Degradable and Dissolvable Thin-Film Materials for the Applications of New-Generation Environmental-Friendly Electronic Devices
Abstract
Featured Application
Abstract
1. Introduction
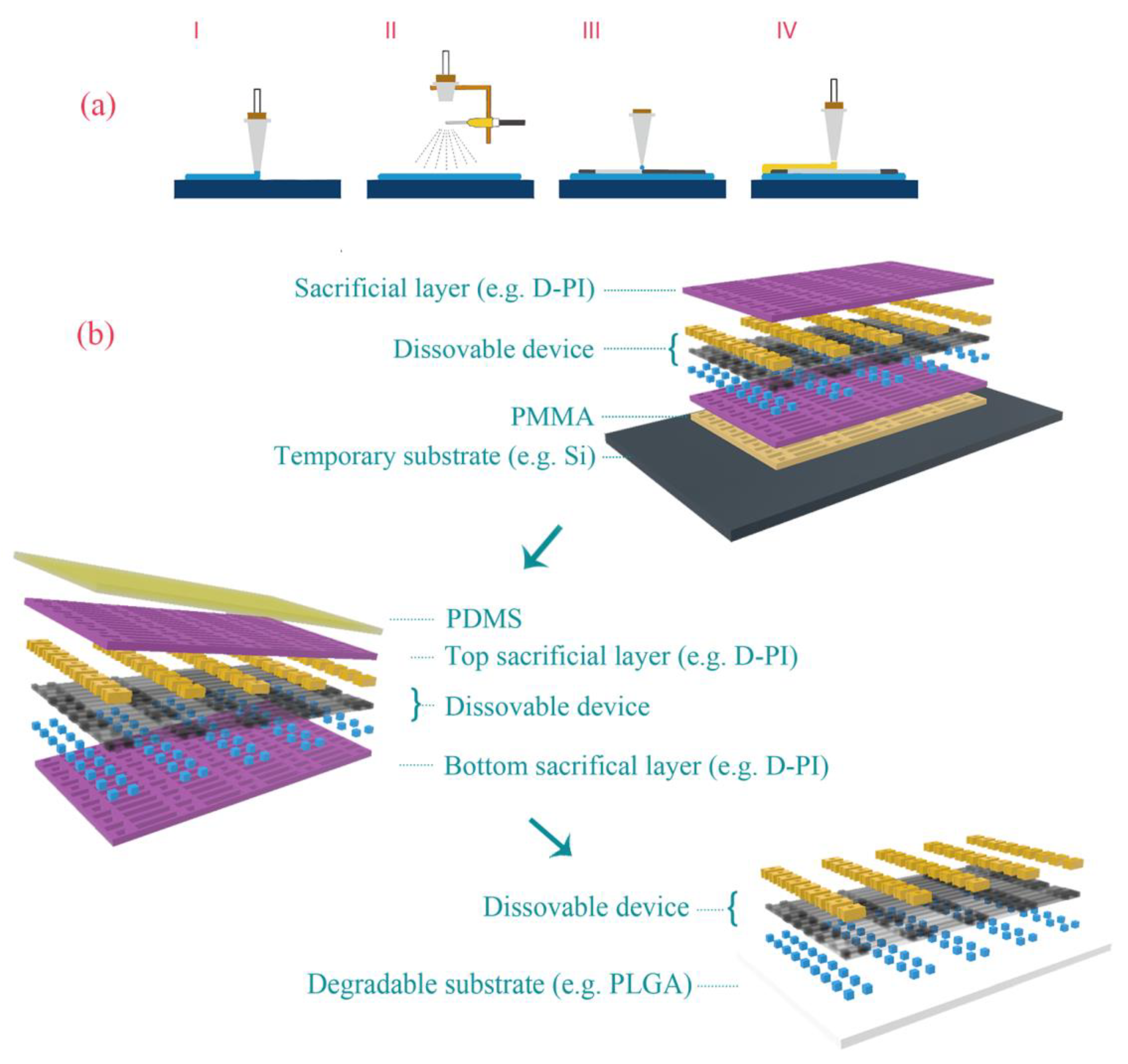
2. Preparation of Thin-Film Materials
3. Thin-Film Materials and Their Applications
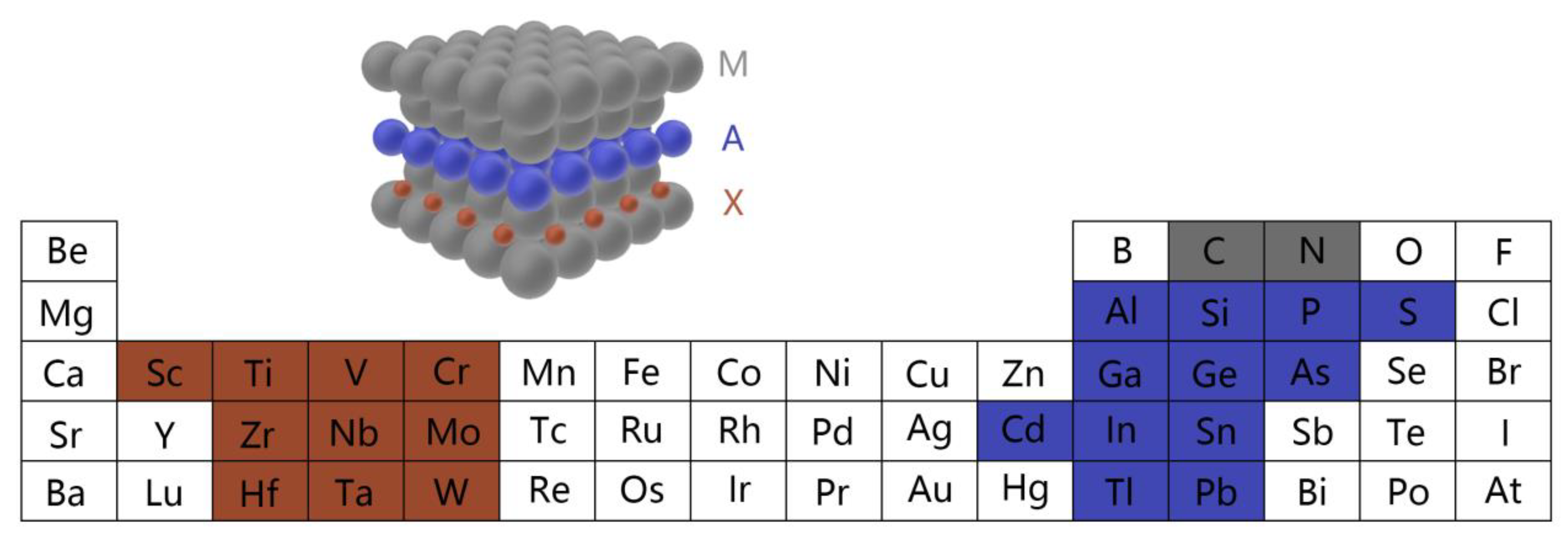
3.1. Inorganic Thin-Film Materials
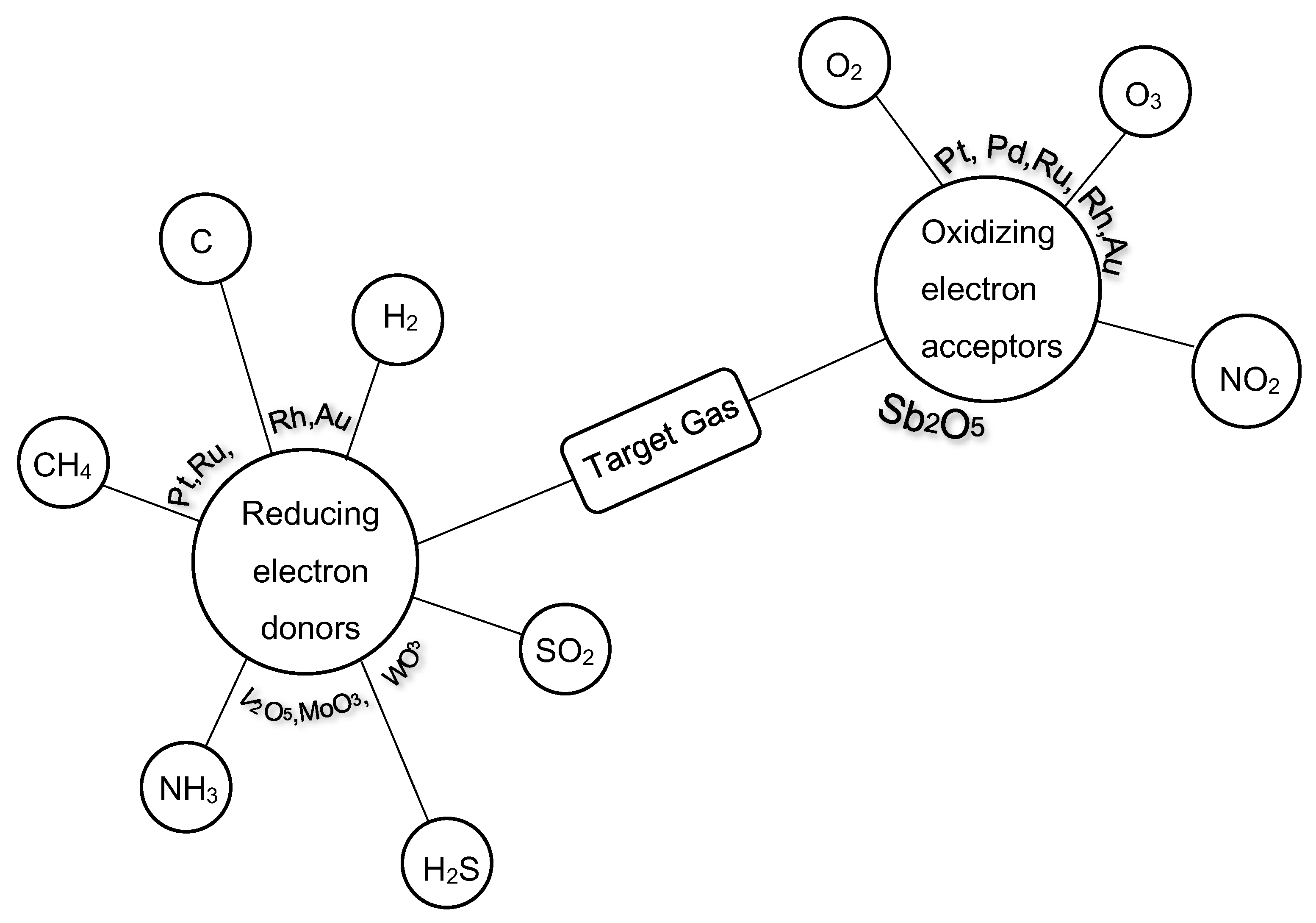
3.1.1. Metal Oxide Thin-Films
Binary Metal Oxides
Complex Metal Oxides
3.1.2. Halide Perovskite Thin-Films
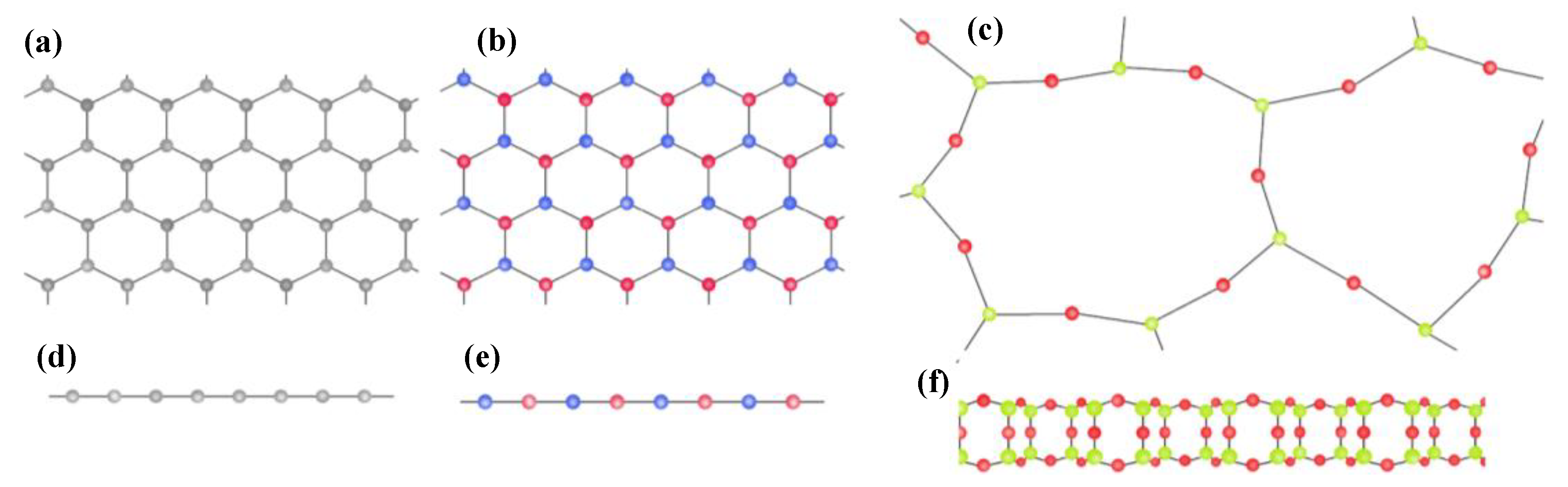
3.1.3. Two-Dimensional (2D) Thin-Film Materials
Graphene
Transition Metal Dichalcogenide (TMDC)
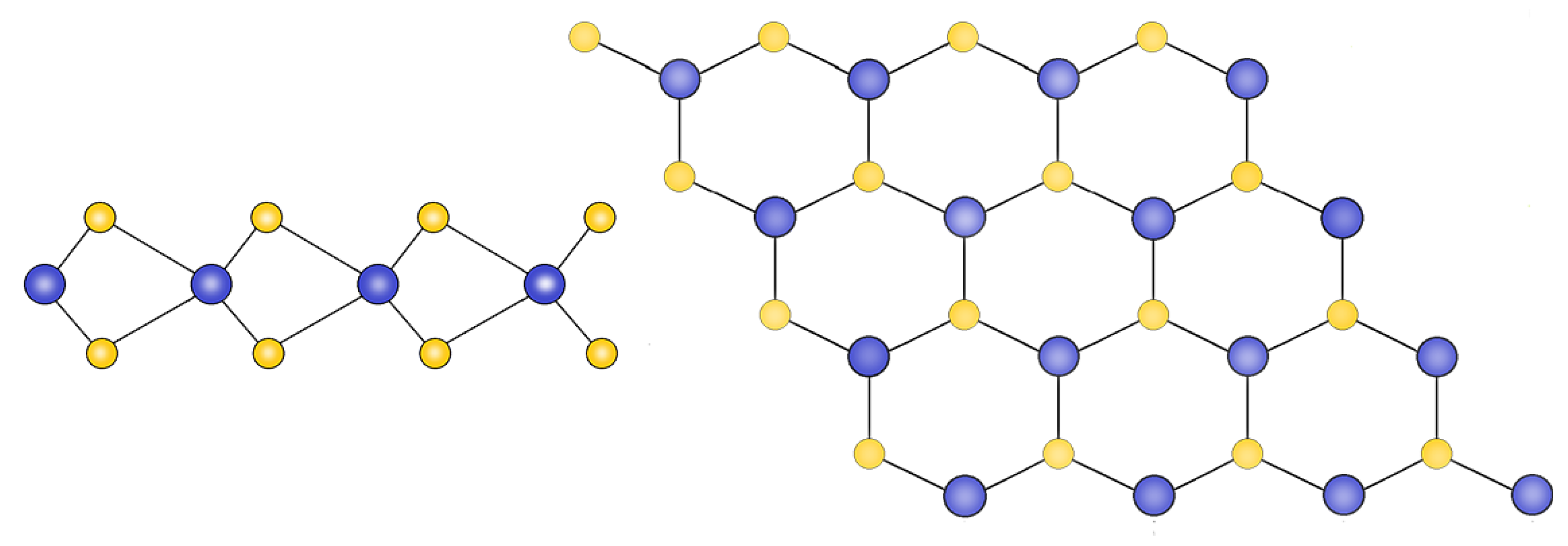
MXene
3.2. Organic Thin-Films
3.2.1. Small Molecule and Polymer Thin-Films
3.2.2. Inorganic-Organic Hybrid Thin-Films
4. Environment-Friendly Electronic Devices
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Xue, M.; Xu, Z. Application of Life Cycle Assessment on Electronic Waste Management: A Review. Environ. Manag. 2017, 59, 693–707. [Google Scholar] [CrossRef]
- Yu, X.; Shou, W.; Mahajan, B.K.; Huang, X.; Pan, H. Materials, processes, and facile manufacturing for bioresorbable electronics: A review. Adv. Mater. 2018, 30, 1707624. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, Y.-S.; Amsden, J.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G.; Zakin, M.R.; Rogers, J.A. Silicon electronics on silk as a path to bioresorbable, implantable devices. Appl. Phys. Lett. 2009, 95, 133701. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Tao, H.; Kim, D.H.; Cheng, H.; Song, J.K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.S.; et al. A physically transient form of silicon electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.-W.; Jain, H.; Kang, S.-K.; Su, Y.; et al. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Kong, D.; Yin, L. Recent progress on biodegradable materials and transient electronics. Bioact. Mater. 2018, 3, 322–333. [Google Scholar] [CrossRef]
- Fu, K.K.; Wang, Z.; Dai, J.; Carter, M.; Hu, L. Transient Electronics: Materials and Devices. Chem. Mater. 2016, 28, 3527–3539. [Google Scholar] [CrossRef]
- Liu, D.; Yin, Y.; Cheng, H. Physically transient memristor based on the permeation of water at the interface of electrode and substrate. J. Alloy. Compd. 2019, 810, 151957. [Google Scholar] [CrossRef]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.; Rogers, J.A. High-performance biodegradable/transient electronics on biodegradable polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef]
- Irimia-Vladu, M. Green electronics: Biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 2014, 43, 588–610. [Google Scholar] [CrossRef]
- Kang, S.K.; Koo, J.; Lee, Y.K.; Rogers, J.A. Advanced Materials and Devices for Bioresorbable Electronics. Acc. Chem. Res. 2018, 51, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Yuming, W.; Alfred, W. An Introduction to Physics and Technology of Thin Films; World Scientific: Singapore, 1994. [Google Scholar]
- Wuttig, M.; Kanel, H. Physics and Technology Of Thin Films, Iwtf 2003-Proceedings of The International Workshop; World Scientific: Singapore, 2004. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films: Applications-Led Growth of Functional Materials; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Kang, S.-K.; Hwang, S.-W.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.-H.; Huang, Y.; Rogers, J.A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Comini, E.; Ferroni, M.; Guidi, V.; Faglia, G.; Martinelli, G.; Sberveglieri, G. Nano-structured mixed oxides compounds for gas sensing applications. Sensor. Actuator B Chem. 2002, 84, 26–32. [Google Scholar] [CrossRef]
- Novoselov, S.K. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- McDonnell, S.J.; Wallace, R.M. Atomically-thin layered films for device applications based upon 2D TMDC materials. Thin Solid Films 2016, 616, 482–501. [Google Scholar] [CrossRef]
- Berzina, T.; Erokhina, S.; Camorani, P.; Konovalov, O.; Erokhin, V.; Fontana, M.P. Electrochemical control of the conductivity in an organic memristor: A time-resolved X-ray fluorescence study of ionic drift as a function of the applied voltage. ACS Appl. Mater. Interfaces 2009, 1, 2115–2118. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bao, Z. Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 2010, 22, 651–655. [Google Scholar] [CrossRef]
- Luan, L.; Royal, M.W.; Evans, R.; Fair, R.B.; Jokerst, N.M. Chip Scale Optical Microresonator Sensors Integrated With Embedded Thin Film Photodetectors on Electrowetting Digital Microfluidics Platforms. IEEE Sens. J. 2012, 12, 1794–1800. [Google Scholar] [CrossRef]
- Koppens, F.H.; Mueller, T.; Avouris, P.; Ferrari, A.C.; Vitiello, M.S.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793. [Google Scholar] [CrossRef]
- Hu, L.; Choi, J.W.; Yang, Y.; Jeong, S.; La Mantia, F.; Cui, L.-F.; Cui, Y. Highly conductive paper for energy-storage devices. Proc. Natl. Acad. Sci. USA 2009, 106, 21490–21494. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.H. Transition metal oxide thin films for nonvolatile resistive random access memory applications. J. Ceram. Soc. Jpn. 2009, 117, 929–934. [Google Scholar] [CrossRef]
- Klasens, H.A.; Koelmans, H. A tin oxide field-effect transistor. Solid State Electron. 1964, 7, 701–702. [Google Scholar] [CrossRef]
- Koezuka, H.; Tsumura, A.; Ando, T. Field-effect transistor with polythiophene thin film. Synthetic Met. 1987, 18, 699–704. [Google Scholar] [CrossRef]
