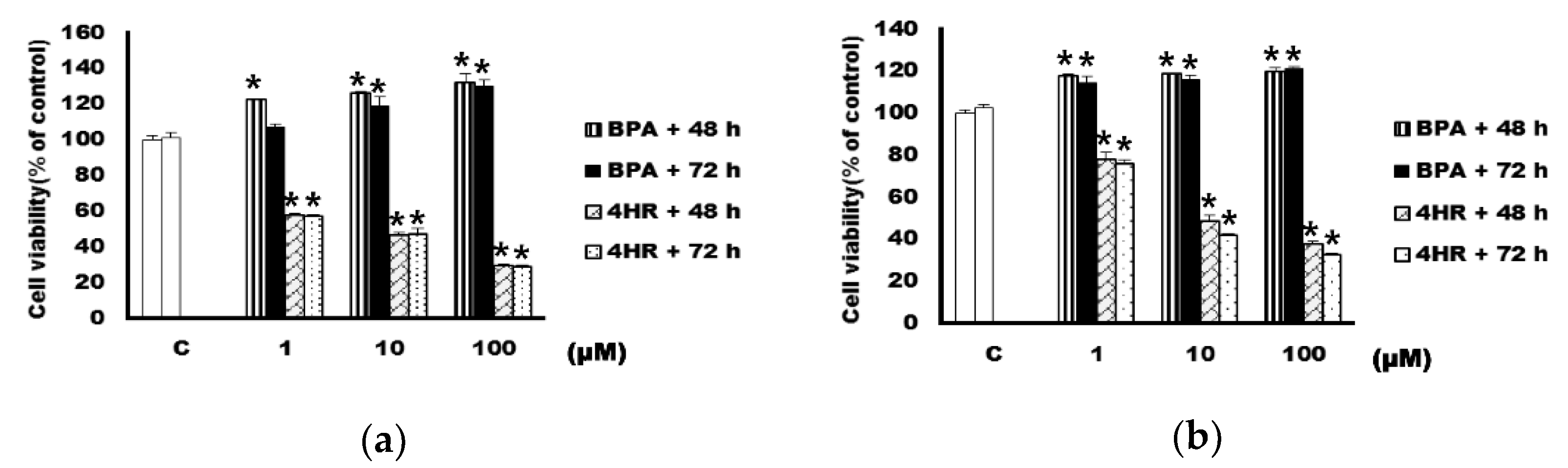
3.1. The Application of 4HR Suppressed the Proliferation of Both MCF-7 and SK-BR-3 Cells
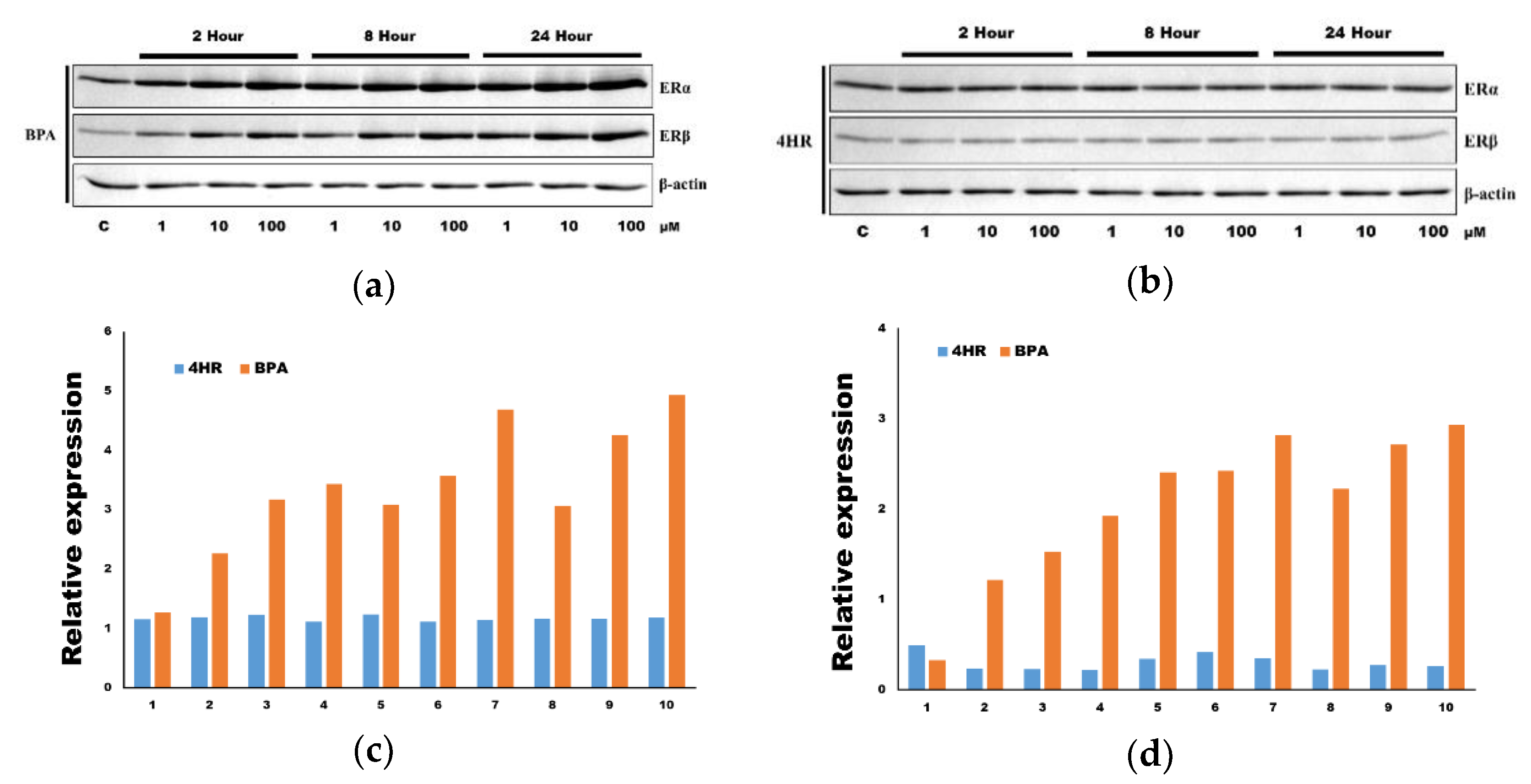
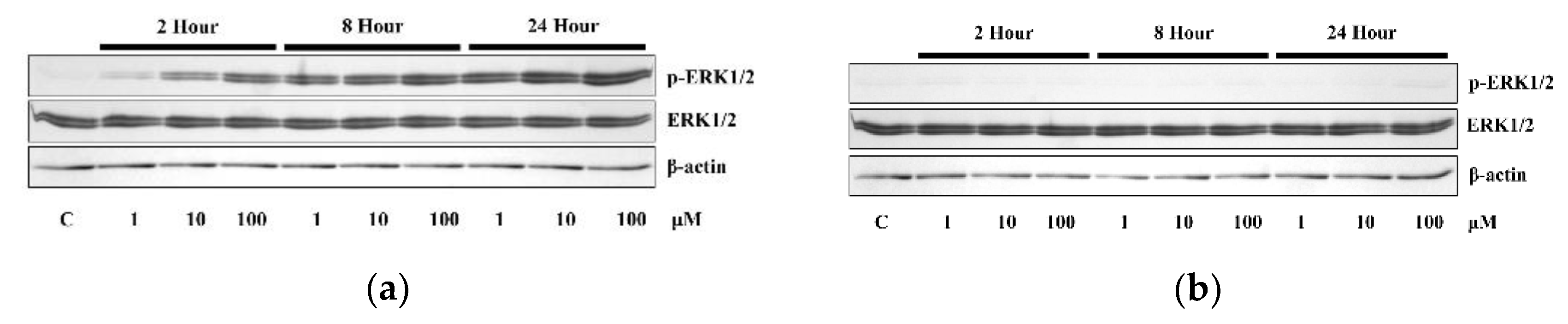
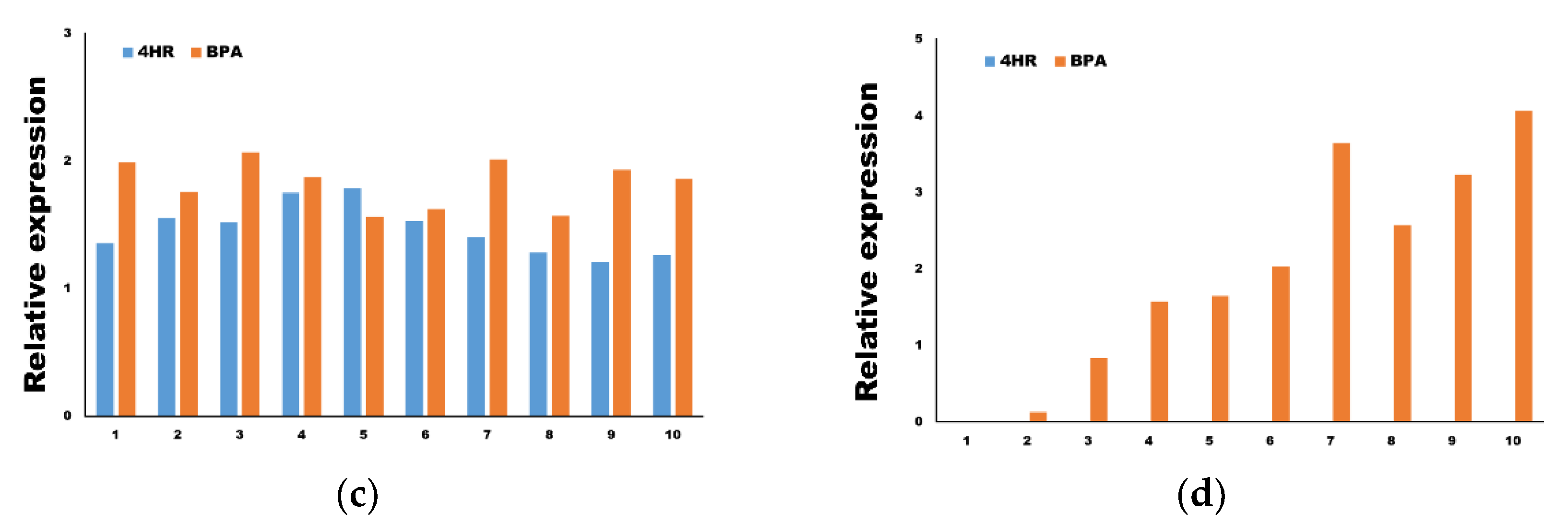
3.2. The Application of 4HR Did Not Increase ERα Expression and p-ERK
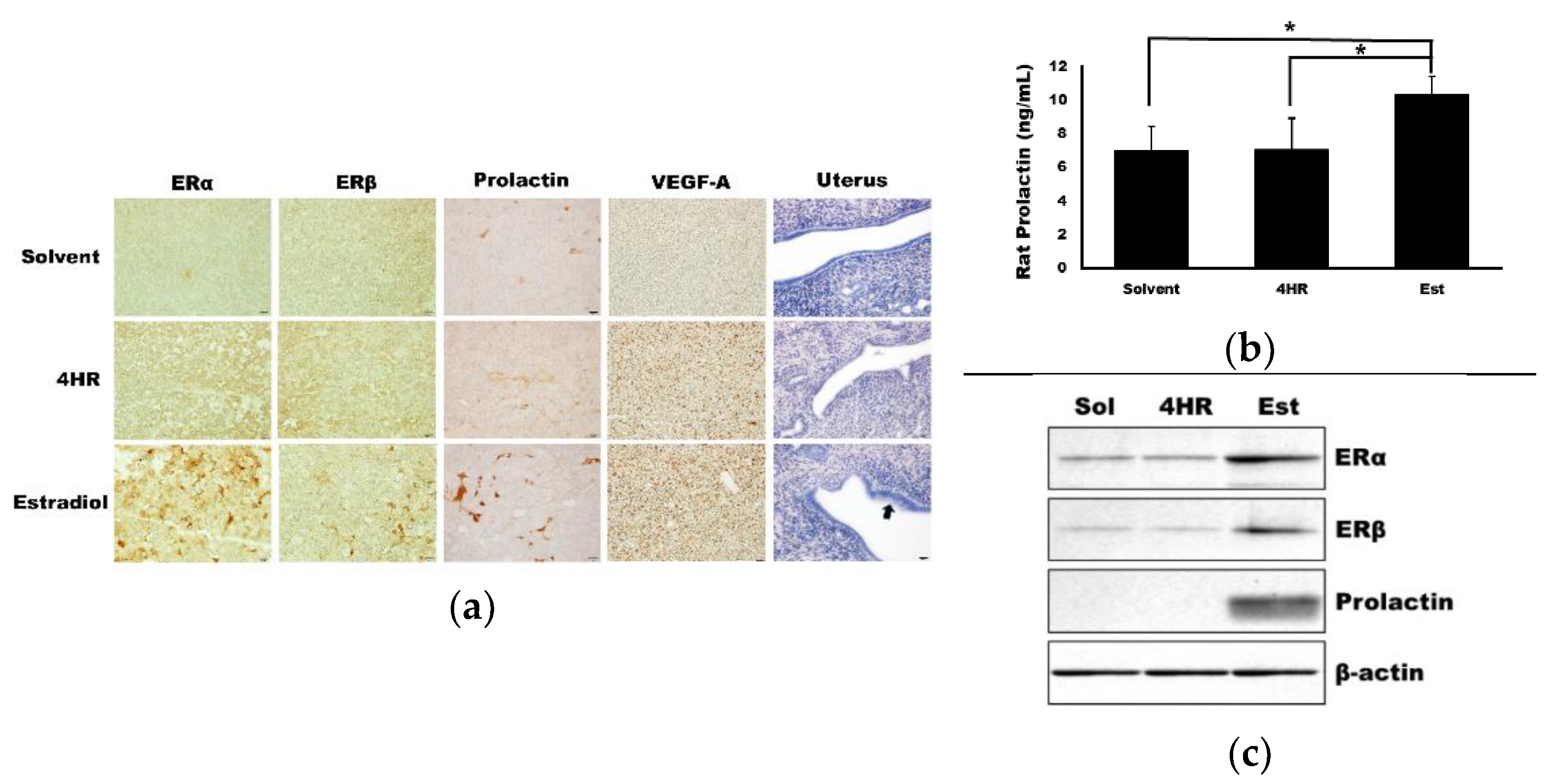
3.3. 4HR Did Not Increase Prolactin and ERα Expression in the Pituitary Gland
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Evans, R.T.; Baker, P.J.; Coburn, R.A.; Fischman, S.L.; Genco, R.J. In vitro antiplaque effects of antiseptic phenols. J. Periodontol. 1977, 48, 156–162. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.M.; Lee, J.S.; Gang, S.R.; Lim, H.S.; Kim, M.; Lee, O.H. Development and validation of an analytical method for the determination of 4-hexylresorcinol in food. Food Chem. 2016, 190, 1086–1092. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Berna, J.; Rodriguez-Lopez, J.N.; Tomas, V.; Garcia-Canovas, F. Tyrosinase-catalyzed hydroxylation of 4-hexylresorcinol, an antibrowning and depigmenting agent: A kinetic study. J. Agric. Food Chem. 2015, 63, 7032–7040. [Google Scholar] [CrossRef]
- Kim, S.G.; Choi, J.Y. 4-hexylresorcinol exerts antitumor effects via suppression of calcium oscillation and its antitumor effects are inhibited by calcium channel blockers. Oncol. Rep. 2013, 29, 1835–1840. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, S.G.; Park, Y.W.; Kweon, H.; Kim, J.Y.; Rotaru, H. Cisplatin and 4-hexylresorcinol synergise to decrease metastasis and increase survival rate in an oral mucosal melanoma xenograft model: A preliminary study. Tumour Biol. 2013, 34, 1595–1603. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, S.G.; Park, N.R.; Choi, J.Y. Porcine bone incorporated with 4-hexylresorcinol increases new bone formation by suppression of the nuclear factor kappa B signaling pathway. J. Craniofac. Surg. 2018, 29, 1983–1990. [Google Scholar] [CrossRef]
- Kweon, H.; Kim, S.G.; Choi, J.Y. Inhibition of foreign body giant cell formation by 4-hexylresorcinol through suppression of diacylglycerol kinase delta gene expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.Y.; Kim, D.W.; Choi, J.Y.; Kim, S.G. 4-Hexylresorcinol and silk sericin increase the expression of vascular endothelial growth factor via different pathways. Sci. Rep. 2019, 9, 3448. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L.; Mark-Lee, W.F. A review on the effects of bisphenol A and its derivatives on skeletal health. Int. J. Med. Sci. 2018, 15, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, G.; Carnevali, O.; Franzoni, M.F.; Cottone, E.; Lutz, I.; Kloas, W.; Yamamoto, K.; Kikuyama, S.; Polzonetti-Magni, A.M. Environmental estrogens and reproductive biology in amphibians. Gen. Comp. Endocrinol. 2002, 126, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Ankley, G.T.; Segner, H.; Tyler, C.R. Screening and testing for endocrine disruption in fish-biomarkers as “signposts,” not “traffic lights,” in risk assessment. Environ. Health Perspect. 2006, 114 (Suppl. 1), 106–114. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Hu, T.; Lin, C.; Xiong, Q.; Liu, F.; Yuan, J.; Zhao, X.; Wang, R. Resveratrol downregulates PCSK9 expression and attenuates steatosis through estrogen receptor α-mediated pathway in L02cells. Eur. J. Pharmacol. 