Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application
Abstract
1. Introduction
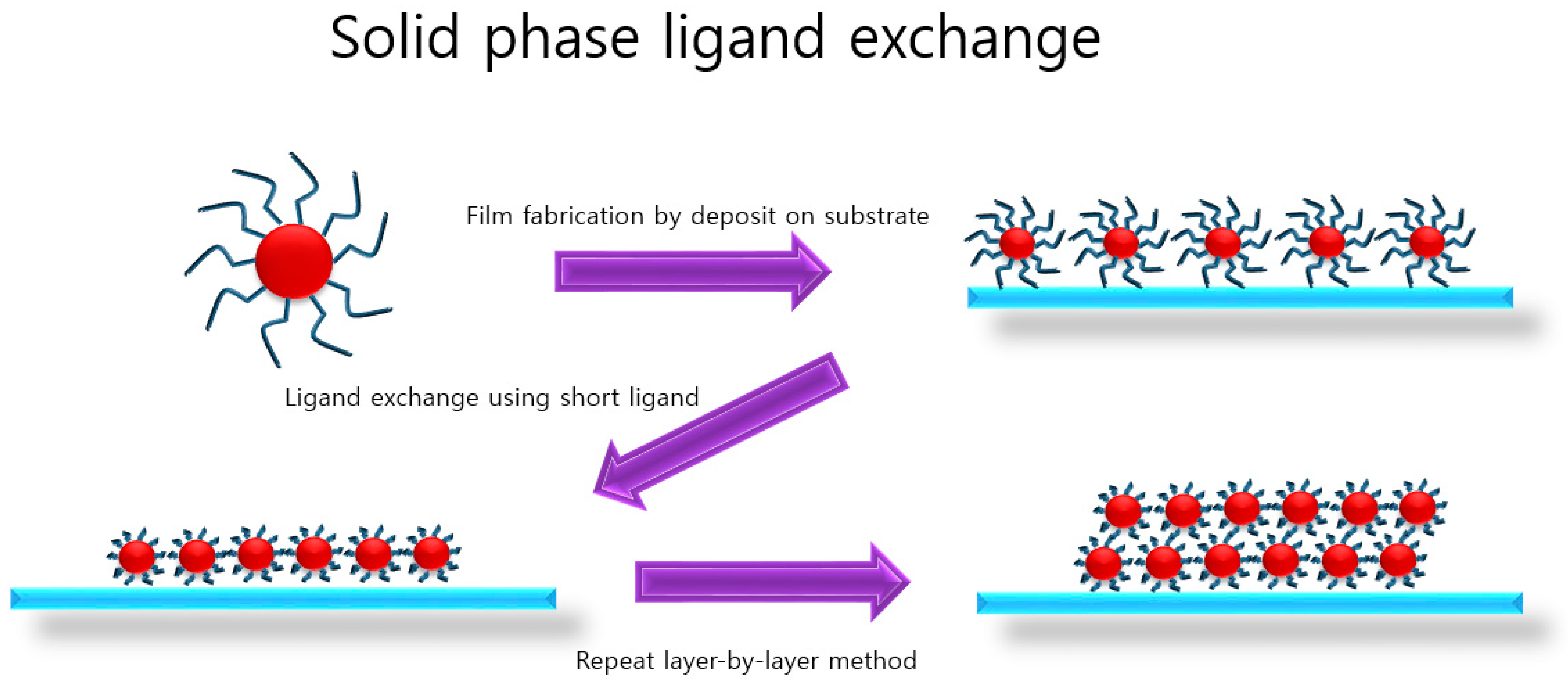
2. Solid-State Ligand Exchange
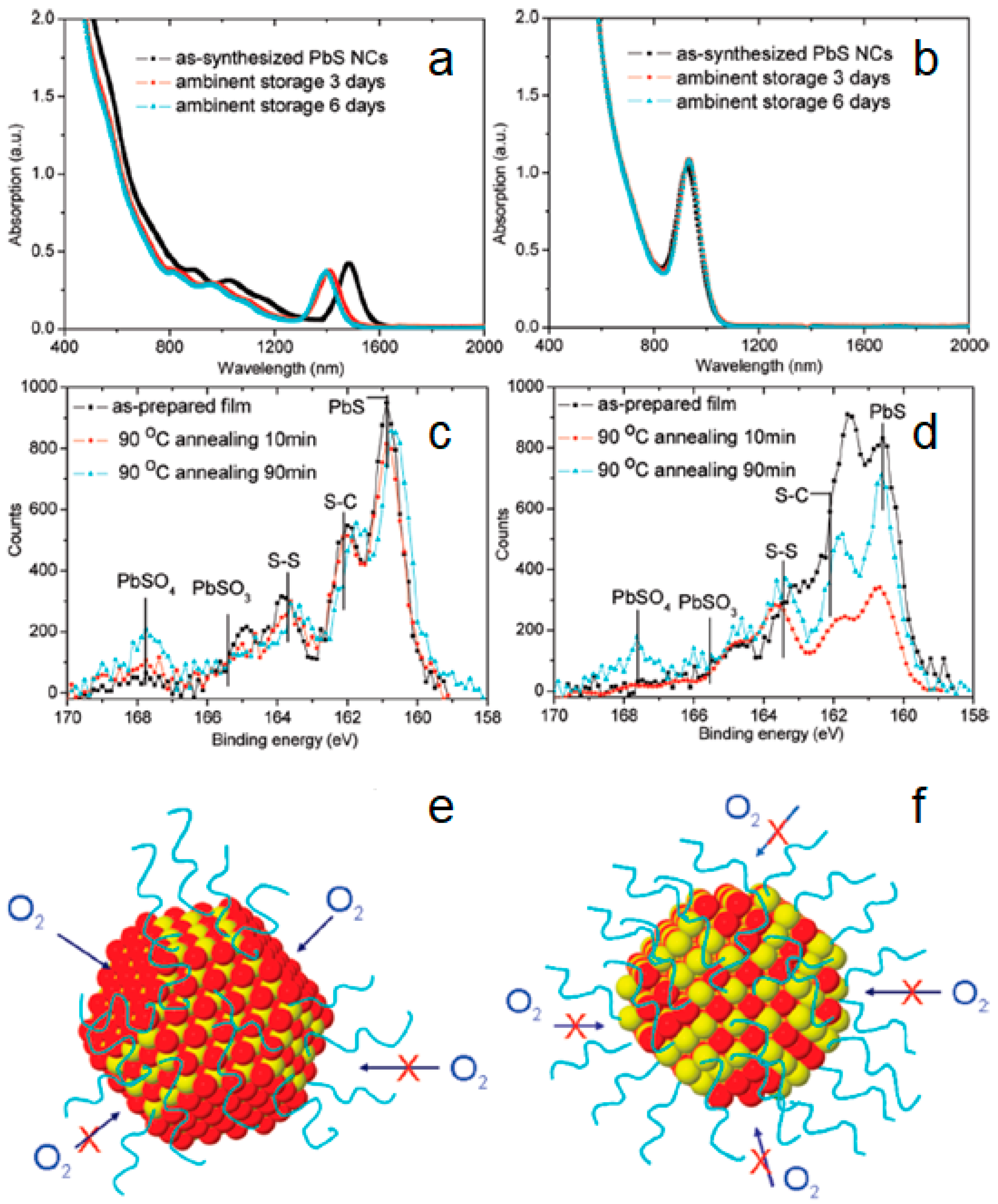
2.1. Air-Stable PbS CQDs via Solid-State Ligand Exchange Process
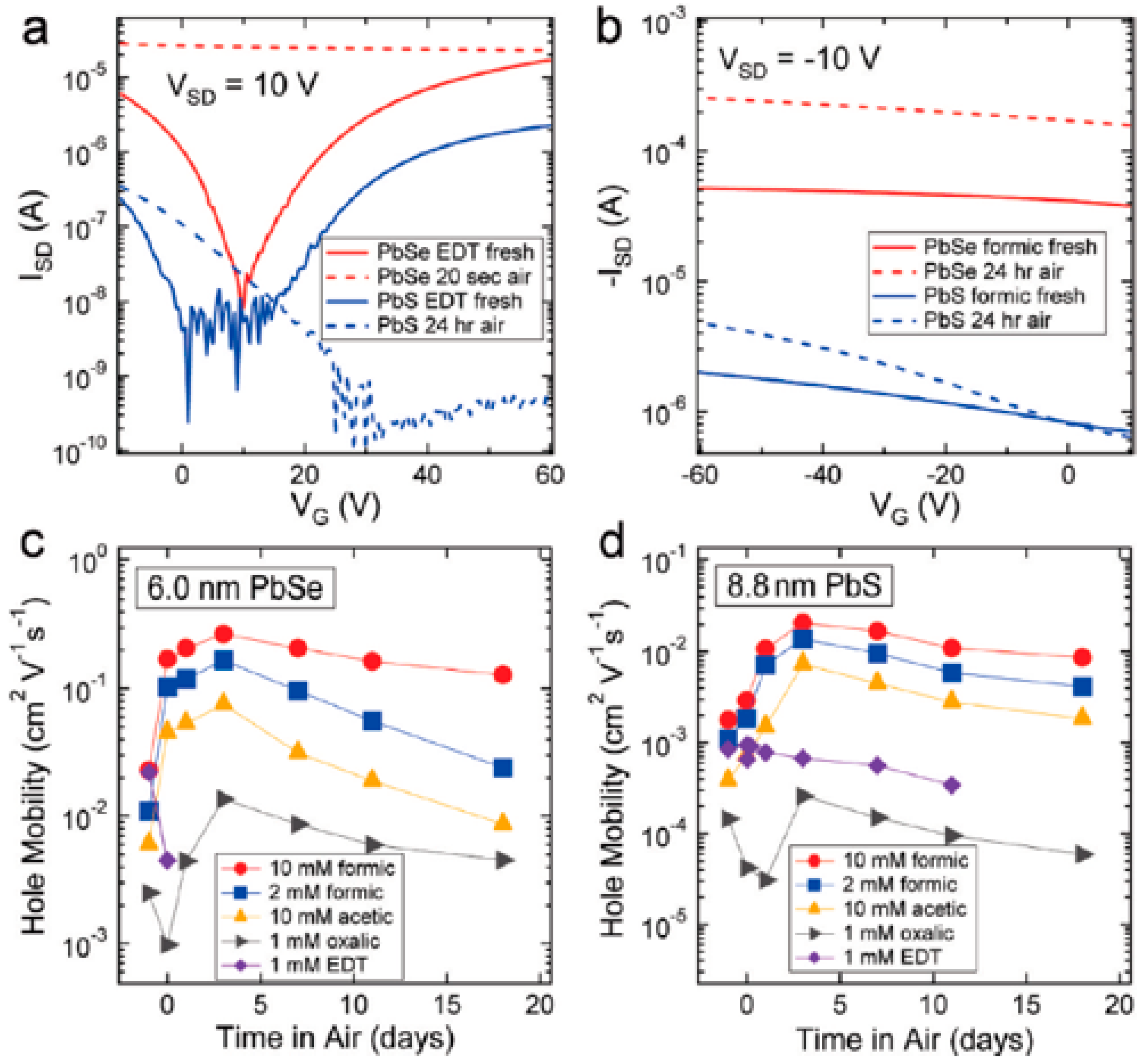
2.2. Carrier Mobility of PbS CQDs Depending on Ligand Species
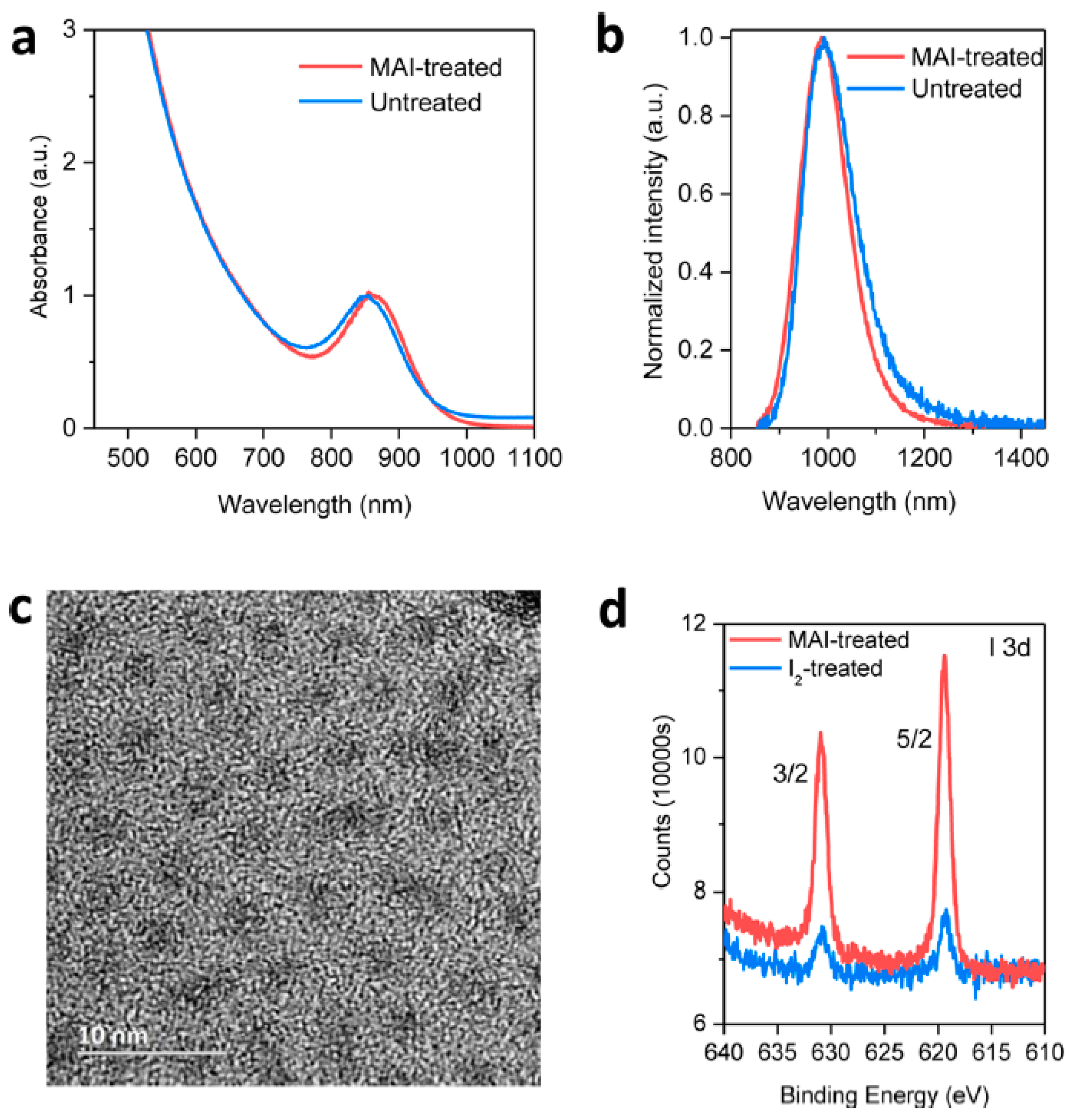
2.3. Halide Ligand to Eliminate Trap States
2.4. Solvent-Polarity-Eengineered Halide Passivation
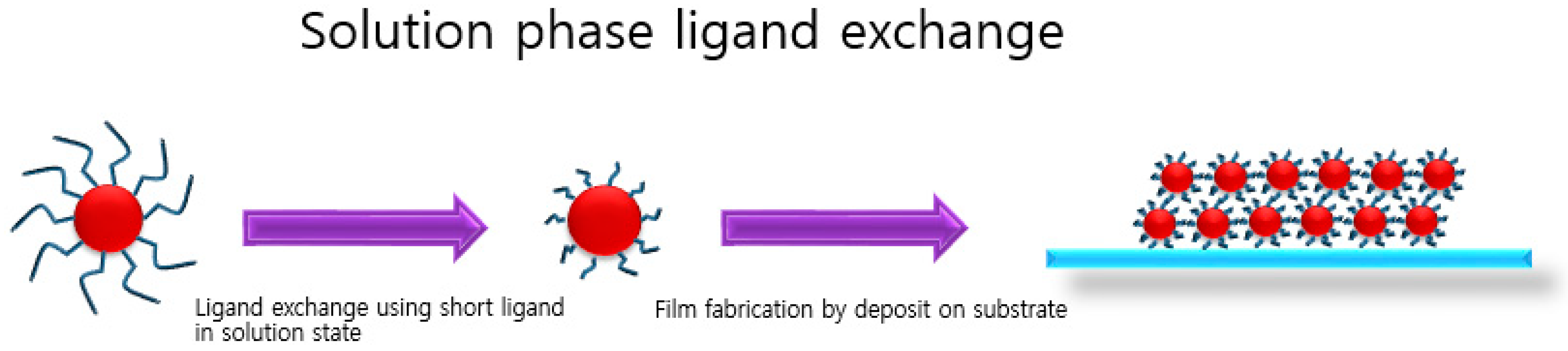
3. Solution-Phase Ligand Exchange
3.1. Directly Usable PbS CQD Inks by Solution-Phase Ligand Exchange
3.2. Flat Energy Landscape and High Packing Density in Quantum Dots
3.3. Tuning Reactivity of Ligands
3.4. Improved Diffusion Length of Quantum Dot Film by Matrix Approach
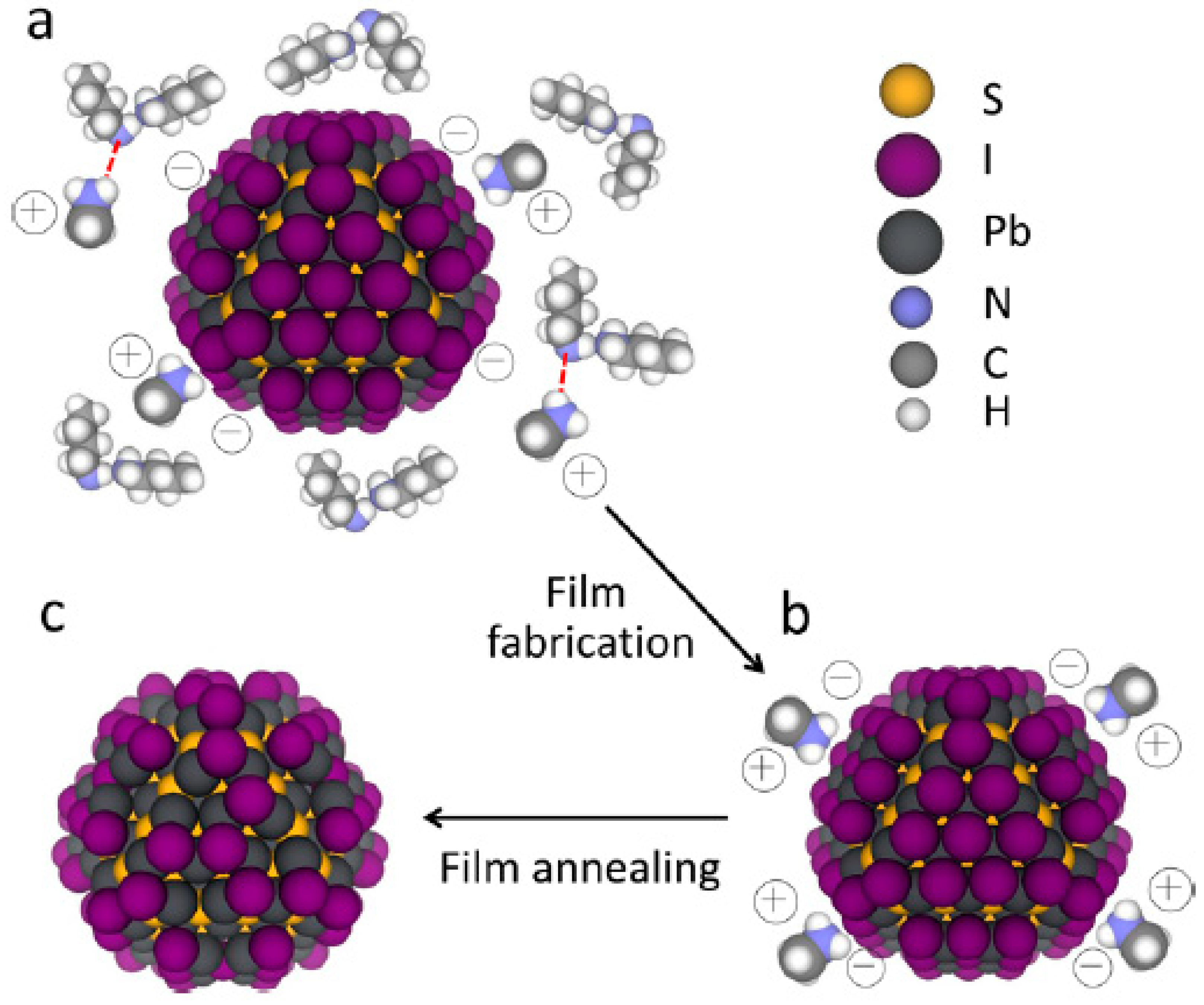
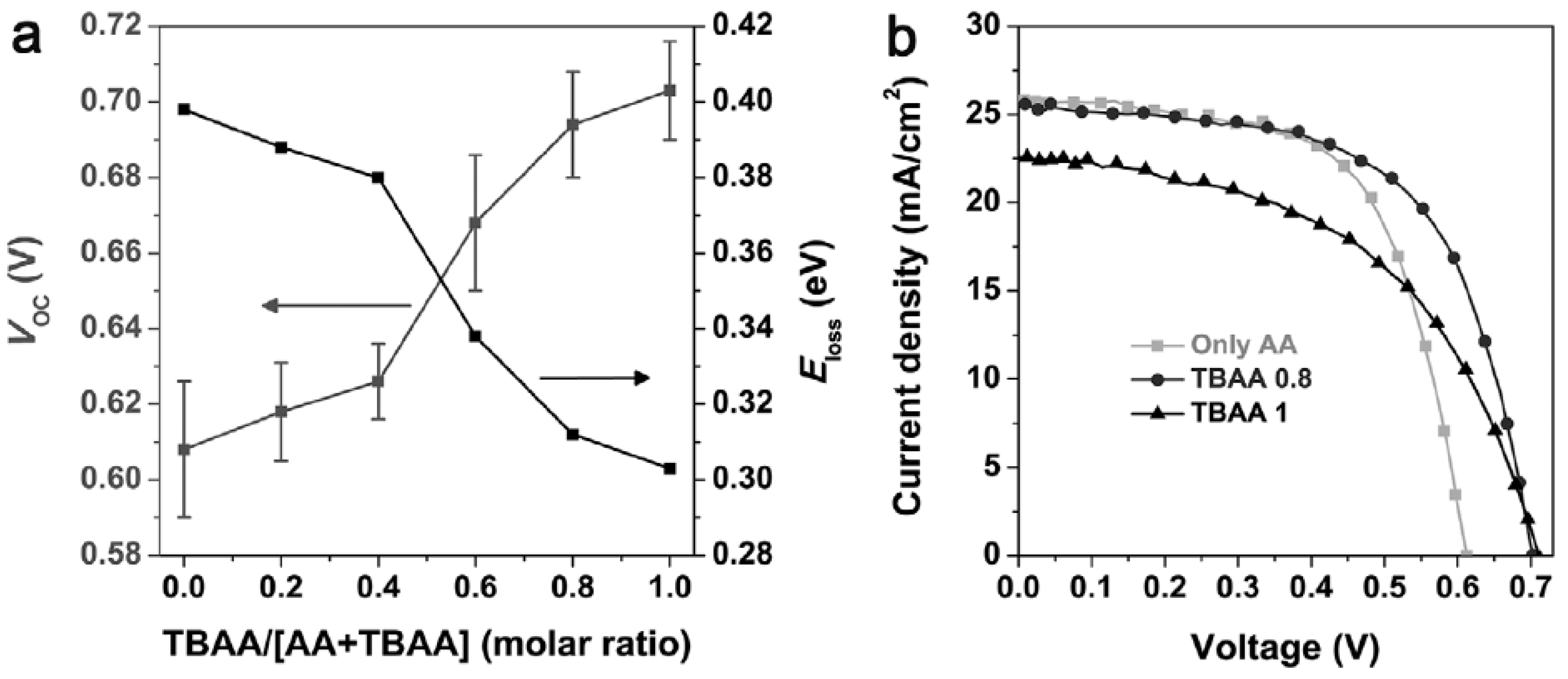
3.5. Acid-Assisted Ligand Exchange for Infrared Quantum Dots
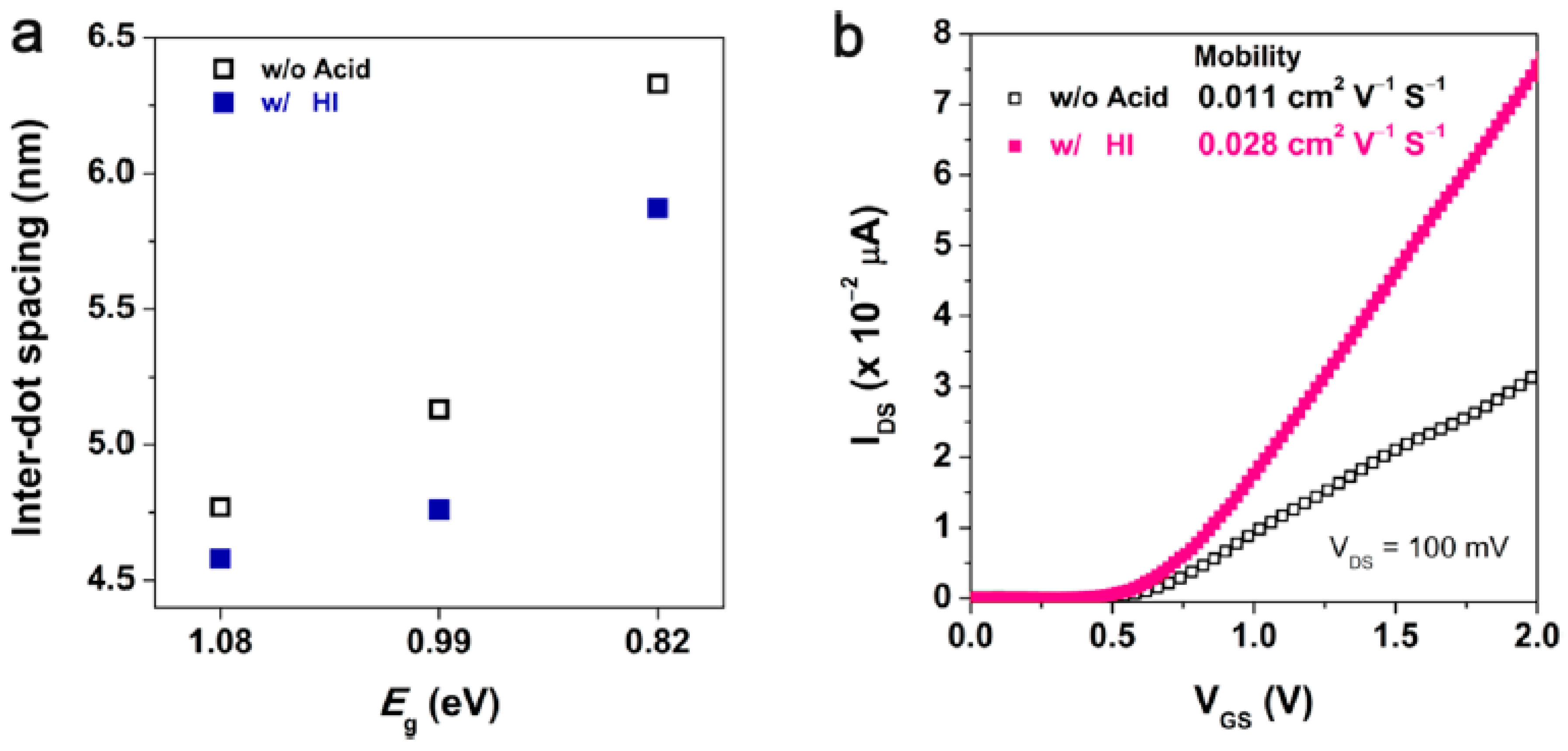
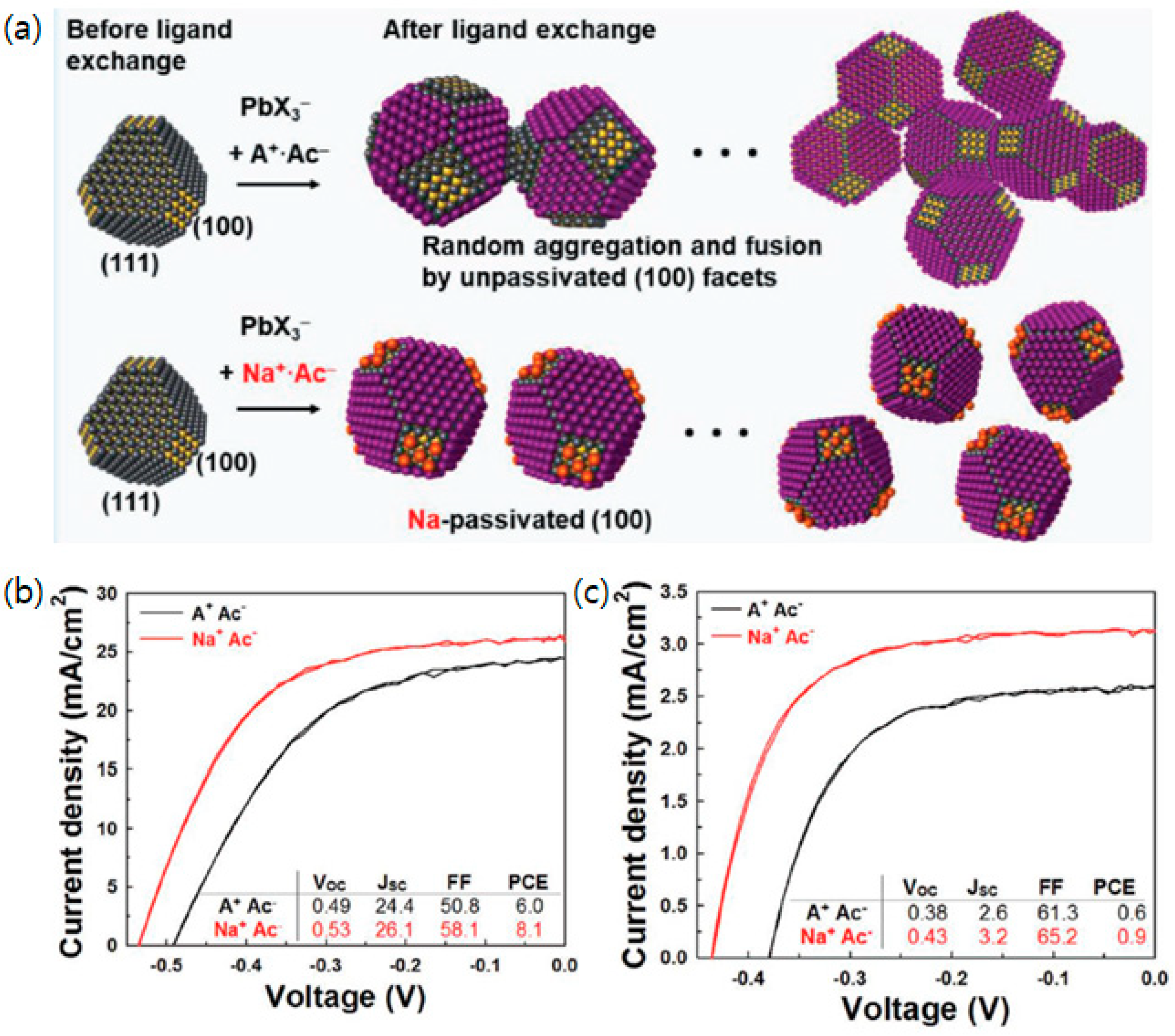
3.6. A Facet-Specific Quantum Dot Passivation Strategy for Infrared Quantum Dots
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in three-dimensional microscopic semiconductor crystals. JETP Lett. 1982, 34, 345–349. [Google Scholar]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Klimov, V.I. Spectral and dynamical properties of multiexcitons in semiconductor nanocrystals. Annu. Rev. Phys. Chem. 2007, 58, 635–673. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Guyot-Sionnest, P. Slow electron cooling in colloidal quantum dots. Science 2008, 322, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Niemal, M.; Norris, D.J.; Kuno, M.; Bawendi, M.G.; Efros, A.L.; Rosen, M. Observation of the “dark exciton” in CdSe quantum dots. Phys. Rev. Lett. 1995, 75, 37223731. [Google Scholar]