- Wasa, K.; Kitabatake, M.; Adachi, H. Thin Film Materials Technology: Sputtering of Control Compound Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Loh, K.J.; Kim, J.; Lynch, J.P.; Kam, N.W.S.; Kotov, N.A. Multifunctional layer-by-layer carbon nanotube–polyelectrolyte thin films for strain and corrosion sensing. Smart Mater. Struct. 2007, 16, 429–438. [Google Scholar] [CrossRef]
- Ling, M.M.; Bao, Z. Thin Film Deposition, Patterning, and Printing in Organic Thin Film Transistors. Chem. Mater. 2004, 16, 4824–4840. [Google Scholar] [CrossRef]
- Freund, L.B.; Suresh, S. Thin Film Materials: Stress, Defect Formation and Surface Evolution; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Böer, K.W. Handbook of the Physics of Thin-Film Solar Cells; Springer Science & Business: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Hu, X.; Li, G.; Yu, J.C. Design, fabrication, and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir 2010, 26, 3031–3039. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Patthanasettakul, V.; Lomas, T.; Chindaudom, P. Ion-assisted e-beam evaporated gas sensor for environmental monitoring. Sci. Technol. Adv. Mater. 2005, 6, 26. [Google Scholar] [CrossRef]
- Baraton, M.-I. Sensors for Environment, Health and Security: Advanced Materials and Technologies; Springer Science & Business Media: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Boyadjiev, S.; Georgieva, V.; Vergov, L.; Szilágyi, I.M. QCM gas sensor characterization of ALD-grown very thin TiO2 films. J. Phys. Conf. Ser. 2018, 992, 012054. [Google Scholar] [CrossRef]
- Mannion, J.M.; Locklair, W.D.; Powell, B.A.; Husson, S.M. Alpha spectroscopy substrates based on thin polymer films. J. Radioanal. Nucl. Chem. 2015, 307, 2339–2345. [Google Scholar] [CrossRef]
- Yang, G.; Jung, W.; Ahn, S.-J.; Lee, D. Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes. Appl. Sci. 2019, 9, 1030. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Lee, C.H.; Cheng, H.; Jeong, J.-W.; Kang, S.-K.; Kim, J.-H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Degradable and Resorbable Biomaterials. In Biomaterials Science: An Introduction to Materials, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 179–195. [Google Scholar]
- Dagdeviren, C.; Hwang, S.W.; Su, Y.; Kim, S.; Cheng, H.; Gur, O.; Haney, R.; Omenetto, F.G.; Huang, Y.; Rogers, J.A. Transient, biocompatible electronics and energy harvesters based on ZnO. Small 2013, 9, 3398–3404. [Google Scholar] [CrossRef]
- Martin, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materi-als: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef]
- Kern, W. Thin Film Processes II; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Petty, M.C. Organic Thin Film Deposition Techniques. Encycl. Nanosci. Nanotechnol. 2004, 8, 295–304. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Jung, H.Y.; Oh, S.C.; Lee, H. Resistive Switching Characteristics and Failure Analysis of TiO2 Thin Film Deposited by RF Magnetron Sputtering System. J. Electrochem. Soc. 2011, 158, H178–H182. [Google Scholar] [CrossRef]
- Pan, C.; Ma, T. High-quality transparent conductive indium oxide films prepared by thermal evaporation. Appl. Phys. Lett. 1980, 37, 163–165. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F. Characterization of copper oxide thin films deposited by the thermal evaporation of cuprous oxide (Cu2O). Vacuum 2008, 82, 623–629. [Google Scholar] [CrossRef]
- Markeev, A.; Chouprik, A.; Egorov, K.; Lebedinskii, Y.; Zenkevich, A.; Orlov, O. Multilevel resistive switching in ternary HfxAl1−xOy oxide with graded Al depth profile. Microelectron. Eng. 2013, 109, 342–345. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lu, Y.; Zhu, H.; Zhang, J.; Yue, S.; Li, S.; Zhuge, F.; Ye, Z.; Lu, J. Memristors based on amorphous ZnSnO films. Mater. Lett. 2019, 249, 169–172. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Lee, S.; Kim, T.; Jang, B.; Lee, W.-Y.; Song, K.; Kim, H.; Do, G.; Hwang, S.; Chung, S.; Jang, J. Impact of Device Area and Film Thickness on Performance of Sol-gel Processed ZrO2 RRAM. IEEE Electron Device Lett. 2018, 39, 668–671. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Late, D.J.; Shaikh, P.A.; Khare, R.; Kashid, R.V.; Chaudhary, M.; More, M.A.; Ogale, S.B. Pulsed Laser-Deposited MoS2 Thin Films on W and Si: Field Emission and Photoresponse Studies. ACS Appl. Mater. Interfaces 2014, 6, 15881–15888. [Google Scholar] [CrossRef]
- Poh, S.M.; Tan, S.J.R.; Wang, H.; Song, P.; Abidi, I.H.; Zhao, X.; Dan, J.; Chen, J.; Luo, Z.; Pennycook, S.J.; et al. Molecular-Beam Epitaxy of Two-Dimensional In2Se3 and Its Giant Electroresistance Switching in Ferroresistive Memory Junction. Nano Lett. 2018, 18, 6340–6346. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Z.-Z.; Li, H.-O.; Song, X.-X.; Deng, G.-W.; Cao, G.; Xiao, M.; Guo, G.-P. Quantum dot behavior in transition metal dichalcogenides nanostructures. Front. Phys. Beijing 2017, 12, 128502. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Bhattacharya, G.; Saha, S.; Barman, A.; Roy, S.S. Grape extract assisted green synthesis of reduced graphene oxide for water treatment application. Mater. Lett. 2015, 160, 355–358. [Google Scholar] [CrossRef]
- Hao, L.; Wang, H.; Chen, R. Organic–inorganic hybrid hydrophobic Mg(OH)2−xFx–MTES coating with ultraviolet durability and high visible transmittance. J. Mater. Sci. 2019, 54, 13569–13578. [Google Scholar] [CrossRef]
