Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Thiourea and Calcium Citrate for Lithium-Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
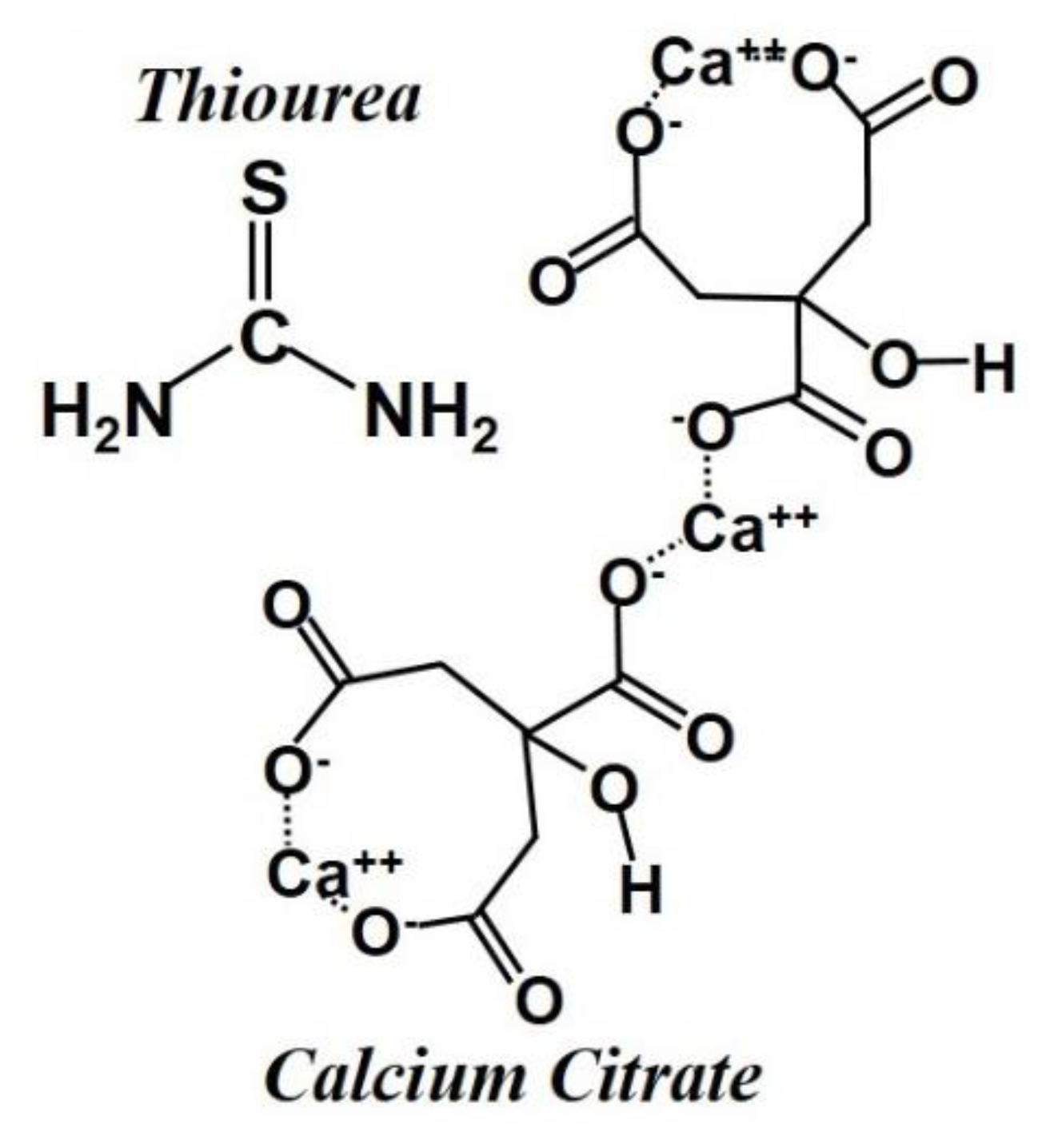
2.1. Synthesis of the Mesoporous Carbon–Sulfur Composites
2.2. Characterization
2.3. Fabrication of Electrode and Electrochemical Measurements
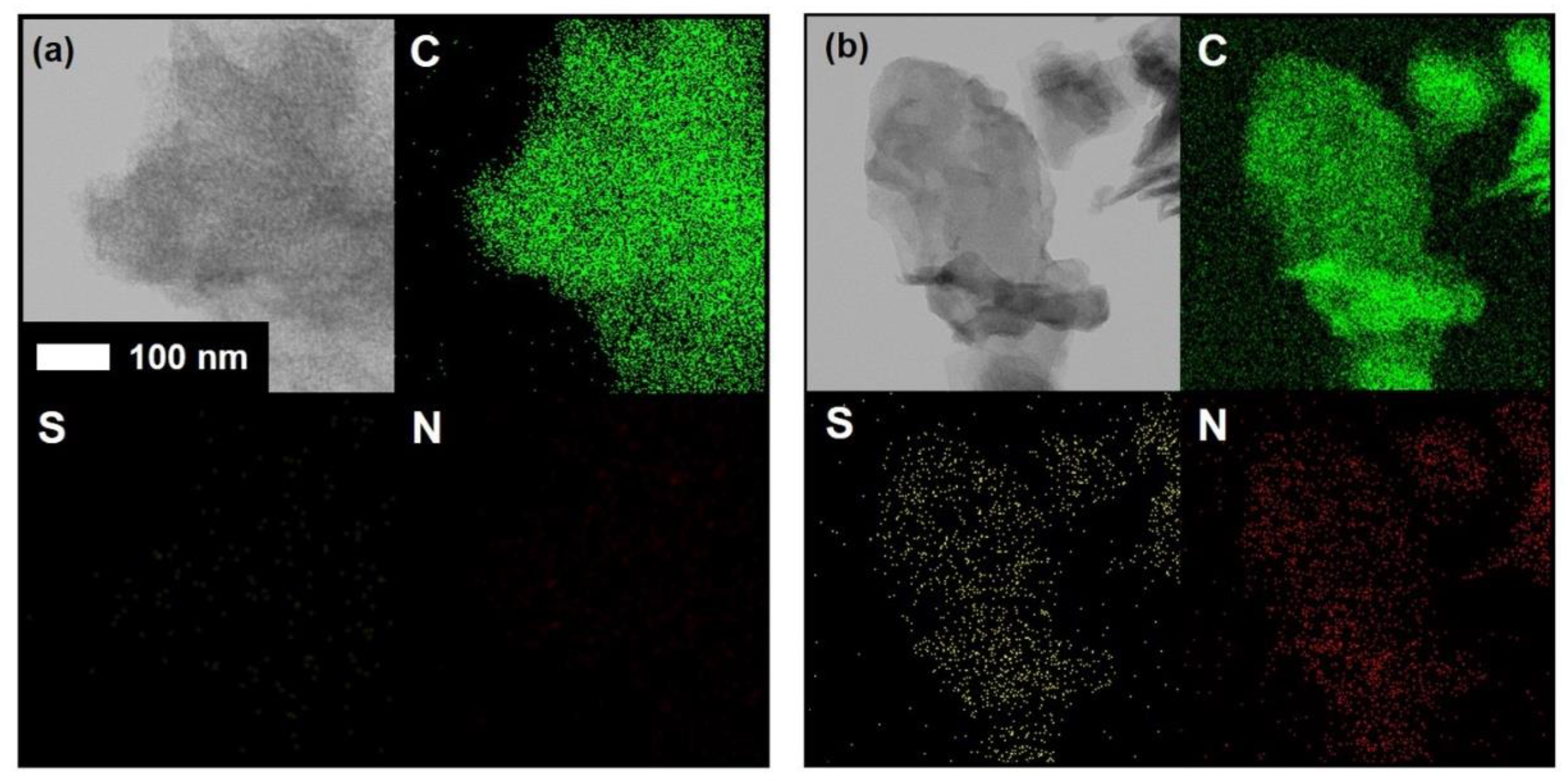
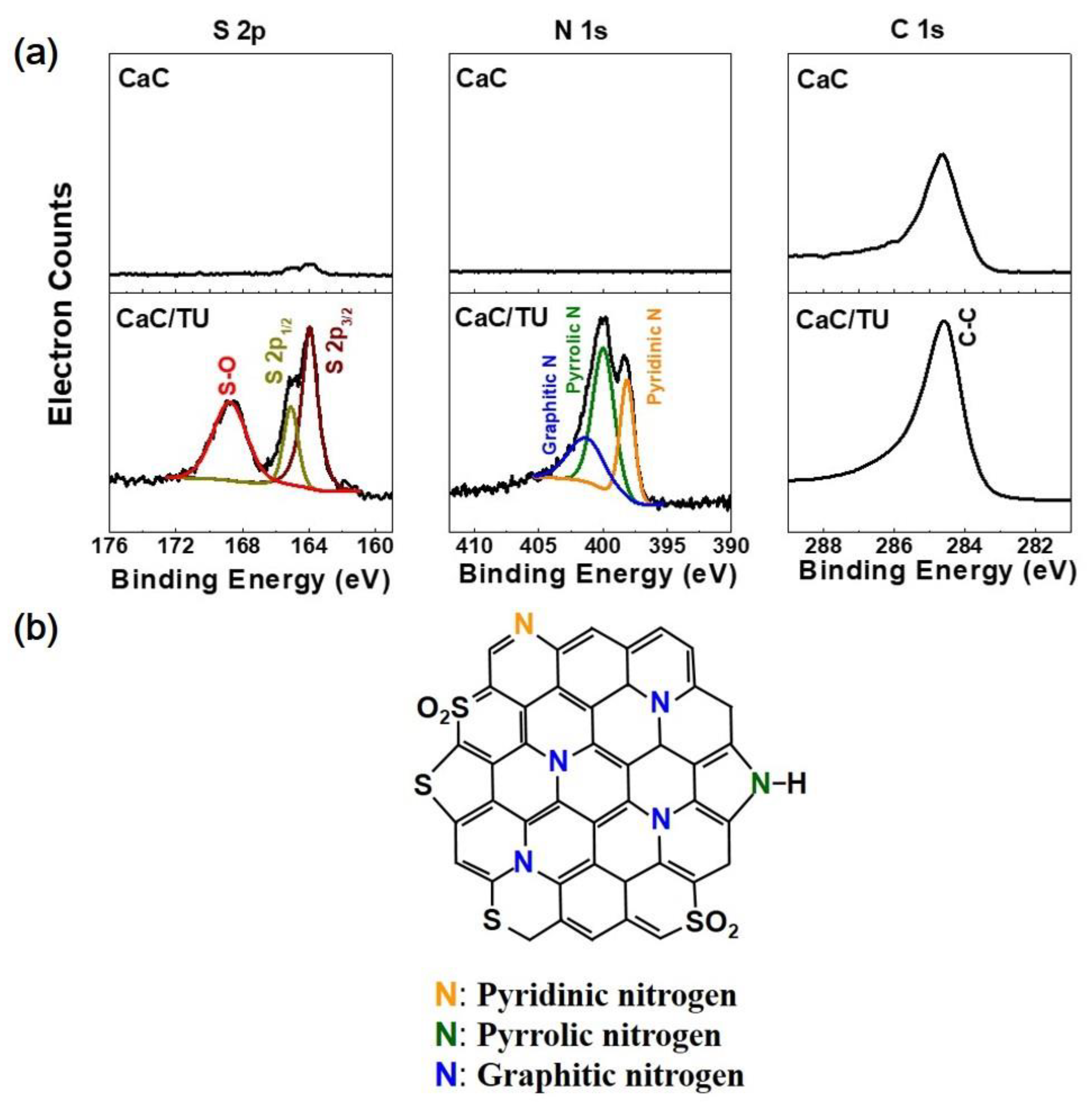
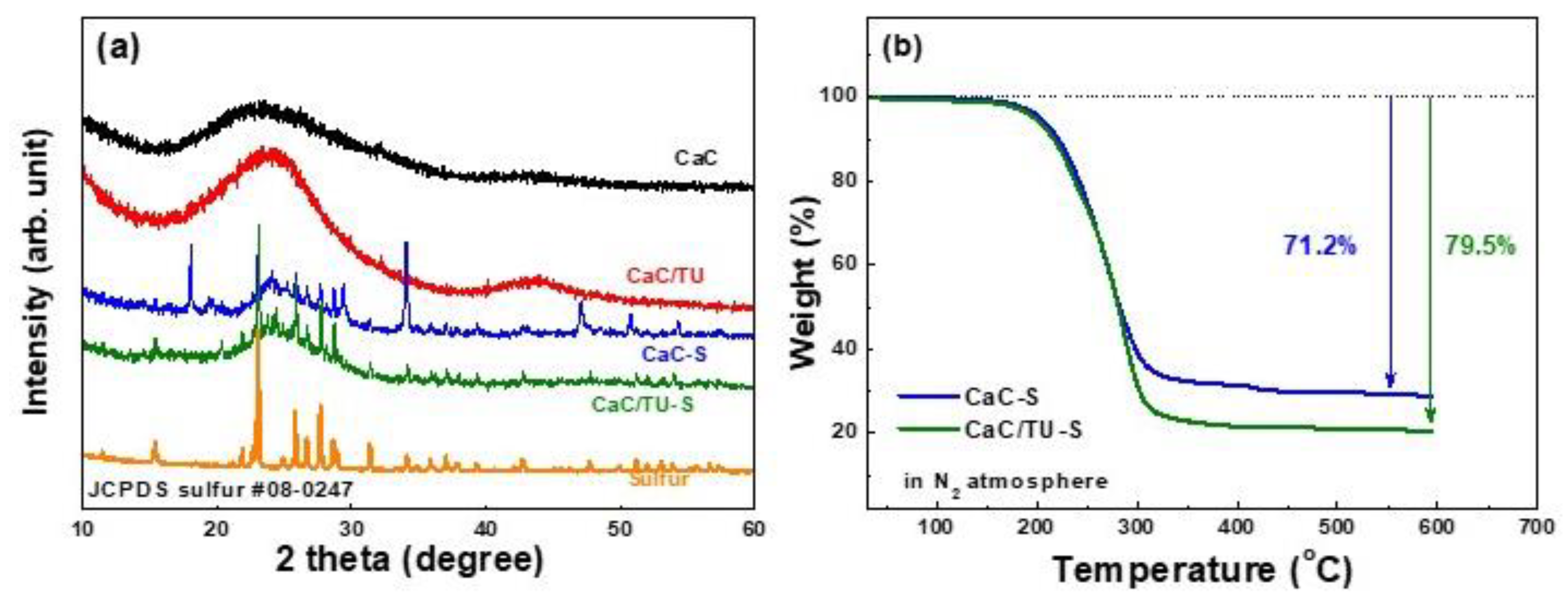
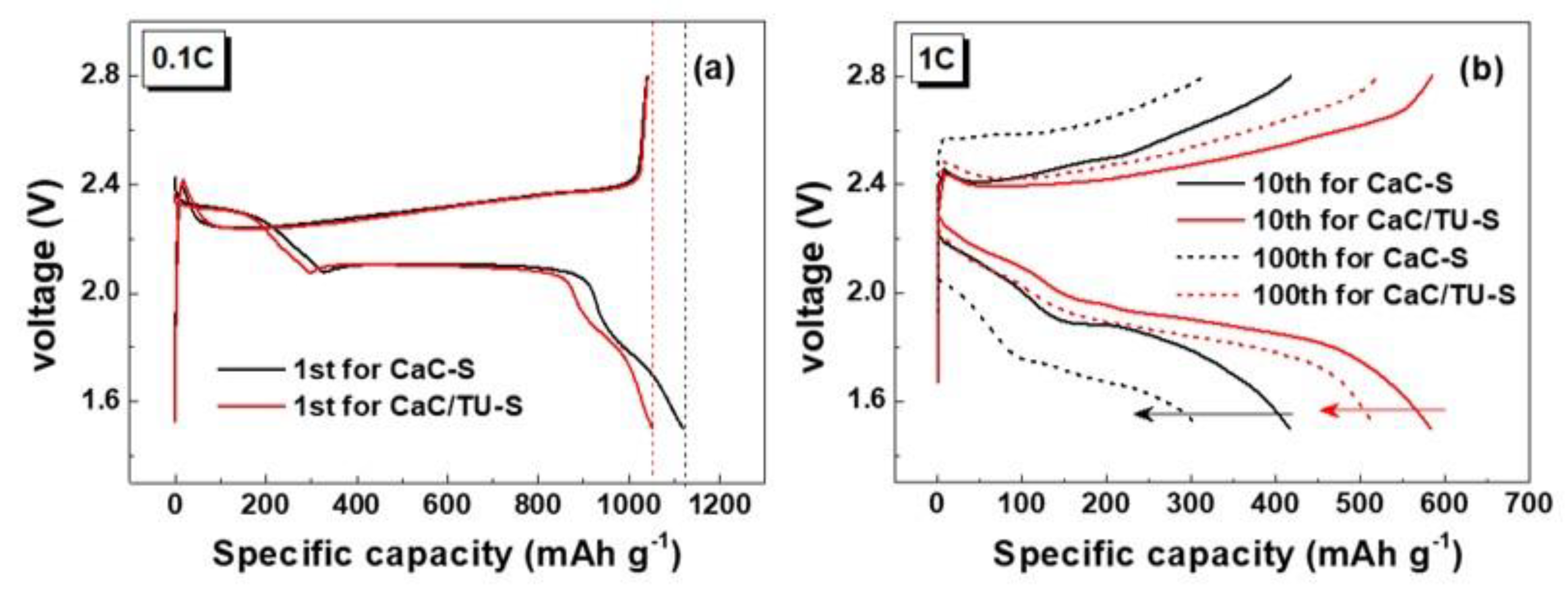
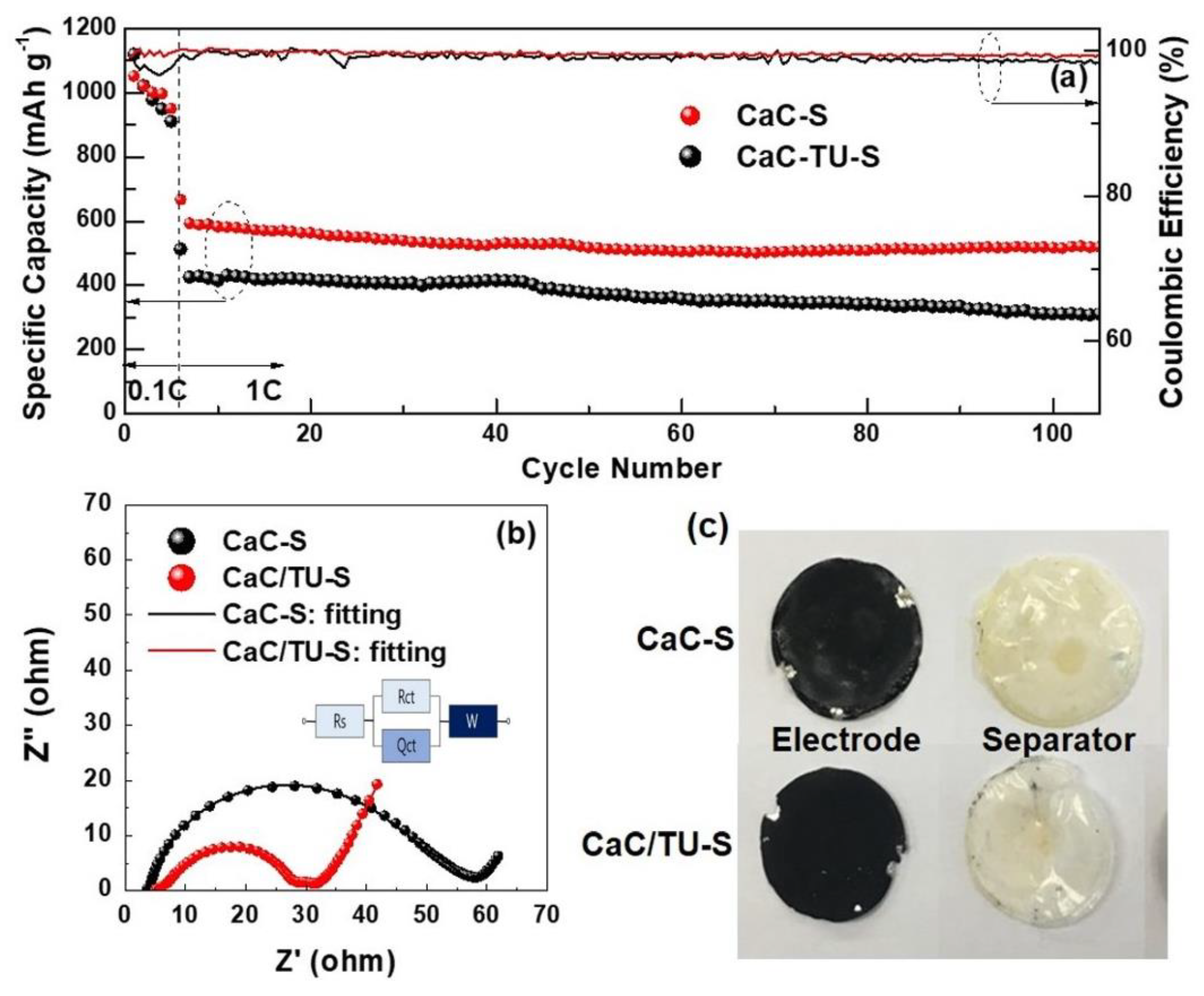
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, X.; Nazar, L.F. Advances in Li–S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zeng, Q.; Zhou, G.; Yin, L.; Li, F.; Cheng, H.-M.; Gentle, I.R.; Lu, G.Q.M. Carbon–sulfur composites for Li–S batteries: Status and prospects. J. Mater. Chem. A 2013, 1, 9382–9394. [Google Scholar] [CrossRef]
- Oh, C.; Yoon, N.; Choi, J.; Choi, Y.; Ahn, S.; Lee, J.K. Enhanced Li–S battery performance based on solution-impregnation-assisted sulfur/mesoporous carbon cathodes and a carbon-coated separator. J. Mater. Chem. A 2017, 5, 5750–5760. [Google Scholar] [CrossRef]