- Choi, J.J.; Wenger, W.N.; Hoffman, R.S.; Lim, Y.-F.; Luria, J.; Jasieniak, J.; Marohn, J.A.; Hanrath, T. Solution-Processed Nanocrystal Quantum Dot Tandem Solar Cells. Adv. Mater. 2011, 23, 3144–3148. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, D.; Voznyy, O.; Levina, L.; Hoogland, S.; Kemp, K.W.; Ip, A.H.; Thon, S.M.; Sargent, E.H. Engineering Colloidal Quantum Dot Solids within and beyond the Mobility-Invariant Regime. Nat. Commun. 2014, 5, 3803. [Google Scholar] [CrossRef]
- Johnston, K.W.; Pattantyus-Abraham, A.G.; Clifford, J.P.; Myrskog, S.H.; MacNeil, D.D.; Levina, L.; Sargent, E.H. Schottky-Quantum Dot Photovoltaics for Efficient Infrared Power Conversion. Appl. Phys. Lett. 2008, 92, 151115. [Google Scholar] [CrossRef]
- Ma, W.; Luther, J.M.; Zheng, H.; Wu, Y.; Alivisatos, A.P. Photovoltaic Devices Employing Ternary PbSxSe1-x Nanocrystals. Nano Lett. 2009, 9, 1699–1703. [Google Scholar] [CrossRef]
- Tang, J.; Brzozowski, L.; Barkhouse, A.; Wang, X.; Debnath, R.; Wolowiec, R.; Palmiano, E.; Levina, L.; Pattantyus-Abraham, A.G.; Jamakosmanovic, D.; et al. Quantum Dot Photovoltaics in the Extreme Quantum Confinement Regime: The Surface-Chemical Origins of Exceptional Air- and Light-Stability. ACS Nano 2010, 2, 869–878. [Google Scholar] [CrossRef]
- Moreels, L.; Fritzinger, B.; Martins, J.C.; Hens, Z. Surface Chemistry of Collodial PbSe Nanocrystals. J. Am. Chem. Soc. 2008, 130, 15081–15086. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Levina, L.; Fisher, A.; Sargent, E.H. Engineering the Temporal Response of Photoconductive Photodetectors via Selective Introduction of Surface Trap States. Nano Lett. 2008, 8, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Sargent, E.H. PbS Colloidal Quantum Dot Photoconductive Photodetectors: Transport, Traps and Gain. Appl. Phys. Lett. 2007, 91, 173505. [Google Scholar] [CrossRef]
- Fan, D.B.; Thomas, P.J.; O’Brien, P. Pyramidal Lead Sulfide Crystallites with High Energy {113} Facets. J. Am. Chem. Soc. 2008, 130, 10892–10894. [Google Scholar] [CrossRef]
- Warner, J.H.; Cao, H. Shape Control of PbS Nanocrystals Using Multiple Surfactants. Nanotechnology 2008, 19, 305605. [Google Scholar] [CrossRef]