- Macedo, A.G.; Christopholi, L.P.; Gavim, A.E.X.; de Deus, J.F.; Teridi, M.A.M.; Yusoff, A.R.; da Silva, W.J. Perylene derivatives for solar cells and energy harvesting: A review ofmaterials, challenges and advances. J. Mater. Sci. Mater. Electron. 2019, 30, 15803–15824. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Flores-Castañeda, M.; González, E.C.; Ruiz-Aguilar, I.; Camps, E.; Cruces, M.P.; Pimentel, E.; Camacho-López, M. Preparation and characterization of organic nanoparticles by laser ablation in liquids technique and their biological activity. Mater. Res. Express. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Alvarez-Venicio, V.; Arcos-Ramos, R.O.; Hernandez-Rojas, J.A.; Guerra-Pulido, J.O.; Basiuk, V.A.; Rivera, M.; Carreon-Castro, M.D.P. Preparation and Characterization of a Novel Organic Semiconductor Indacenedithiophene Derivative and the Corresponding Langmuir-Blodgett Thin Films. J. Nanosci. Nanotechnol. 2019, 19, 7244–7250. [Google Scholar] [CrossRef]
- You, M.; Li, W.; Pan, Y.; Fei, P.; Wang, H.; Zhang, W.; Zhi, L.; Meng, J. Preparation and characterization of antibacterial polyamine-based cyclophosphazene nanofiltration membranes. J. Membr. Sci. 2019, 592, 1–11. [Google Scholar] [CrossRef]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef]
- Lewis, G.D.A.; Zhang, Y.; Schlenker, C.W.; Ryu, K.; Thompson, M.E.; Zhou, C. Continuous, Highly Flexible, and Transparent Graphene Films by Chemical Vapor Deposition for Organic Photovoltaics. ACS Nano 2010, 4, 2865–2873. [Google Scholar] [CrossRef]
- Seul, M.; Sammon, M.J. Preparation of surfactant multilayer films on solid substrates by deposition from organic solution. Thin Solid Films 1990, 185, 287–305. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Kramer, R. All-Printed Flexible and Stretchable Electronics. Adv. Mater. 2017, 29, 1604965. [Google Scholar] [CrossRef]
- Inui, T.; Mandamparambil, R.; Araki, T.; Abbel, R.; Koga, H.; Nogi, M.; Suganuma, K. Laser-induced forward transfer of high-viscosity silver precursor ink for non-contact printed electronics. RSC Adv. 2015, 5, 77942–77947. [Google Scholar] [CrossRef]
- Mandal, S.; Purohit, G.; Katiyar, M. Inkjet Printed Organic Thin Film Transistors: Achievements and Challenges. Mater. Sci. Forum 2013, 736, 250–274. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Central Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Park, G.; Cheng, H.; Song, J.K.; Kang, S.K.; Yin, L.; Kim, J.H.; Omenetto, F.G.; Huang, Y.; Lee, K.M.; et al. 25th anniversary article: Materials for high-performance biodegradable semiconductor devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Deschler, F.; Gao, S.; Friend, R.H.; Cheetham, A.K. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2017, 2, 16099. [Google Scholar] [CrossRef]
- Kang, S.-K.; Hwang, S.-W.; Yu, S.; Seo, J.-H.; Corbin, E.A.; Shin, J.; Wie, D.S.; Bashir, R.; Ma, Z.; Rogers, J.A. Biodegradable Thin Metal Foils and Spin-On Glass Materials for Transient Electronics. Adv. Funct. Mater. 2015, 25, 1789–1797. [Google Scholar] [CrossRef]
- Rudraswamy, S.B.; Bhat, N. Optimization of RF Sputtered Ag-Doped BaTiO3-CuO Mixed Oxide Thin Film as Carbon Dioxide Sensor for Environmental Pollution Monitoring Application. IEEE Sens. J. 2016, 16, 5145–5151. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, W.; Qian, L.; Chen, S.; Shi, G. High-performance gas sensors based on a thiocy-anate ion-doped organometal halide perovskite. Phys. Chem. Chem. Phys. 2017, 19, 12876–12881. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.K.; Gratzel, M.; Ahmad, S. Perovskite as light harvester: A game changer in photovoltaics. Angew. Chem. Int. Edit. 2014, 53, 2812–2824. [Google Scholar] [CrossRef]
- Cheng, X.-F.; Qian, W.-H.; Wang, J.; Yu, C.; He, J.-H.; Li, H.; Xu, Q.-F.; Chen, D.-Y.; Li, N.-J.; Lu, J.-M. Environmentally Robust Memristor Enabled by Lead-Free Double Perovskite for High-Performance Information Storage. Small 2019, 1905731. [Google Scholar] [CrossRef]
- Lin, Q.; Hu, W.; Zang, Z.; Zhou, M.; Du, J.; Wang, M.; Han, S.; Tang, X. Transient Resistive Switc-hing Memory of CsPbBr3 Thin Films. Adv. Electron. Mater. 2018, 4, 1700596. [Google Scholar] [CrossRef]
- Chen, R.; Xu, J.; Lao, M.; Liang, Z.; Chen, Y.; Zhong, C.; Huang, L.; Hao, A.; Ismail, M. Transient Resistive Switching for Nonvolatile Memory Based on Water-Soluble Cs4PbBr6 Perovskite Films. Phys. Status. Solidi.-R 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; Wu, Y.; Ding, S.; Hou, P.; Song, H.; Wang, J.; Zhong, X. The resistive switchingbehaviors of a flexible device based on a SrTiO3 film. EPL-Europhys. Lett. 2019, 127, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Singh, V.V.; Sattayasamitsathit, S.; Orozco, J.; Kaufmann, K.; Dong, R.; Gao, W.; Jurado-Sanchez, B.; Fedorak, Y.; Wang, J. Water-Driven Micromotors for Rapid Photocatalytic Degradation of Biological and Chemical Warfare Agents. ACS Nano 2014, 8, 11118–11125. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Gupta, S.K.; Joshi, A.; Kaur, M. Development of gas sensors using ZnO nanostructures. J. Chem. Sci. 2010, 122, 57–62. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef]
- Bhat, S.S.; Qurashi, A.; Khanday, F.A. ZnO nanostructures based biosensors for cancer and infectious disease applications: Perspectives, prospects and promises. TrAC Trend. Anal. Chem. 2017, 86, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Mater. 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Xu, N.; Liu, L.; Sun, X.; Liu, X.; Han, D.; Wang, Y.; Han, R.; Kang, J.; Yu, B. Characteristics and mechanism of conduction/set process in TiN/ZnO/Pt resistance switching random-access memories. Appl. Phys. Lett. 2008, 92, 232112. [Google Scholar] [CrossRef]