- Ferrero, G.; Sevilla, M.; Fuertes, A. Mesoporous carbons synthesized by direct carbonization of citrate salts for use as high-performance capacitors. Carbon 2015, 88, 239–251. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, K.; Dokko, K.; Watanabe, M. Recent advances in electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 2015, 5, 1500117. [Google Scholar] [CrossRef]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 10663–10665. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathode for rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef]
- Gao, Y.-P.; Zhai, Z.-B.; Huang, K.-J.; Zhang, Y.-Y. Energy storage applications of biomass-derived carbon materials: Batteries and supercapacitors. New J. Chem. 2017, 41, 11456–11470. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Chen, X.Y.; Wang, B. An activation-free protocol for preparing porous carbon from calcium citrate and the capacitive performance. Microporous Mesoporous Mater. 2012, 158, 155–161. [Google Scholar] [CrossRef]
- Fuertes, A.; Ferrero, G.; Sevilla, M. One-pot synthesis of microporous carbons highly enriched in nitrogen and their electrochemical performance. J. Mater. Chem. A 2014, 2, 14439–14448. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: an overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- See, K.A.; Jun, Y.-S.; Gerbec, J.A.; Sprafke, J.K.; Wudl, F.; Stucky, G.D.; Seshadri, R. Sulfur-functionalized mesoporous carbons as sulfur hosts in Li–S batteries: Increasing the affinity of polysulfide intermediates to enhance performance. ACS Appl. Mater. Interfaces 2014, 6, 10908–10916. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.-H.; Kang, F. N and S co-doped porous carbon spheres prepared using l-cysteine as a dual functional agent for high-performance lithium–sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Morishita, T.; Ishihara, K.; Kato, M.; Inagaki, M. Preparation of a carbon with a 2 nm pore size and of a carbon with a bi-modal pore size distribution. Carbon 2007, 45, 209–211. [Google Scholar] [CrossRef]
- Morishita, T.; Ishihara, K.; Kato, M.; Tsumura, T.; Inagaki, M. Mesoporous carbons prepared from mixtures of magnesium citrate with poly (vinyl alcohol). Tanso 2007, 2007, 19–24. [Google Scholar] [CrossRef]