- Joseph, M.L.; Gao, J.; Lloyd, M.T.; Semonin, O.E.; Beard, M.C.; Arthur Nozik, A.J. Stability assessment on a 3% bilayer PbS/ZnO quantum dot heterojunction solar cell. Adv. Mater. 2010, 22, 3704–3707. [Google Scholar]
- Luther, J.M.; Law, M.; Song, Q.; Beard, M.C.; Nozik, A.J. Structural, Optical, and Electrical Properties of Self-Assembled Films of PbSe Nanocrystals Treated with 1,2-Ethanedithiol. ACS Nano 2008, 2, 271–280. [Google Scholar] [CrossRef]
- Luther, J.M.; Law, M.; Song, Q.; Reese, M.O.; Beard, M.C.; Ellingson, R.J.; Nozik, A.J. Schottky Solar Cells Based on Colloidal Nanocrystal Films. Nano Lett. 2008, 8, 3488–3492. [Google Scholar] [CrossRef]
- Law, M.; Beard, M.C.; Choi, S.; Luther, J.M.; Hanna, M.C.; Nozik, A.J. Determining the Internal Quantum Efficiency of PbSe Nanocrystal Solar Cells with the Aid of an Optical Model. Nano Lett. 2008, 8, 3904–3910. [Google Scholar] [CrossRef]
- Zarghami, M.H.; Markelle Gibbs, Y.L.; Gebremichael, E.; Webster, C.; Law, M. p-Type PbSe and PbS Quantum Dot Solids Prepared with Short-Chain Acids and Diacids. ACS Nano 2010, 4, 2475–2485. [Google Scholar] [CrossRef]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed]
- lp, A.H.; Thon, S.M.; Hoogland, S.; Voznyy, O.; Zhitomirsky, D.; Debnath, R.; Levina, L.; Rollny, L.R.; Carey, G.H.; Fischer, A.; et al. Hybrid passivated colloidal quantum dot solids. Nat. Nano 2012, 79, 577–582. [Google Scholar]
- Ning, Z.; Voznyy, O.; Pan, J.; Hoogland, S.; Adinolfi, V.; Xu, J.; Li, M.; Kirmani, A.R.; Sun, J.-P.; Minor, J.; et al. Air-stable n-type colloidal quantum dot solids. Nat. Mater. 2014, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, J.; Church, C.P.; Miller, E.M.; Luther, J.M.; Klimov, V.I.; Beard, M.C. PbSe quantum dot solar cells with more than 6% efficiency fabricated in ambient atmosphere. Nano Lett. 2014, 14, 6010–6015. [Google Scholar] [CrossRef] [PubMed]