- Park, S.Y.; Jung, Y.W.; Hwang, S.H.; Jang, G.H.; Seo, H.; Kim, Y.-C.; Ok, M.-R. Instrument-Free and Autonomous Generation of H2O2 from Mg–ZnO/Au Hybrids for Disinfection and Organic Pollutant Degradations. Met. Mater. Int. 2018, 24, 657–663. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kulkarni, S.B.; Mathe, V.L. A multifunctional ZnO thin film based devices for photoelectrocatalytic degradation of terephthalic acid and CO2 gas sensing applications. Sensor. Actuator B Chem. 2018, 274, 1–9. [Google Scholar] [CrossRef]
- Liu, G.; Rumyantsev, S.L.; Jiang, C.; Shur, M.S.; Balandin, A.A. Selective Gas Sensing With h-BN Capped MoS2 Heterostructure Thin-Film Transistors. IEEE Electron Device Lett. 2015, 36, 1202–1204. [Google Scholar] [CrossRef]
- Juliasih, N.; Buchari; Noviandri, I. Application of ZnO Nanoparticle as Sulphide Gas Sensor Using UV/VIS/NIR-Spectrophotometer. J. Phys. Conf. Ser. 2017, 824, 012020. [Google Scholar] [CrossRef]
- Buvailo, A.I.; Xing, Y.; Hines, J.; Dollahon, N.; Borguet, E. TiO2/LiCl-based nanostructured thin film for humidity sensor applications. ACS Appl. Mater. Interfaces 2011, 3, 528–533. [Google Scholar] [CrossRef]
- Park, J.I.; Jang, Y.; Bae, J.S.; Yoon, J.H.; Lee, H.U.; Wakayama, Y.; Kim, J.P.; Jeong, Y. Effect of thickness- dependent structural defects on electrical stability of MoS2 thin film transistors. J. Alloys Compd. 2020, 814, 152134. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, D.H.; Park, J.; Park, K.I.; Lee, K.J.; Jeong, C.K. Modulation of surface physics and chemistry in triboelectric energy harvesting technologies. Sci. Technol. Adv. Mater. 2019, 20, 758–773. [Google Scholar] [CrossRef]
- Barai, H.R.; Rahman, M.M.; Roy, M.; Barai, P.; Joo, S.W. A calcium doped binary strontium-copper oxide electrode material for high-performance supercapacitors. Mater. Sci. Semicond. Proc. 2019, 90, 245–251. [Google Scholar] [CrossRef]
- Yang, L.F.; Zhao, Y.G.; Zhang, S.; Li, P.S.; Gao, Y.; Yang, Y.J.; Huang, H.L.; Miao, P.X.; Liu, Y.; Chen, A.T.; et al. Bipolar loop-like non-volatile strain in the (001)-oriented Pb(Mg1/3Nb2/3)O3-PbTiO3 single crystals. Sci. Rep. UK 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Sun, J.; Dang, B.; Gao, H.; Yang, M.; Ma, X.; Hao, Y. Solution-Processed Physi-cally Transient Resistive Memory Based on Magnesium Oxide. IEEE Electron Device Lett. 2019, 40, 193–195. [Google Scholar] [CrossRef]
- Rhee, S.J.; Kang, C.Y.; Choi, C.H.; Zhang, M.; Lee, J.C. Material and electrical analysis of hafnium titania bilayer dielectric metal-oxide-semiconductor field-effect transistors. J. Vac. Sci. Technol. B 2005, 23, 2561–2563. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, J.; Hu, W.; He, Y.; Yang, J.; He, J.; Gao, Y.; Wan, Q. Coplanar Multigate MoS2 Electric-Double-Layer Transistors for Neuromorphic Visual Recognition. ACS Appl. Mater. Interfaces 2018, 10, 25943–25948. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y. Improvement on the Stability of Amorphous Indium Gallium Zinc Oxide Thin Film Transistors Using Amorphous Oxide Multilayer Source/Drain Electrodes. Trans. Electr. Electron. Mater. 2016, 17, 143–145. [Google Scholar] [CrossRef][Green Version]
- Wang, J.T.; Liu, Y.F.; Chen, X.T.; Chen, C.; Chen, P.; Wang, Z.K.; Duan, Y. Functional Metal Oxides in Perovskite Solar Cells. ChemPhysChem 2019, 20, 2580–2586. [Google Scholar] [CrossRef]
- Jin, S.H.; Kang, S.K.; Cho, I.T.; Han, S.Y.; Chung, H.U.; Lee, D.J.; Shin, J.; Baek, G.W.; Kim, T.I.; Lee, J.H.; et al. Water-Soluble Thin Film Transistors and Circuits Based on Amorphous Indium-Gallium-Zinc Oxide. ACS Appl. Mater. Interfaces 2015, 7, 8268–8274. [Google Scholar] [CrossRef]
- Kim, T.W.; Cho, W.J. Effects of Al Addition on Resistive-Switching Characteristics of Solution Processed Zn-Sn-O ReRAMs. J. Nanosci. Nanotechnol. 2019, 19, 6099–6105. [Google Scholar] [CrossRef]
- Barman, A.; Kar-Narayan, S.; Mukherjee, D. Caloric Effects in Perovskite Oxides. Adv. Mater. Interfaces 2019, 6, 1–31. [Google Scholar] [CrossRef]
- Raza, E.; Aziz, F.; Ahmad, Z. Stability of organometal halide perovskite solar cells and role of HTMs: Recent developments and future directions. RSC Adv. 2018, 8, 20952–20967. [Google Scholar] [CrossRef]
- Kaji, H.; Kondo, H.; Fujii, T.; Arita, M.; Takahashi, Y. Effect of Electrode and Interface Oxide on the Property of ReRAM Composed of Pr0.7Ca0.3MnO3. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2010; p. 012032. [Google Scholar]
- Janousch, M.; Meijer, G.I.; Staub, U.; Delley, B.; Karg, S.F.; Andreasson, B.P. Role of oxygen vac-ancies in Cr-doped SrTiO3 for resistance-change memory. Adv. Mater. 2007, 19, 2232–2235. [Google Scholar] [CrossRef]
- Mirzababaei, J.; Chuang, S. La0.6Sr0.4Co0.2Fe0.8O3 Perovskite: A Stable Anode Catalyst for Direct Methane Solid Oxide Fuel Cells. Catalysts 2014, 4, 146–161. [Google Scholar] [CrossRef]
- Muralt, P.; Polcawich, R.G.; Trolier-McKinstry, S. Piezoelectric Thin Films for Sensors, Actuators, and Energy Harvesting. MRS Bull. 2009, 34, 658–664. [Google Scholar] [CrossRef]
- Todaro, M.T.; Guido, F.; Algieri, L.; Mastronardi, V.M.; Desmaele, D.; Epifani, G.; De Vittorio, M. Biocompatible, Flexible, and Compliant Energy Harvesters Based on Piezoelectric Thin Films. IEEE. Trans. Nanotechnol. 2018, 17, 220–230. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X. Luminescent perovskite quantum dots: Synthesis, microstructures, optical properties and applications. J. Mater. Chem. C 2019, 7, 1413–1446. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Jiang, Z.; Zhang, Y.; Wu, S.; Pan, H.; Khisro, N.; Chen, X. Photoresponse of nonvolatile resistive memory device based on all-inorganic perovskite CsPbBr3 nanocrystals. J. Phys. D Appl. Phys. 2019, 52, 1–7. [Google Scholar] [CrossRef]
