Investigation of Airborne Molecular Contamination in Cleanroom Air Environment through Portable Soft X-Ray Radiolysis Detector
Abstract
1. Introduction
2. Materials and Methods
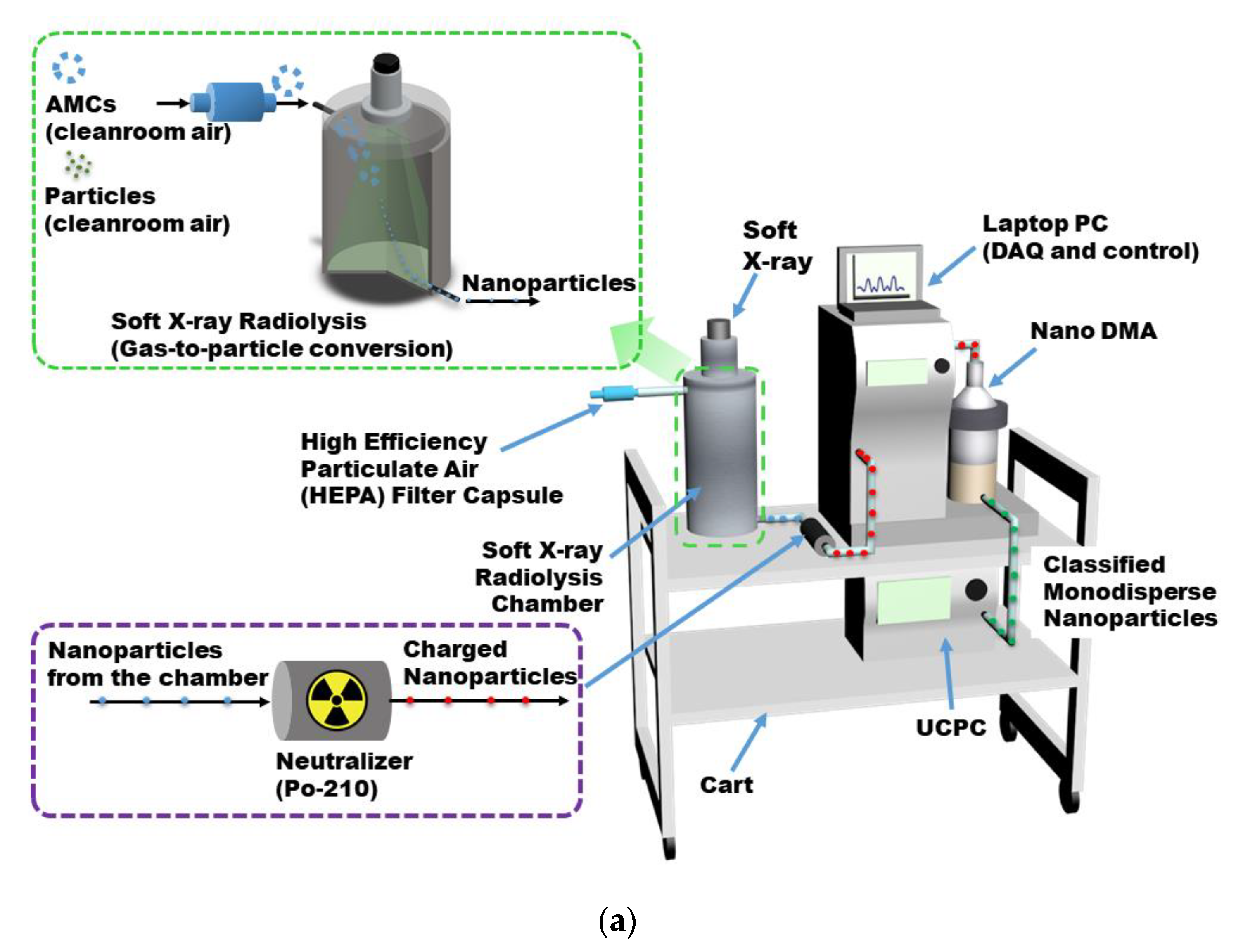
2.1. Portable AMC Detector Deploying Soft X-Ray Radiolysis
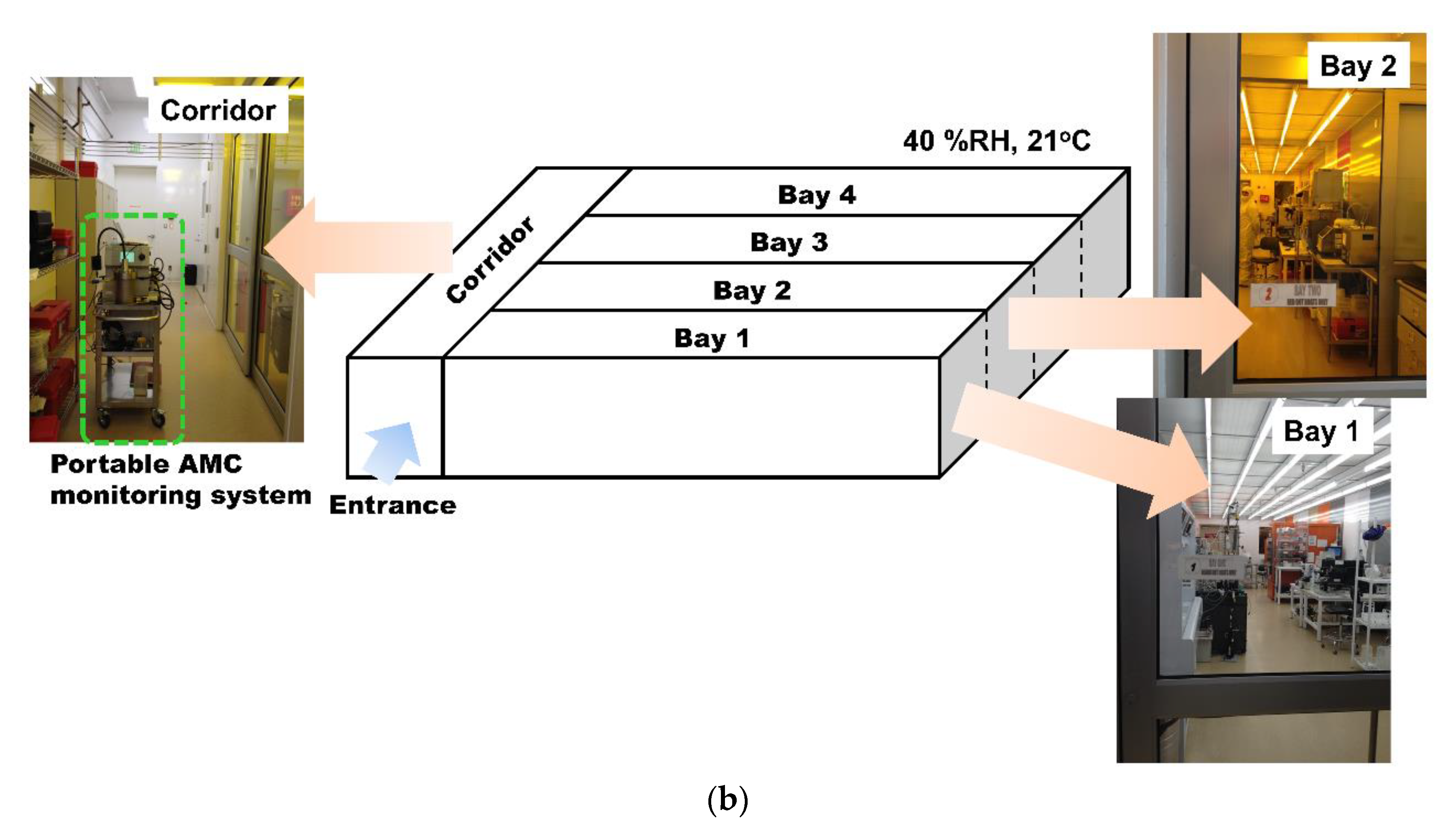
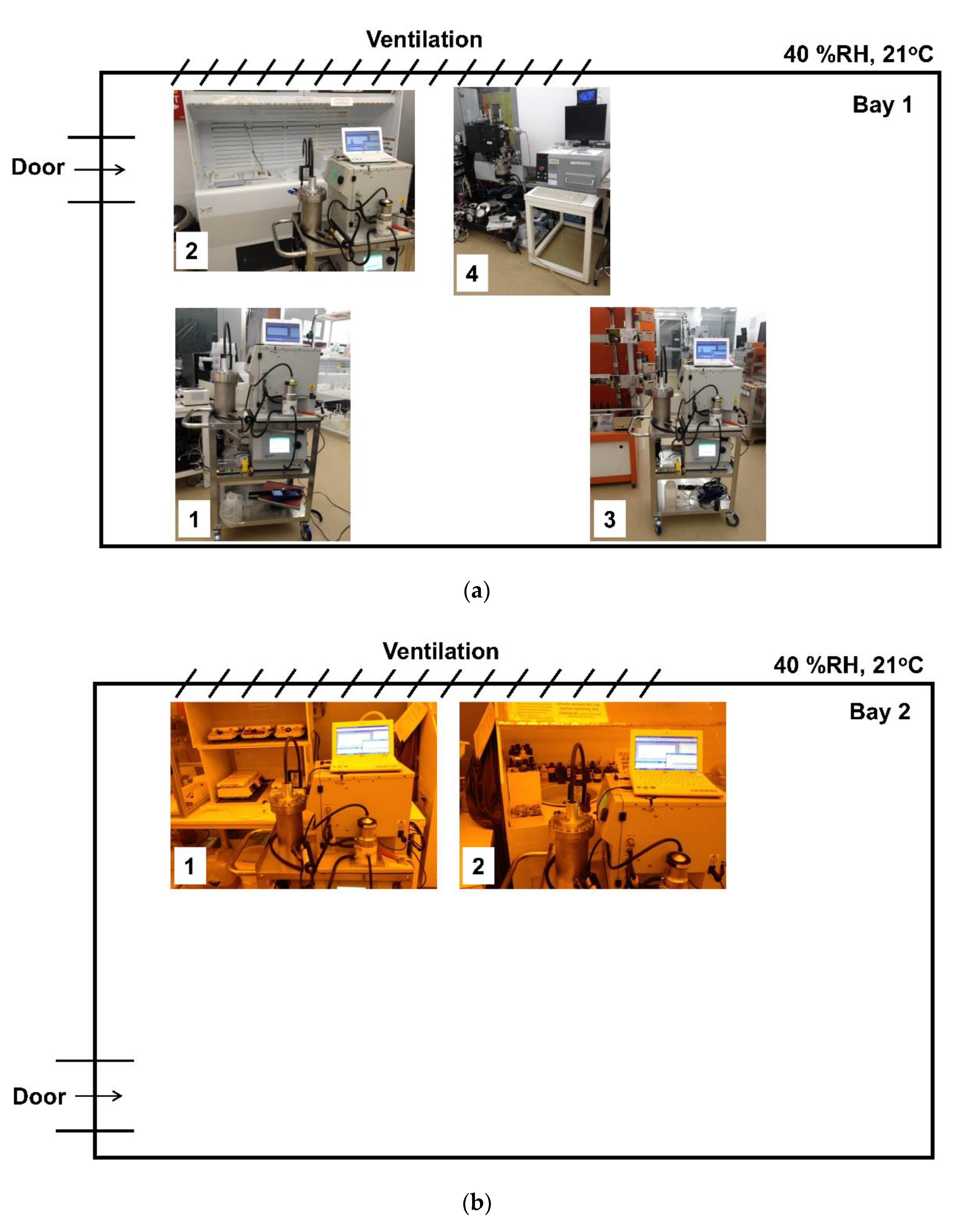
2.2. AMC Monitoring Sites in A Cleanroom at the University of Minnesota
2.3. Estimation of AMC from the Measured Size Distributions of Nanoparticles Synthesized through Soft X-Ray Radiolysis
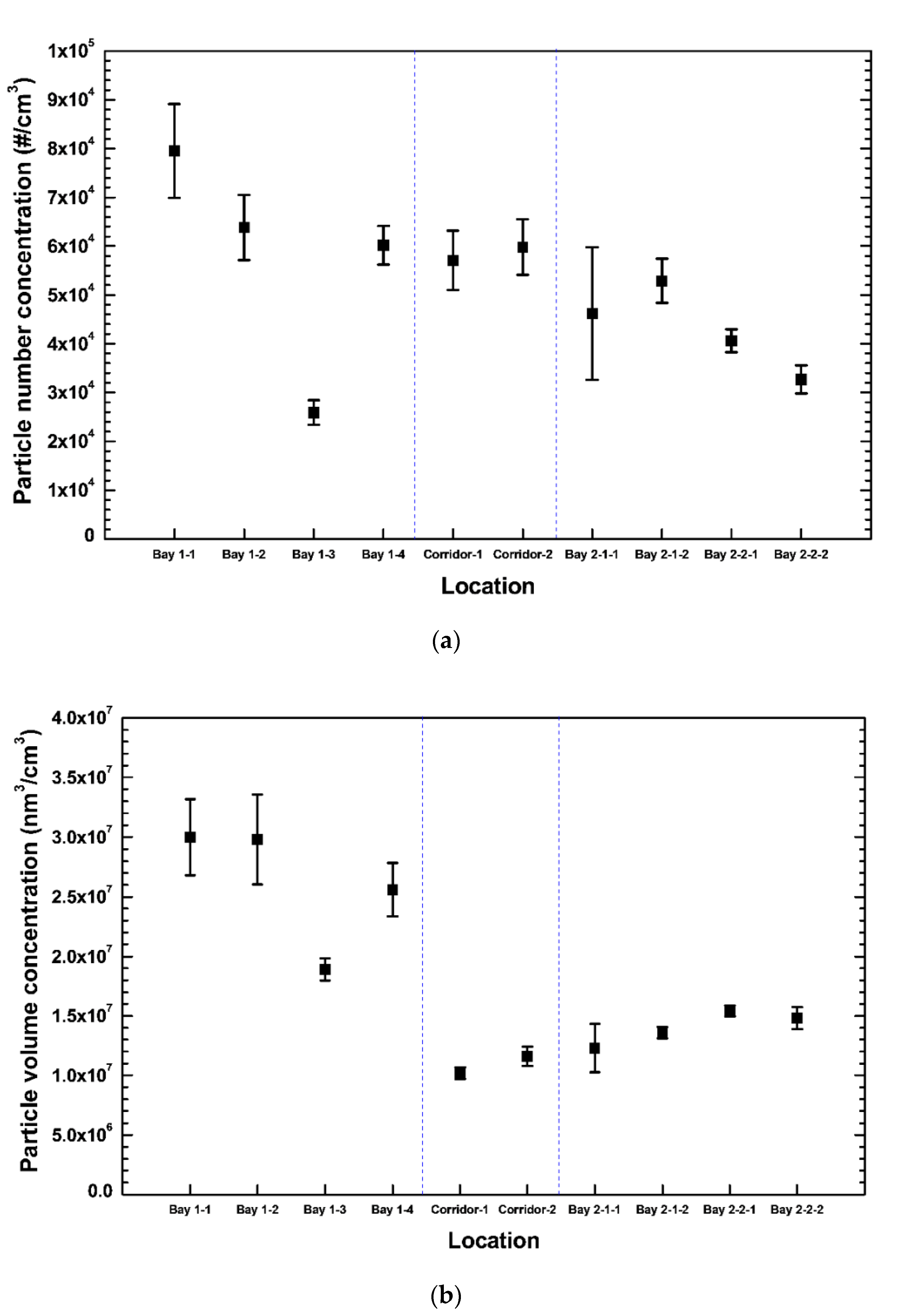
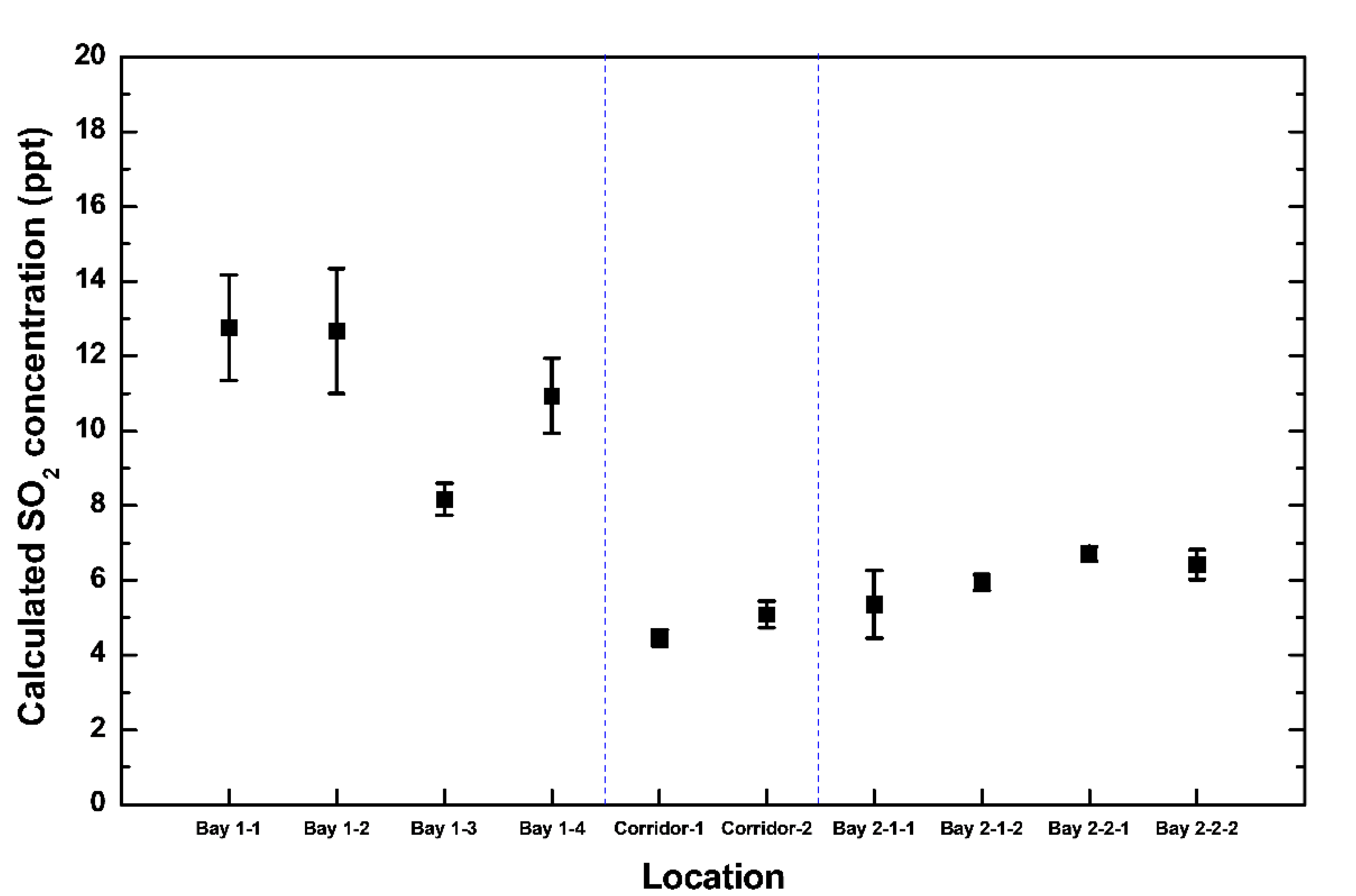
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Den, W.; Bai, H.; Kang, Y. Organic Airborne Molecular Contamination in Semiconductor Fabrication Clean Rooms. J. Electrochem. Soc. 2006, 153, G149. [Google Scholar] [CrossRef]
- Weineck, G.; Zastera, D.; Dallas, A.J. AMC Control in Photolithography: The Past Decade in Review. Proc. SPIE 2010, 7638, 76383H. [Google Scholar]