- Crisp, R.W.; Kroupa, D.M.; Marshall, A.R.; Miller, E.M.; Zhang, J.; Beard, M.C.; Luther, J.M. Metal halide solid-state surface treatment for high efficiency PbS and PbSe QD solar cells. Sci. Rep. 2015, 5, 9945. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Voznyy, O.; Kiani, A.; García de Arquer, F.P.; Abbas, A.S.; Kim, G.-H.; Liu, M.; Yang, Z.; Walters, G.; Xu, J.; et al. Passivation using molecular halides increases quantum dot solar cell performance. Adv. Mater. 2016, 28, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Voznyy, O.; García de Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% Certified Colloidal Quantum Dot Solar Cells via Solvent-Polarity-Engineered Halide Passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Talapin, D.V.; Murray, C.B. PbSe Nanocrystals Solids for n- and p-Channel Thin Film Field-Effect Transistors. Science 2005, 310, 86–89. [Google Scholar] [CrossRef]
- Wang, X.; Koleilat, G.; Tang, J.; Liu, H.; Kramer, I.J.; Debnath, R.; Brzozowski, L.; Barkhouse, D.A.R.; Levina, L.; Hoogland, S.; et al. Tandem Colloidal Quantum Dot Solar Cells Employing a Graded Recombination Layer. Nat. Photonics 2011, 5, 480–484. [Google Scholar] [CrossRef]
- Ning, Z.; Zhitomirsky, D.; Adinolfi, V.; Sutherland, B.; Xu, J.; Voznyy, O.; Maraghechi, P.; Lan, X.; Hoogland, S.; Ren, Y.; et al. Graded Doping for Enhanced Colloidal Quantum Dot Photovoltaics. Adv. Mater. 2013, 25, 1719–1723. [Google Scholar] [CrossRef]
- Tang, J.; Liu, H.; Zhitomirsky, D.; Hoogland, S.; Wang, X.; Furukawa, M.; Levina, L.; Sargent, E.H. Quantum Junction Solar Cells. Nano Lett. 2012, 12, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Zhitomirsky, D.; Furukawa, M.; Tang, J.; Stadler, P.; Hoogland, S.; Voznyy, O.; Liu, H.; Sargent, E.H. N-Type Colloidal-Quantum-Dot Solids for Photovoltaics. Adv. Mater. 2012, 24, 6181–6185. [Google Scholar] [CrossRef] [PubMed]