- Branton, P.J.; Hall, P.G.; Sing, K.S.; Reichert, H.; Schüth, F.; Unger, K.K. Physisorption of argon, nitrogen and oxygen by MCM-41, a model mesoporous adsorbent. J. Chem. Soc. Faraday Trans. 1994, 90, 2965–2967. [Google Scholar] [CrossRef]
- Yuan, S.; Bao, J.L.; Wang, L.; Xia, Y.; Truhlar, D.G.; Wang, Y. Graphene-Supported Nitrogen and Boron Rich Carbon Layer for Improved Performance of Lithium–Sulfur Batteries Due to Enhanced Chemisorption of Lithium Polysulfides. Adv. Energy Mater. 2016, 6, 1501733. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Tian, Y.; Su, Y.; Wang, J.; Yang, W.; Li, N.; Chen, S.; Bao, L. 3D coral-like nitrogen-sulfur co-doped carbon-sulfur composite for high performance lithium-sulfur batteries. Sci. Rep. 2015, 5, 13340. [Google Scholar] [CrossRef] [PubMed]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen-and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design principles for heteroatom-doped nanocarbon to achieve strong anchoring of polysulfides for lithium–sulfur batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, X.; Li, G.R.; Gao, X.P. Enhancement of long stability of sulfur cathode by encapsulating sulfur into micropores of carbon spheres. Energy Environ. Sci. 2010, 3, 1531. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Tian, T.; Cao, S.; Coxon, P.R.; Xi, K.; Fairen-Jimenez, D.; Vasant Kumar, R.; Cheetham, A.K. Graphene-wrapped sulfur/metal organic framework-derived microporous carbon composite for lithium sulfur batteries. APL Mater. 2014, 2, 124109. [Google Scholar] [CrossRef]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium—Sulfur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Zhu, L.; Yang, C. Microporous carbon derived from Apricot shell as cathode material for lithium–sulfur battery. Microporous Mesoporous Mater. 2015, 204, 235–241. [Google Scholar] [CrossRef]
- Xue, M.; Chen, C.; Tan, Y.; Ren, Z.; Li, B.; Zhang, C. Mangosteen peel-derived porous carbon: Synthesis and its application in the sulfur cathode for lithium sulfur battery. J. Mater. Sci. 2018, 53, 11062–11077. [Google Scholar] [CrossRef]
- Vu, D.-L.; Seo, J.-S.; Lee, H.-Y.; Lee, J.-W. Activated carbon with hierarchical micro–mesoporous structure obtained from rice husk and its application for lithium–sulfur batteries. RSC Adv. 2017, 7, 4144–4151. [Google Scholar] [CrossRef]
- Wang, D.; Fu, A.; Li, H.; Wang, Y.; Guo, P.; Liu, J.; Zhao, X.S. Mesoporous carbon spheres with controlled porosity for high-performance lithium-sulfur batteries. J. Power Sources 2015, 285, 469–477. [Google Scholar] [CrossRef]

| CaC | CaC/TU | |
|---|---|---|
| S.A., a (m2 g−1) | 816 | 929 |
| VP, b (cm3 g−1) | 1.39 | 1.76 |
| DP, c (nm) | 7.20 | 7.30 |
| Material for Carbonization | Activation Temperature (°C) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| Pomelo peel | 600 | 1533 | 0.837 | C dis = 750 mAh g−1 Cycle number = 100 Rate = 0.2 C | [29] |
| Apricot shells | 750 | 2269 | 1.05 | C dis = 613 mAh g−1 Cycle number = 200 Rate = 1 C | [30] |
| Mangosteen peel | 800 | 3244 | 1.56 | C dis = 509 mAh g−1 Cycle number = 500 Rate = 0.5 C | [31] |
| Wheat straw | 850 | 1066 | 0.62 | C dis = 445 mAh g−1 Cycle number = 200 Rate = 1 C | [32] |
| Sodium alginate | No activation | 1270 | 4.1 | C dis = 857 mAh g−1 Cycle number = 100 Rate = 0.2 C | [33] |