- Liu, D.; Lin, Q.; Zang, Z.; Wang, M.; Wangyang, P.; Tang, X.; Zhou, M.; Hu, W. Flexible All-Inorganic Perovskite CsPbBr3 Nonvolatile Memory Device. ACS Appl. Mater. Interfaces 2017, 9, 6171–6176. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hersam, M.C. 2D materials for quantum information science. Nat. Rev. Mater. 2019, 4, 669–684. [Google Scholar] [CrossRef]
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef]
- Xia, F.; Wang, H.; Xiao, D.; Dubey, M.; Ramasubramaniam, A. Two-dimensional material nanophotonics. Nat. Photonics. 2014, 8, 899–907. [Google Scholar] [CrossRef]
- Akinwande, D.; Petrone, N.; Hone, J. Two-dimensional flexible nanoelectronics. Nat. Commun. 2014, 5, 5678. [Google Scholar] [CrossRef] [PubMed]
- Cepellotti, A.; Fugallo, G.; Paulatto, L.; Lazzeri, M.; Mauri, F.; Marzari, N. Phonon hydrodynamics in two-dimensional materials. Nat. Commun. 2015, 6, 6400. [Google Scholar] [CrossRef] [PubMed]
- Doussal, P.L.; Radzihovsky, L. Self-consistent theory of polymerized membranes. Phys. Rev. Lett. 1992, 69, 1209–1212. [Google Scholar] [CrossRef] [PubMed]
- Büchner, C.; Wang, Z.-J.; Burson, K.M.; Willinger, M.G.; Heyde, M.; Schloegl, R.; Freund, H.-J. A Large-Area Transferable Wide Band Gap 2D Silicon Dioxide Layer. ACS Nano 2016, 10, 7982–7989. [Google Scholar] [CrossRef]
- Cassabois, G.; Valvin, P.; Gil, B. Hexagonal boron nitride is an indirect bandgap semiconductor. Nat. Photon. 2016, 10, 262–266. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Transition Metal Carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two- dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Khazaei, M.; Arai, M.; Sasaki, T.; Chung, C.-Y.; Venkataramanan, N.S.; Estili, M.; Sakka, Y.; Kawazoe, Y. Novel Electronic and Magnetic Properties of Two-Dimensional Transition Metal Carbides and Nitrides. Adv. Funct. Mater. 2013, 23, 2185–2192. [Google Scholar] [CrossRef]
- Ghidiu, M.; Naguib, M.; Shi, C.; Mashtalir, O.; Pan, L.; Zhang, B.; Yang, J.; Gogotsi, Y.; Billinge, S.J.; Barsoum, M.W. Synthesis and characterization of two-dimensional Nb4C3 (MXene). Chem. Commun. 2014, 50, 9517–9520. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Barsoum, M.W. Two-Dimensional, Ordered, Double Tran-sition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y. Chemical vapour deposition: Transition metal carbides go 2D. Nat. Mater. 2015, 14, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Şahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Monolayer honeycomb structures of group-IV elements and III-V binary compounds: First-principles calculations. Phys. Rev. B 2009, 80, 155453. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Computational identification of single-layer CdO for electronic and optical applications. Appl. Phys. Lett. 2013, 103, 212102. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.-B.; Chen, N.-K.; Xie, S.-Y.; Tian, W.Q.; Chen, Y.; Xia, H.; Zhang, S.B.; Sun, H.-B. Monolayer II-VI semiconductors: A first-principles prediction. Phys. Rev. B 2015, 92, 115307. [Google Scholar] [CrossRef]
- Lebegue, S.; Eriksson, O. Electronic structure of two-dimensional crystals from ab initio theory. Phys. Rev. B 2009, 79, 115409. [Google Scholar] [CrossRef]
- Ashton, M.; Paul, J.; Sinnott, S.B.; Hennig, R.G. Topology-Scaling Identification of Layered Solids and Stable Exfoliated 2D Materials. Phys. Rev. Lett. 2017, 118, 106101. [Google Scholar] [CrossRef]
- Zhou, L.; Kou, L.; Sun, Y.; Felser, C.; Frauenheim, T. New Family of Quantum Spin Hall Insulators in Two-dimensional Transition-Metal Halide with Large Nontrivial Band Gaps. Nano Lett. 2015, 15, 7867–7872. [Google Scholar] [CrossRef]
- Debbichi, L.; Kim, H.; Björkman, T.; Eriksson, O.; Lebègue, S. First-principles investigation of two-dimensional trichalcogenide and sesquichalcogenide monolayers. Phys. Rev. B 2016, 93, 245307. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, S.; Ryu, S.; Bhang, S.H.; Kim, J.; Yoon, J.K.; Park, Y.H.; Cho, S.P.; Lee, S.; Hong, B.H. Graphene-regulated cardiomyogenic differentiation process of mesenchymal stem cells by enhancing the expression of extracellular matrix proteins and cell signaling molecules. Adv. Healthc. Mater. 2014, 3, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; Oudjedi, L.; Piponnier, M.; Bussy, C.; Prato, M.; Kostarelos, K.; Lounis, B.; Bianco, A.; Cognet, L. Direct visualization of carbon nanotube degradation in primary cells by photothermal imaging. Nanoscale 2017, 9, 4642–4645. [Google Scholar] [CrossRef] [PubMed]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The Enzymatic Oxidation of Graphene Oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Kotchey, G.P.; Hasan, S.A.; Kapralov, A.A.; Ha, S.H.; Kim, K.; Shvedova, A.A.; Kagan, V.E.; Star, A. A Natural Vanishing Act: The Enzyme-Catalyzed Degradation of Carbon Nanomaterials. Accounts Chem. Res. 2012, 45, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Garadkar, K.; Patil, A.; Hankare, P.; Chate, P.; Sathe, D.; Delekar, S. MoS2: Preparation and their characterization. J. Alloys Compd. 2009, 487, 786–789. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M=Mo, Nb, W, Ta; X=S, Se, Te) monolayers. Physica B 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Amory, C.; Bernede, J.C.; Hamdadou, N. A study of textured non-stoichiometric MoTe2 thin films used as substrates for textured stoichiometric MoS2 thin films. Vacuum 2004, 74, 117. [Google Scholar] [CrossRef]