- Voznyy, O.; Zhitomirsky, D.; Stadler, P.; Ning, Z.; Hoogland, S.; Sargent, E.H. A Charge-Orbital Balance Picture of Doping in Colloidal Quantum Dot Solids. ACS Nano 2012, 6, 8448–8455. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Dong, H.; Zhang, Q.; Voznyy, O.; Sargent, E.H. Solar Cells Based on Inks of n-Type Colloidal Quantum Dots. ACS Nano 2014, 8, 10321–10327. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Scheele, M.; Talapin, D.V. Colloidal Nanocrystals with Molecular Metal Chalcogenide Surface Ligands. Science 2009, 324, 1417–1420. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Bodnarchuk, M.I.; Zaumseil, J.; Lee, J.-S.; Talapin, D.V. Expanding the Chemical Versatility of Colloidal Nanocrystals Capped with Molecular Metal halcogenide Ligands. J. Am. Chem. Soc. 2010, 132, 10085–10092. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of Colloidal Nanocrystals for Electronic and Optoelectronic Applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef]
- Pejova, B.; Abay, B. Nanostructured CdSe films in low size-quantization regime: Temperature dependence of the band gap energy and sub-band gap absorption tails. J. Phys. Chem. C 2011, 115, 23241–23255. [Google Scholar] [CrossRef]
- Pejova, B.; Abay, B.; Bineva, I. Temperature dependence of the band-gap energy and sub-band-gap absorption tails in strongly quantized ZnSe nanocrystals deposited as thin films. J. Phys. Chem. C 2010, 114, 15280–15291. [Google Scholar] [CrossRef]
- Zhitomirsky, D.; Kramer, I.J.; Labelle, A.J.; Fischer, A.; Debnath, R.; Pan, J.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot photovoltaics: The effect of polydispersity. Nano Lett. 2012, 12, 1007–1012. [Google Scholar] [CrossRef]
- Guyot-Sionnest, P. Electrical transport in colloidal quantum; dot films. J. Phys. Chem. Lett. 2012, 3, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Sa-Yakanit, V.; Glyde, H.R. Urbach tails and disorder. Comments Condens. Matter Phys. 1987, 13, 35–48. [Google Scholar]
- Erslev, P.T.; Chen, H.Y.; Gao, J.; Beard, M.C.; Frank, A.J.; van de Lagemaat, J.; Johnson, J.C.; Luther, J.M. Sharp exponential band tails in highly disordered lead sulfide quantum dot arrays. Phys. Rev. B 2012, 86, 155313–155316. [Google Scholar] [CrossRef]