2019, 855, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Amadasi, A.; Mozzarelli, A.; Meda, C.; Maggi, A.; Cozzini, P. Identification of xenoestrogens in food additives by an integrated in silico and in vitro approach. Chem. Res. Toxicol. 2009, 22, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Hexylresorcinol, Compound Summary, U.S. National Library of Medicine, NIH. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3610#section=Metabolism-Metabolites (accessed on 17 February 2020).
- Duan, P.; Hu, C.; Quan, C.; Yu, T.; Zhou, W.; Yuan, M.; Shi, Y.; Yang, K. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: Involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology 2016, 341–343, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Consumer Antiseptic Wash Final Rule. Questions and Answers. 2017. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 28 January 2020).
- Kim, M.K.; Yoon, C.S.; Kim, S.G.; Park, Y.W.; Lee, S.S.; Lee, S.K. Effects of 4-hexylresorcinol on protein expressions in RAW264.7 cells as determined by immunoprecipitation high performance liquid chromatography. Sci. Rep. 2019, 9, 3379. [Google Scholar] [CrossRef]
- Okada, K.; Fujisawa, M. Recovery of spermatogenesis following cancer treatment with cytotoxic chemotherapy and radiotherapy. World J. Mens Health 2019, 37, 166–174. [Google Scholar] [CrossRef]
- Lei, B.; Sun, S.; Zhang, X.; Feng, C.; Xu, J.; Wen, Y.; Huang, Y.; Wu, M.; Yu, Y. Bisphenol AF exerts estrogenic activity in MCF-7cells through activation of Erk and PI3K/Akt signals via GPER signaling pathway. Chemosphere 2019, 220, 362–370. [Google Scholar] [CrossRef]