- Lobert, J.M.; Srivastava, R.; Belanger, F. Airborne molecular contamination: Formation, impact, measurement and removal of nitrous acid (HNO2). In Proceedings of the 29th Annual SEMI Advanced Semiconductor Manufacturing Conference (ASMC), Saratoga Springs, NY, USA, 30 April–3 May 2018; IEEE: New York, NY, USA, 2018; pp. 180–185. [Google Scholar]
- Otto, M. Airborne molecular contamination: Quality criterion for laser and optical components. Proc. SPIE 2015, 9346, 93460F. [Google Scholar]
- Daly, J.G. Contamination and UV lasers: Lessons learned. Proc. SPIE 2015, 9573, 95730C. [Google Scholar]
- Chuang, T.S.; Chang, L.M. To mitigate airborne molecular contamination through ultra-pure air system. Build. Environ. 2013, 59, 153–163. [Google Scholar] [CrossRef]
- Kim, C.; Zuo, Z.; Finger, H.; Haep, S.; Asbach, C.; Fissan, H.; Pui, D.Y.H. Soft X-ray-assisted detection method for airborne molecular contaminations (AMCs). J. Nanopart. Res. 2015, 17, 126. [Google Scholar] [CrossRef]
- Kim, C.; Sul, Y.T.; Pui, D.Y.H. Real-time and online screening method for materials emitting volatile organic compounds. J. Nanopart. Res. 2016, 18, 282. [Google Scholar] [CrossRef]
- Kim, C.; Pui, D.Y.H. Experimental study on the filtration efficiency of activated carbons for 3-30 nm particles. Carbon 2015, 93, 226–229. [Google Scholar] [CrossRef]
- Kim, C.; Kang, S.; Pui, D.Y.H. Removal of airborne sub-3 nm particles using fibrous filters and granular activated carbons. Carbon 2016, 104, 125–132. [Google Scholar] [CrossRef]
- Kim, C.; Lee, H.; Juelfs, A.; Haynes, C.L.; Pui, D.Y.H. The effect of filtered nanoparticles on gas filtration efficiency of granular activated carbons. Carbon 2017, 121, 63–71. [Google Scholar] [CrossRef]
- Kim, C.; Chen, S.-C.; Zhou, J.; Cao, J.; Pui, D.Y.H. Measurements of outgassing from PM2.5 collected in Xi’an, China through Soft X-ray radiolysis. IEEE Trans. Semicond. Manuf. 2019, 32, 259–266. [Google Scholar] [CrossRef]