- Pütz, J.; Aegerter, M.A. Spin deposition of MoSx thin films. Thin Solid Films 1999, 351, 119–124. [Google Scholar] [CrossRef]
- Jäger-Waldau, A.; Lux-Steiner, M.; Jäger-Waldau, R.; Burkhardt, R.; Bucher, E. Composition and morphology of MoSe2 thin films. Thin Solid Films 1990, 189, 339–345. [Google Scholar] [CrossRef]
- Regula, M.; Ballif, C.; Moser, J.; Levy, F. Structural, chemical, and electrical characterisation of reactively sputtered WSx thin films. Thin Solid Films 1996, 280, 67–75. [Google Scholar] [CrossRef]
- Fleischauer, P.D. Fundamental aspects of the electronic structure, materials properties and lubri-cation performance of sputtered MoS2 films. Thin Solid Films 1987, 154, 309–322. [Google Scholar] [CrossRef]
- Delphine, S.M.; Jayachandran, M.; Sanjeeviraja, C. Pulsed electrodeposition and characterization of molybdenum diselenide thin film. Mater. Res. Bull. 2005, 40, 135–147. [Google Scholar] [CrossRef]
- Mccain, M.N.; He, B.; Sanati, J.; Wang, Q.J.; Marks, T.J. Aerosol-Assisted Chemical Vapor Deposition of Lubricating MoS2 Films. Ferrous Substrates and Titanium Film Doping. Chem. Mater. 2013, 20, 5438–5443. [Google Scholar] [CrossRef]
- Jiménez, D. Drift-diffusion model for single layer transition metal dichalcogenide field-effect transistors. Appl. Phys. Lett. 2012, 101, 243501. [Google Scholar] [CrossRef]
- Cao, W.; Kang, J.; Liu, W.; Banerjee, K. A Compact Current–Voltage Model for 2D Semiconductor Based Field-Effect Transistors Considering Interface Traps, Mobility Degradation, and Inefficient Doping Effect. IEEE Trans. Electron. Devices 2014, 61, 4282–4290. [Google Scholar] [CrossRef]
- Najam, F.; Tan, M.L.P.; Ismail, R.; Yu, Y.S. Two-dimensional (2D) transition metal dichalcogenide semiconductor field-effect transistors: The interface trap density extraction and compact model. Semicond. Sci. Technol. 2015, 30, 075010. [Google Scholar] [CrossRef]
- Wang, Z.; von dem Bussche, A.; Qiu, Y.; Valentin, T.M.; Gion, K.; Kane, A.B.; Hurt, R.H. Chemical dissolution pathways of MoS2 nanosheets in biological and environmental media. Environ. Sci. Technol. 2016, 50, 7208–7217. [Google Scholar] [CrossRef]
- Ivanovskii, A.L.; Enyashin, A.N. Graphene-like transition-metal nanocarbides and nanonitrides. Russ. Chem. Rev. 2013, 82, 735–746. [Google Scholar] [CrossRef]
- Zha, X.J.; Zhao, X.; Pu, J.H.; Tang, L.S.; Ke, K.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Yang, M.B.; Yang, W. Flexible Anti-Biofouling MXene/Cellulose Fibrous Membrane for Sustainable Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2019, 11, 36589–36597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Zhou, Z. MXene-based materials for electrochemical energy storage. J. Energy Chem. 2018, 27, 73–85. [Google Scholar] [CrossRef]
- Sun, S.; Chan, L.; Hafez, A.M.; Zhu, H.; Wu, S.J.C.E.J. Two-dimensional MXenes for Energy Storage. Chem. Eng. J. 2017, 338, 22–36. [Google Scholar] [CrossRef]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-Based Composites: Synthesis and Applications in Rechargeable Batteries and Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Shein, I.R.; Ivanovskii, A.L. Graphene-like titanium carbides and nitrides Tin+1Cn,Tin+1Nn (n=1, 2, and 3) from de-intercalated MAX phases: First-principles probing of their structural, electronic propertiesand relative stability. Comp. Mater. Sci. 2012, 65, 104–114. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Ghorbani-Asl, M.; Arai, M.; Sasaki, T.; Liang, Y.; Yunoki, S. Nearly free electron states in MXenes. Phys. Rev. B. 2016, 93, 205125. [Google Scholar] [CrossRef]
- Kurtoglu, M.; Naguib, M.; Gogotsi, Y.; Barsoum, M.W. First principles study of two-dimensional early transition metal carbides. MRS Commun. 2012, 2, 133–137. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; Wu, Q.; Ye, X.; Zhou, A.; Sun, D.; Wang, L.; Liu, B.; He, J. Two-dimensional Sc2C: A reversible and high-capacity hydrogen storage material predicted by first-principles calculations. Int. J. Hydrog. Energy 2014, 39, 10606–10612. [Google Scholar] [CrossRef]
- Shibata, Y.; Tsutsumi, J.y.; Matsuoka, S.; Matsubara, K.; Yoshida, Y.; Chikamatsu, M.; Hasegawa, T. Uniaxially oriented polycrystalline thin films and air-stable n-type transistors based on donor-acceptor semiconductor (diC8BTBT)(FnTCNQ) [n = 0, 2, 4]. Appl. Phys. Lett. 2015, 106, 1–4. [Google Scholar] [CrossRef]
- Yang, T.; Liang, B.; Cheng, Z.; Li, C.; Lu, G.; Wang, Y. Construction of Efficient Deep-Red/Near-Infrared Emitter Based on a Large π-Conjugated Acceptor and Delayed Fluorescence OLEDs with External Quantum Efficiency of over 20%. J. Phys. Chem. C 2019, 123, 18585–18592. [Google Scholar] [CrossRef]