- Kagan, C.R.; Murray, C.B. Charge transport in strongly coupled quantum dot solids. Nat. Nanotech 2015, 10, 1013–1026. [Google Scholar] [CrossRef]
- Yang, Z.; Janmohamed, A.; Lan, X.; de Arquer, F.P.G.; Voznyy, O.; Yassitepe, E.; Kim, G.; Ning, Z.; Gong, X.; Comin, R.; et al. Colloidal quantum dot photovoltaics enhanced by perovskite shelling. Nano Lett. 2015, 15, 7539–7543. [Google Scholar] [CrossRef]
- Liu, M.; Voznyy, O.; Sabatini, R.; de Arquer, F.P.G.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic–inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nature Mater 2017, 16, 258–263. [Google Scholar] [CrossRef]
- Chuang, C.-H.M.; Maurano, A.; Brandt, R.E.; Hwang, G.W.; Jean, J.; Buonassisi, T.; Bulović, V.; Bawendi, M.G. Open-circuit voltage deficit, radiative sub-bandgap states, and prospects in quantum dot solar cells. Nano Lett. 2015, 15, 3286–3294. [Google Scholar] [CrossRef]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Science 2016, 352, aad4424. [Google Scholar] [CrossRef]
- Carey, G.H.; Kramer, I.J.; Kanjanaboos, P.; Moreno-Bautista, G.; Voznyy, O.; Rollny, L.; Tang, J.A.; Hoogland, S.; Sargent, E.H. Electronically active impurities in colloidal quantum dot solids. ACS Nano 2014, 8, 11763–11769. [Google Scholar] [CrossRef]
- Jo, J.W.; Kim, Y.; Choi, J.; Pelayo García de Arquer, F.; Walters, G.; Sun, B.; Ouellette, O.; Kim, J.; Proppe, A.H.; Quintero-Bermudez, R.; et al. Enhanced Open-Circuit Voltage in Colloidal Quantum Dot Photovoltaics via Reactivity-Controlled Solution-Phase Ligand Exchange. Adv. Mater. 2017, 29, 1703627. [Google Scholar] [CrossRef]
- Dolzhnikov, D.S.; Zhang, H.; Jang, J.; Son, J.S.; Panthani, M.G.; Shibata, T.; Chattopadhyay, S.; Talapin, D.V. Composition-matched molecular ‘solders’ for semiconductors. Science 2015, 347, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Gong, X.; Comin, R.; Walters, G.; Fan, F.; Voznyy, O.; Yassitepe, E.; Buin, A.; Hoogland, S.; Sargent, E.H. Quantum-dot-in-perovskite