- Bengtson, D.A.; Henshel, D.S. Environmental Toxicology and Risk Assessment: Biomarkers and Risk Assessment. 5th Volume; American Society for Testing and Materials: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Bigsby, R.M.; Caperell-Grant, A.; Madhukar, B.V. Xenobiotics released from fat during fasting produce estrogenic effects in ovariectomized mice. Cancer Res. 1997, 57, 865–869. [Google Scholar]
- Zhao, Q.; Howard, E.W.; Parris, A.B.; Ma, Z.; Xing, Y.; Yang, X. Bisphenol AF promotes estrogen receptor-positive breast cancer cell proliferation through amphiregulin-mediated crosstalk with receptor tyrosine kinase signaling. PLoS ONE 2019, 14, e0216469. [Google Scholar] [CrossRef]
- Hess-Wilson, J.K.; Boldison, J.; Weaver, K.E.; Knudsen, K.E. Xenoestrogen action in breast cancer: Impact on ER-dependent transcription and mitogenesis. Breast Cancer Res. Treat. 2006, 96, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Handel, A.E. Bioinformatics analysis of estrogen-responsive genes. Methods Mol. Biol. 2016, 1366, 29–39. [Google Scholar] [PubMed]
- Wang, T.; Liu, B.; Guan, Y.; Gong, M.; Zhang, W.; Pan, J.; Liu, Y.; Liang, R.; Yuan, Y.; Ye, L. Melatonin inhibits the proliferation of breast cancer cells induced by bisphenol A via targeting estrogen receptor-related pathways. Thorac. Cancer 2018, 9, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.L.; Rios-Arce, N.D.; Atkinson, S.; Bierhalter, H.; Schoenherr, D.; Bazil, J.N.; McCabe, L.R.; Parameswaran, N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol. Rep. 2017, 5, e13263. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E.; Haseman, J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: Low versus high dose effects. Reprod. Toxicol. 2004, 18, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Goloubkova, T.; Ribeiro, M.F.; Rodrigues, L.P.; Cecconello, A.L.; Spritzer, P.M. Effects of xenoestrogen bisphenol A on uterine and pituitary weight, serum prolactin levels and immunoreactive prolactin cells in ovariectomized Wistar rats. Arch. Toxicol. 2000, 74, 92–98. [Google Scholar] [CrossRef]
- Ronchetti, S.A.; Miler, E.A.; Duvilanski, B.H.; Cabilla, J.P. Cadmium mimics estrogen-driven cell proliferation and prolactin secretion from anterior pituitary cells. PLoS ONE 2013, 8, e81101. [Google Scholar] [CrossRef]
- Buteau-Lozano, H.; Velasco, G.; Cristofari, M.; Balaguer, P.; Perrot-Applanat, M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J. Endocrinol. 2008, 196, 399–412. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Liu, X.; Wang, X.; Zhou, A.; Liu, P. Dynamic analysis on the calcium oscillation model considering the influences of mitochondria. Biosystems 2018, 163, 36–46. [Google Scholar] [CrossRef]
- Whyte, J.; Bergin, O.; Bianchi, A.; McNally, S.; Martin, F. Key signalling nodes in mammary gland development and cancer. Mitogen-activated protein kinase signaling in experimental models of breast cancer progression and in mammary gland development. Breast Cancer Res. 2009, 11, 209. [Google Scholar] [CrossRef]
- Nagao, T.; Saito, Y.; Usumi, K.; Yoshimura, S.; Ono, H. Low-dose bisphenol A does not affect reproductive organs in estrogen-sensitive C57BL/6N mice exposed at the sexually mature, juvenile, or embryonic stage. Reprod. Toxicol. 2002, 16, 123–130. [Google Scholar] [CrossRef]