- Wu, C.; Wu, T.; Yang, Y.; McLeod, J.A.; Wang, Y.; Zou, Y.; Zhai, T.; Li, J.; Ban, M.; Song, T.; et al. Alternative Type Two-Dimensional-Three-Dimensional Lead Halide Perovskite with Inorganic Sodium Ions as a Spacer for High-Performance Light-Emitting Diodes. ACS Nano 2019, 13, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Wu, Q.; Song, F.; Sun, J.; Yang, M.; Ma, X.; Wang, H.; Hao, Y. A bio-inspired physically transient/biodegradable synapse for security neuromorphic computing based on memristors. Nanoscale 2018, 10, 20089–20095. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, T.; Campos, A.; Mas-Torrent, M. Solution-processed thin films of a charge transfer complex for ambipolar field-effect transistors. J. Mater. Chem. C 2019, 7, 10257–10263. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Yin, L. Materials and Devices for Biodegradable and Soft Biomedical Electronics. Materials 2018, 11, 2108. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin Film Composite Membrane for OilyWaste Water Treatment: Recent Advances and Challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Matsuura, T. Thin Film Composite and/or Thin Film Nanocomposite Hollow Fiber Membrane for Water Treatment, Pervaporation, and Gas/Vapor Separation. Polymers 2018, 10, 1050. [Google Scholar] [CrossRef]
- Pang, Y.; Xi, F.; Luo, J.; Liu, G.; Guo, T.; Zhang, C. An alginate film-based degradable triboelectric nanogenerator. RSC Adv. 2018, 8, 6719–6726. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Zhan, Y.; Ding, X.; Wang, M.; Wang, X. Preparing the Degradable, Flame-Retardant and Low Dielectric Constant Nanocomposites for Flexible and Miniaturized Electronics with Poly(lactic acid), Nano ZIF-8@GO and Resorcinol Di(phenyl phosphate). Materials 2018, 11, 1756. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, L.Q.; Wang, W.; Hui, X.; Liu, Z.P.; Wan, Q. Biodegradable oxide synaptic transistors gated by a biopolymer electrolyte. J. Mater. Chem. C 2016, 4, 7744–7750. [Google Scholar] [CrossRef]
- Aldana, A.A.; Toselli, R.; Strumia, M.C.; Martinelli, M. Chitosan films modified selectively on oneside with dendritic molecules. J. Mater. Chem. 2012, 22, 22670–22677. [Google Scholar] [CrossRef]
- Liang, D.-K.; Chen, Y.-H.; Xu, W.; Ji, X.-C.; Tong, Y.; Wu, G.-D. Ultralow-voltage albumen-gated electric-double-layer thin film transistors. Acta Phys. Sin. 2018, 67, 237302. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Li, H.; Zhang, C.; Fan, J.; Mai, Y. C60 additive-assisted crystallization in CH3NH3Pb0.75Sn0.25I3 perovskite solar cells with high stability and efficiency. Nanoscale 2017, 9, 13967–13975. [Google Scholar] [CrossRef] [PubMed]
- Azarian, M.H.; Boochathum, P.; Kongsema, M. Biocompatibility and biodegradability of filler encapsulated chloroacetated natural rubber/polyvinyl alcohol nanofiber for wound dressing. Mater. Sci. Eng. C 2019, 103, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.N.; Jaafar, J.; Ismail, A.F.; Abd Mutalib, M.; Jamil, S.M. Incorporation of N-doped TiO2 nanorods in regenerated cellulose thin films fabricated from recycled newspaper as a green portable photocatalyst. Carbohydr. Polym. 2015, 133, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ay, S.B.; Perkgoz, N.K. Nanotechnological Advances in Catalytic Thin Films for Green Large-Area Surfaces. J. Nanomater. 2015, 2015, 1–20. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2, 2375–2548. [Google Scholar] [CrossRef]
- Shi, X.; Liao, Y.-M.; Lin, H.-Y.; Tsao, P.-W.; Wu, M.-J.; Lin, S.-Y.; Hu, H.-H.; Wang, Z.; Lin, T.-Y.; Lai, Y.-C. Dissolvable and Recyclable Random Lasers. ACS Nano 2017, 11, 7600–7607. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Jin, L.; Li, Y.; Li, J.; Gan, G.; Wei, M.; Li, M.; Liao, Y. Controllably degradable transient electronic antennas based on water-soluble PVA/TiO2 films. J. Mater. Sci. 2017, 53, 2638–2647. [Google Scholar] [CrossRef]
- Park, M.-J.; Lee, J.-S. Foldable and Biodegradable Energy-Storage Devices on Copy Papers. Adv. Electron. Mater. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Troshin, P.A.; Reisinger, M.; Shmygleva, L.; Kanbur, Y.; Schwabegger, G.; Bodea, M.; Schwödiauer, R.; Mumyatov, A.; Fergus, J.W.; et al. Biocompatible and Biodegradable Materials for Organic Field-Effect Transistors. Adv. Funct. Mater. 2010, 20, 4069–4076. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Kim, D.-H.; Tao, H.; Kim, T.-i.; Kim, S.; Yu, K.J.; Panilaitis, B.; Jeong, J.-W.; Song, J.-K.; Omenetto, F.G.; et al. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater. 2013, 23, 4087–4093. [Google Scholar] [CrossRef]
- Guo, J.; Liu, J.; Yang, B.; Zhan, G.; Kang, X.; Tian, H.; Tang, L.; Chen, X.; Yang, C. Low-voltage transient/biodegradable transistors based on free-standing sodium alginate membranes. IEEE Electron Device Lett. 2015, 36, 576–578. [Google Scholar] [CrossRef]
- Chua, L. Memristor: The missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Hickmott, T.W. Low-Frequency Negative Resistance in Thin Anodic Oxide Films. J. Appl. Phys. 1962, 33, 2669–2682. [Google Scholar] [CrossRef]
- Gibbons, J.F.; Beadle, W.E. Switching properties of thin NiO films. Solid State Electron. 1964, 7, 785–790. [Google Scholar] [CrossRef]
- Kwon, D.-H.; Kim, K.M.; Jang, J.H.; Jeon, J.M.; Lee, M.H.; Kim, G.H.; Li, X.-S.; Park, G.-S.; Lee, B.; Han, S.; et al. Atomic structure of conducting nanofilaments in TiO2 resistive switching memory. Nat. Nanotechnol. 2010, 5, 148–153. [Google Scholar] [CrossRef]
- Chang, W.-Y.; Lai, Y.-C.; Wu, T.-B.; Wang, S.-F.; Chen, F.; Tsai, M.-J. Unipolar resistive switching characteristics of ZnO thin films for nonvolatile memory applications. Appl. Phys. Lett. 2008, 92, 022110. [Google Scholar] [CrossRef]
- Jeong, K.W.; Do, Y.H.; Yoon, K.S.; Kim, C.O.; Hong, J.P. Resistive switching characteristics of unique binary-oxide MgOx films. J. Korean Phys. Soc. 2006, 48, 1501–1504. [Google Scholar]
- Kim, S.; Biju, K.P.; Jo, M.; Jung, S.; Hwang, H. Effect of Scaling WOx-Based RRAMs on Their Resistive Switching Characteristics. IEEE Electron Device Lett. 2011, 32, 671–673. [Google Scholar] [CrossRef]