solids. Nature 2015, 523, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, R.H.; Lee, E.M.Y.; Weidman, M.C.; Willard, A.P.; Tisdale, W.A. Charge carrier hopping dynamics in homogeneously broadened PbS quantum dot solids. Nano Lett. 2017, 17, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Ip, A.H.; Kiani, A.; Kramer, I.J.; Voznyy, O.; Movahed, H.F.; Levina, L.; Adachi, M.M.; Hoogland, S.; Sargent, E.H. Infrared colloidal quantum dot photovoltaics via coupling enhancement and agglomeration suppression. ACS Nano 2015, 9, 8833–8842. [Google Scholar] [CrossRef]
- Carey, G.H.; Levina, L.; Comin, R.; Voznyy, O.; Sargent, E.H. Record charge carrier diffusion length in colloidal quantum dot solids via mutual dot-to-dot surface passivation. Adv. Mater. 2015, 27, 3325–3330. [Google Scholar] [CrossRef]
- Akselrod, G.M.; Deotare, P.B.; Thompson, N.J.; Lee, J.; Tisdale, W.A.; Baldo, M.A.; Menon, V.M.; Bulović, V. Visualization of exciton transport in ordered and disordered molecular solids. Nat. Commun. 2014, 5, 4646. [Google Scholar] [CrossRef]
- Xu, J.; Voznyy, O.; Liu, M.; Kirmani, A.R.; Walters, G.; Munir, R.; Abdelsamie, M.; Proppe, A.H.; Sarkar, A.; Garcia de Arquer, F.P.; et al. 2D matrix engineering for homogeneous quantum dot coupling in photovoltaic solids. Nat. Nanotechnol. 2018, 13, 456–462. [Google Scholar] [CrossRef]
- Blancon, C. Extremely efficient internal exciton dissociation through edge states in layered 2D perovskites. Science 2017, 355, 1288–1292. [Google Scholar] [CrossRef]
- Congreve, D.N.; Weidman, M.C.; Seitz, M.; Paritmongkol, W.; Dahod, N.S.; Tisdale, W.A. Tunable light-emitting diodes utilizing quantum-confined layered perovskite emitters. ACS Photonics 2017, 4, 476–481. [Google Scholar] [CrossRef]
- Kiani, A.; Sutherland, B.R.; Kim, Y.; Ouellette, O.; Levina, L.; Walters, G.; Dinh, C.T.; Liu, M.; Voznyy, O.; Lan, X.; et al. Single-step colloidal quantum do films for infrared solar harvesting. Appl. Phys. Lett. 2016, 109, 183105. [Google Scholar] [CrossRef]