- Chhabra, R.S.; Huff, J.E.; Haseman, J.; Hall, A.; Baskin, G.; Cowan, M. Inhibition of some spontaneous tumors by 4-hexylresorcinol in F344/N rats and B6C3F1 mice. Fundam. Appl. Toxicol. 1988, 11, 685–690. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, D.W.; Lee, S.K.; Kim, S.G.; Kim, T.W. The administration of 4-hexylresorcinol accelerates orthodontic tooth movement and increases the expression level of bone turnover markers in ovariectomized rats. Int. J. Mol. Sci. 2020, 21, 1526. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-J.; Oh, J.-H.; Seok, H.; Jo, Y.-Y.; Kim, D.-W.; Garagiola, U.; Choi, J.-Y.; Kim, S.-G. 4-Hexylresorcinol Exhibits Different Characteristics to Estrogen. Appl. Sci. 2020, 10, 1737. https://doi.org/10.3390/app10051737
Kang Y-J, Oh J-H, Seok H, Jo Y-Y, Kim D-W, Garagiola U, Choi J-Y, Kim S-G. 4-Hexylresorcinol Exhibits Different Characteristics to Estrogen. Applied Sciences. 2020; 10(5):1737. https://doi.org/10.3390/app10051737
Chicago/Turabian StyleKang, Yei-Jin, Ji-Hyeon Oh, Hyun Seok, You-Young Jo, Dae-Won Kim, Umberto Garagiola, Je-Yong Choi, and Seong-Gon Kim. 2020. "4-Hexylresorcinol Exhibits Different Characteristics to Estrogen" Applied Sciences 10, no. 5: 1737. https://doi.org/10.3390/app10051737
APA StyleKang, Y.-J., Oh, J.-H., Seok, H., Jo, Y.-Y., Kim, D.-W., Garagiola, U., Choi, J.-Y., & Kim, S.-G. (2020). 4-Hexylresorcinol Exhibits Different Characteristics to Estrogen. Applied Sciences, 10(5), 1737. https://doi.org/10.3390/app10051737