- Asamitsu, A.; Tomioka, Y.; Kuwahara, H.; Tokura, Y. Current switching of resistive states in magnetoresistive manganites. Nature 1997, 388, 50–52. [Google Scholar] [CrossRef]
- Strukov, D.B.; Snider, G.S.; Stewart, D.R.; Williams, R.S. The missing memristor found. Nature 2008, 453, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ji, X.; Song, L.; Zhang, Y.; Zhao, R. Enabling Transient Electronics with Degradation on Demand via Light-Responsive Encapsulation of a Hydrogel-Oxide Bilayer. ACS Appl. Mater. Interfaces 2018, 10, 36171–36176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tsang, M.; Chen, I.W. Biodegradable resistive switching memory based on magnesium difluoride. Nanoscale 2016, 8, 15048–15055. [Google Scholar] [CrossRef]
- Hosseini, N.R.; Lee, J.-S. Biocompatible and Flexible Chitosan-Based Resistive Switching Memory with Magnesium Electrodes. Adv. Funct. Mater. 2015, 25, 5586–5592. [Google Scholar] [CrossRef]
- Wu, W.; Han, S.-T.; Venkatesh, S.; Sun, Q.; Peng, H.; Zhou, Y.; Yeung, C.; Li, R.K.Y.; Roy, V.A.L. Biodegradable skin-inspired nonvolatile resistive switching memory based on gold nanoparticles embedded alkali lignin. Org. Electron. 2018, 59, 382–388. [Google Scholar] [CrossRef]
- Song, F.; Wang, H.; Sun, J.; Gao, H.; Wu, S.; Yang, M.; Ma, X.; Hao, Y. ZnO-Based Physically Transient and Bioresorbable Memory on Silk Protein. IEEE Electron Device Lett. 2018, 39, 31–34. [Google Scholar] [CrossRef]
- Liu, D.; Jing, Q.; Cheng, H. Synaptic-functional and fully water-soluble transient memristor made from materials compatible with semiconductor technology. Jpn. J. Appl. Phys. 2019, 58, 060903. [Google Scholar] [CrossRef]
- Zhong, S.; Ji, X.; Zhou, Y.; Zhang, Y.; Ye, A.; Zhao, R. CMOS Compatible Transient Resistive Memory with Prolonged Lifetime. Adv. Mater. Technol. 2019, 4, 1900217. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Wang, Z.; Song, F.; Zhu, Q.; Dang, B.; Gao, H.; Yang, M.; Ma, X.; Hao, Y. Physically Transient Memristive Synapse With Short-Term Plasticity Based on Magnesium Oxide. IEEE Electron Device Lett. 2019, 40, 706–709. [Google Scholar] [CrossRef]
- Ji, X.; Song, L.; Zhong, S.; Jiang, Y.; Lim, K.G.; Wang, C.; Zhao, R. Biodegradable and Flexible Resistive Memory for Transient Electronics. J. Phys. Chem. C 2018, 122, 16909–16915. [Google Scholar] [CrossRef]
- Yan, X.; Li, X.; Zhou, Z.; Zhao, J.; Wang, H.; Wang, J.; Zhang, L.; Ren, D.; Zhang, X.; Chen, J.; et al. Flexible Transparent Organic Artificial Synapse Based on the Tungsten/Egg Albumen/Indium Tin Oxide/Polyethylene Terephthalate Memristor. ACS Appl. Mater. Interfaces 2019, 11, 18654–18661. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Ma, X.; Hao, Y.; Chen, X. Physically Transient Resistive Switching Memory Based on Silk Protein. Small 2016, 12, 2715–2719. [Google Scholar] [CrossRef] [PubMed]
| Group | Transient Resistive-Switching Structure | Trigger | Application |
|---|---|---|---|
| Dang B. [173] | W/MgO/ZnO/Mo | PBS a or DI water b | Bio-inspired artificial synapse |
| Zhong S. [203] | Mg/MgO/W | DI water or PBS under ultraviolet light | RRAM c |
| Song F. [207] | Mg/ZnO/W and Mg/ZnO/Mg with silk fibroin substrates | DI water or PBS | Memory |
| Liu D. [208] | Cu/a-Si d/W on a poly(vinyl alcohol) substrate | Water | Memristor |
| Song F. [100] | Mg/MgO/W | Water | RRAM |
| Zhong S. [209] | W/Cu/SiO2/W | Water or PBS | Resistive memory |
| Zhang Z. [204] | Fe/MgF2/Mg | DI water | RRAM |
| Sun J. [210] | W/Ag/MgO/Ag/W | DI water | Memristive Synapse |
| Liu D. [8] | Ni/MgO/Ni on SiO2/Si substrate | DI water after exfoliating the substrate | Nonvolatile bipolar memory |
| Liu R. [180] | InZnO/Chitosan/ITO/Graphene/PET | DI water | synaptic transistor |
| Li Q. [80] | Ag/CsPbBr3/PEDOT:PSS/ITO | DI water | Nonvolatile memory |
| Hosseini, N.R. [205] | Mg/Ag-doped chitosan/Mg on plastic substrate | Water | Nonvolatile memory |
| Wu W. [206] | Al/Au NPs: alkali lignin e/Al | Proteinase K aqueous solution | Nonvolatile memory |
| Ji X. [211] | W/silk fibroin/Mg | PBS | Memory |
| Yan X. [212] | W/egg albumen/ITO/PET f | Water | Artificial synaptic device |
| Wang H. [213] | Au/Mg/fibroin/Mg | DI water or PBS | resistive-switching memory |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shi, M.; Luo, Y.; Zhou, L.; Loh, Z.R.; Oon, Z.J.; Lian, X.; Wan, X.; Chong, F.B.L.; Tong, Y. Degradable and Dissolvable Thin-Film Materials for the Applications of New-Generation Environmental-Friendly Electronic Devices. Appl. Sci. 2020, 10, 1320. https://doi.org/10.3390/app10041320
Liu X, Shi M, Luo Y, Zhou L, Loh ZR, Oon ZJ, Lian X, Wan X, Chong FBL, Tong Y. Degradable and Dissolvable Thin-Film Materials for the Applications of New-Generation Environmental-Friendly Electronic Devices. Applied Sciences. 2020; 10(4):1320. https://doi.org/10.3390/app10041320
Chicago/Turabian StyleLiu, Xiaoyan, Mingmin Shi, Yuhao Luo, Lvyang Zhou, Zhi Rong Loh, Zhi Jian Oon, Xiaojuan Lian, Xiang Wan, Fred Beng Leng Chong, and Yi Tong. 2020. "Degradable and Dissolvable Thin-Film Materials for the Applications of New-Generation Environmental-Friendly Electronic Devices" Applied Sciences 10, no. 4: 1320. https://doi.org/10.3390/app10041320
APA StyleLiu, X., Shi, M., Luo, Y., Zhou, L., Loh, Z. R., Oon, Z. J., Lian, X., Wan, X., Chong, F. B. L., & Tong, Y. (2020). Degradable and Dissolvable Thin-Film Materials for the Applications of New-Generation Environmental-Friendly Electronic Devices. Applied Sciences, 10(4), 1320. https://doi.org/10.3390/app10041320