- Fan, J.Z.; Liu, M.; Voznyy, O.; Sun, B.; Levina, L.; Quintero-Hoogland, S.; Sargent, E.H. Halide Re-Shelled Quantum Dot Inks for Infrared Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 37536–37541. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.W.; Choi, J.; Garcia de Arquer, F.P.; Seifitokaldani, A.; Sun, B.; Kim, Y.; Ahn, H.; Fan, J.; Quintero-Bermudez, R.; Kim, J.; et al. Acid-Assisted Ligand Exchange Enhances Coupling in Colloidal Quantum Dot Solids. Nano Lett. 2018, 18, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Ko, J.H.; Kim, Y.H.; Jeong, S. Steric-hindrance-driven shape transition in PbS quantum dots: Understanding size-dependent stability. J. Am. Chem. Soc. 2013, 135, 5278–5281. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef]
- Kim, Y.; Che, F.; Jo, J.W.; Choi, J.; García de Arquer, F.P.; Voznyy, O.; Sun, B.; Kim, J.; Choi, M.J.; Quintero-Bermudez, R.; et al. A Facet-Specific Quantum Dot Passivation Strategy for Colloid Management and Efficient Infrared Photovoltaics. Adv. Mater. 2019, 31, 1805580. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.R.; Park, J.Y.; Lee, D.H.; Kim, Y.; Choi, J. Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application. Appl. Sci. 2020, 10, 975. https://doi.org/10.3390/app10030975
You HR, Park JY, Lee DH, Kim Y, Choi J. Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application. Applied Sciences. 2020; 10(3):975. https://doi.org/10.3390/app10030975
Chicago/Turabian StyleYou, Hyung Ryul, Jin Young Park, Duck Hoon Lee, Younghoon Kim, and Jongmin Choi. 2020. "Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application" Applied Sciences 10, no. 3: 975. https://doi.org/10.3390/app10030975
APA StyleYou, H. R., Park, J. Y., Lee, D. H., Kim, Y., & Choi, J. (2020). Recent Research Progress in Surface Ligand Exchange of PbS Quantum Dots for Solar Cell Application. Applied Sciences, 10(3), 975. https://doi.org/10.3390/app10030975