- Licciardello, A.; Puglisi, O.; Pignataro, S. Effect of organic contaminants on the oxidation kinetics of silicon at room temperature. Appl. Phys. Lett. 1986, 48, 41–43. [Google Scholar] [CrossRef]
- Schueler, B.W. Microscope imaging by time-of-flight secondary ion mass spectrometry. Microsc. Microanal. Microstruct. 1992, 3, 119–139. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Lindinger, W.; Jordan, A. Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998, 27, 347. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Herbig, J.; Märk, L.; Schottkowsky, R.; Seehauser, H.; Sulzer, P.; Märk, T.D. An online ultra-high sensitivity Proton-transfer-reaction mass-spectrometer combined with switchable reagent ion capability (PTR + SRI − MS). Int. J. Mass Spectrom. 2009, 286, 32–38. [Google Scholar] [CrossRef]
- Hinsberg, W.D.; MacDonald, S.A.; Clecak, N.J.; Snyder, C.D. Airborne Contamination of a Chemically Amplified Resist. 2. Effect of Polymer Film Properties on Contamination Rate. Chem. Mater. 1994, 6, 481–488. [Google Scholar] [CrossRef]
- Hada, H.; Watanabe, T.; Kinoshita, H.; Komano, H. Outgassing Analysis in EUV Resist. J. Photopolym. Sci. Technol. 2005, 18, 475–480. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, S.; Santillan, J.J.; Oizumi, H.; Itani, T. EUV resist outgassing release characterization and analysis. Microelectron. Eng. 2009, 86, 479–482. [Google Scholar] [CrossRef]
- Kirkby, J.; Curtius, J.; Almeida, J.; Dunne, E.; Duplissy, J.; Ehrhart, S.; Franchin, A.; Gagné, S.; Ickes, L.; Kürten, A.; et al. Role of sulphuric acid, ammonia and galactic cosmic rays in atmospheric aerosol nucleation. Nature 2011, 476, 429–433. [Google Scholar] [CrossRef]
- Wood, W.P.; Castleman, A.W.J.; Tang, I.N. Mechanisms of aerosol formation from SO2. J. Aerosol Sci. 1975, 6, 367–374. [Google Scholar] [CrossRef]
- Lehtipalo, K.; Yan, C.; Dada, L.; Bianchi, F.; Xiao, M.; Wagner, R.; Stolzenburg, D.; Ahonen, L.R.; Amorim, A.; Baccarini, A.; et al. Multicomponent new particle formation from sulfuric acid, ammonia, and biogenic vapors. Sci. Adv. 2018, 4, eaau5363. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Cho, K.; Pui, D.Y.H. Investigation of Airborne Molecular Contamination in Cleanroom Air Environment through Portable Soft X-Ray Radiolysis Detector. Appl. Sci. 2020, 10, 978. https://doi.org/10.3390/app10030978
Kim C, Cho K, Pui DYH. Investigation of Airborne Molecular Contamination in Cleanroom Air Environment through Portable Soft X-Ray Radiolysis Detector. Applied Sciences. 2020; 10(3):978. https://doi.org/10.3390/app10030978
Chicago/Turabian StyleKim, Changhyuk, Kyungil Cho, and David Y. H. Pui. 2020. "Investigation of Airborne Molecular Contamination in Cleanroom Air Environment through Portable Soft X-Ray Radiolysis Detector" Applied Sciences 10, no. 3: 978. https://doi.org/10.3390/app10030978
APA StyleKim, C., Cho, K., & Pui, D. Y. H. (2020). Investigation of Airborne Molecular Contamination in Cleanroom Air Environment through Portable Soft X-Ray Radiolysis Detector. Applied Sciences, 10(3), 978. https://doi.org/10.3390/app10030978