| Calcium citrate/Thiourea | No activation | 929 | 1.76 | C dis = 590 mAh g−1 Cycle number = 100 Rate = 1 C | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, S.-Y.; Bae, J.-S.; Lee, S.-J.; Kim, H.K.; Jeong, E.D.; Shin, H.-C. Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Thiourea and Calcium Citrate for Lithium-Sulfur Batteries. Appl. Sci. 2020, 10, 1263. https://doi.org/10.3390/app10041263
Choi Y, Lee S-Y, Bae J-S, Lee S-J, Kim HK, Jeong ED, Shin H-C. Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Thiourea and Calcium Citrate for Lithium-Sulfur Batteries. Applied Sciences. 2020; 10(4):1263. https://doi.org/10.3390/app10041263
Chicago/Turabian StyleChoi, Yunju, Sun-Young Lee, Jong-Seong Bae, Sea-Jin Lee, Hyun Kyu Kim, Euh Duck Jeong, and Heon-Cheol Shin. 2020. "Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Thiourea and Calcium Citrate for Lithium-Sulfur Batteries" Applied Sciences 10, no. 4: 1263. https://doi.org/10.3390/app10041263
APA StyleChoi, Y., Lee, S.-Y., Bae, J.-S., Lee, S.-J., Kim, H. K., Jeong, E. D., & Shin, H.-C. (2020). Nitrogen and Sulfur Co-Doped Porous Carbon Derived from Thiourea and Calcium Citrate for Lithium-Sulfur Batteries. Applied Sciences, 10(4), 1263. https://doi.org/10.3390/app10041263




