Preparation of Modified Colloidal Gas Aphrons and Analysis of the Oil Displacement Effect
Abstract
:1. Introduction
2. Method
2.1. Preparation of the Modified CGAs
2.2. Oil Displacement Experiments
3. Results and Discussion
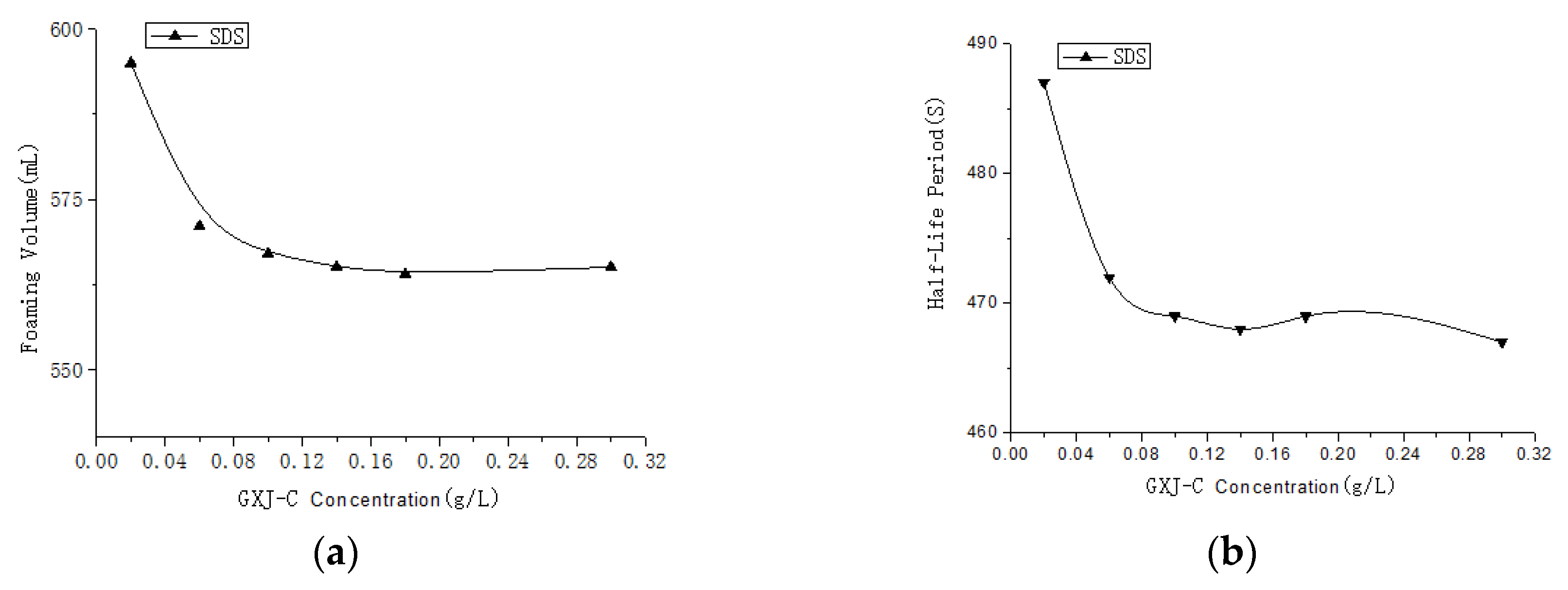
3.1. Selection of the Foaming Agents
3.2. Selection of the Modifier
3.3. Selection of the Modifier Amount
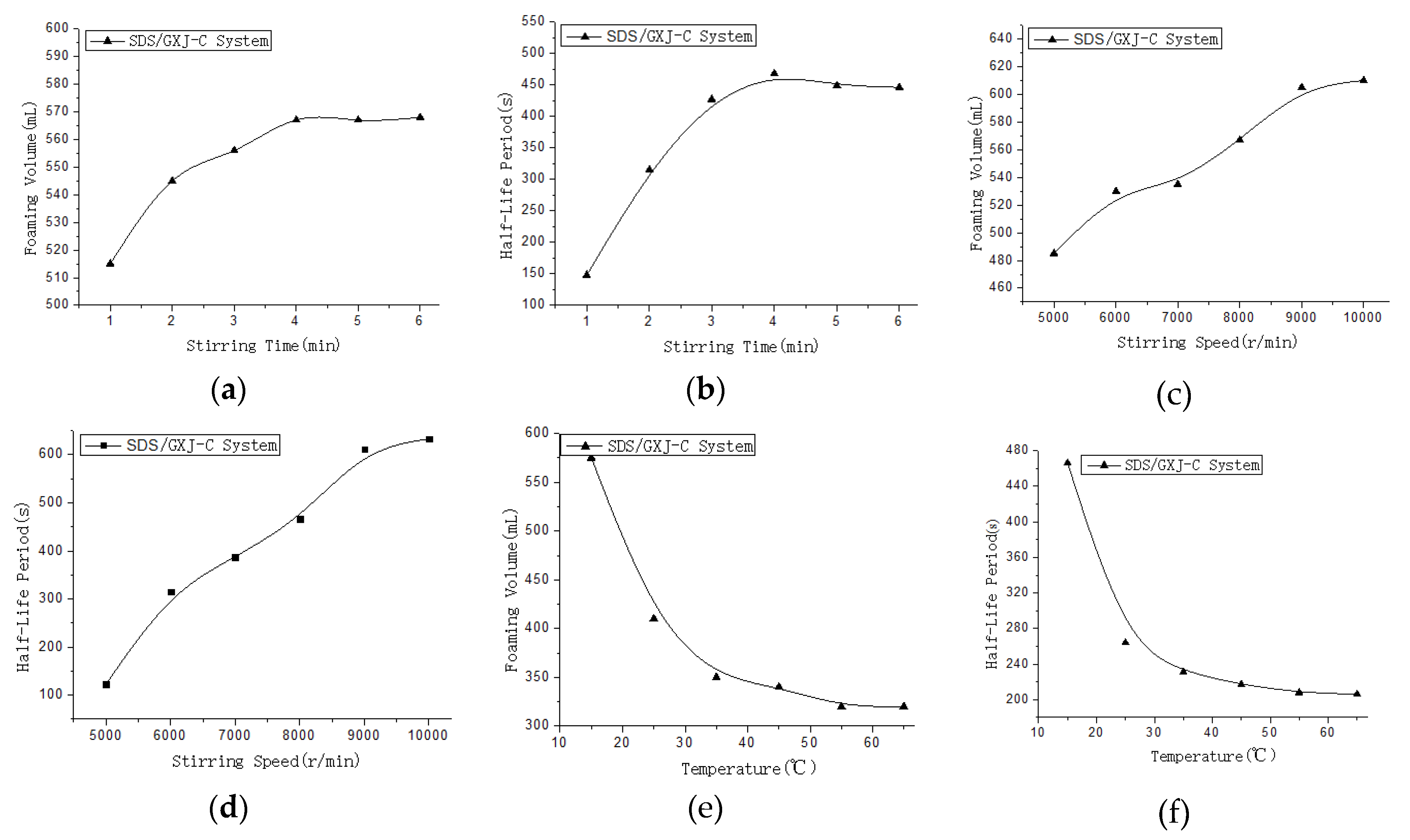
3.4. The Effects of Stirring Time, Speed, and Temperature on the Modified CGA Foam
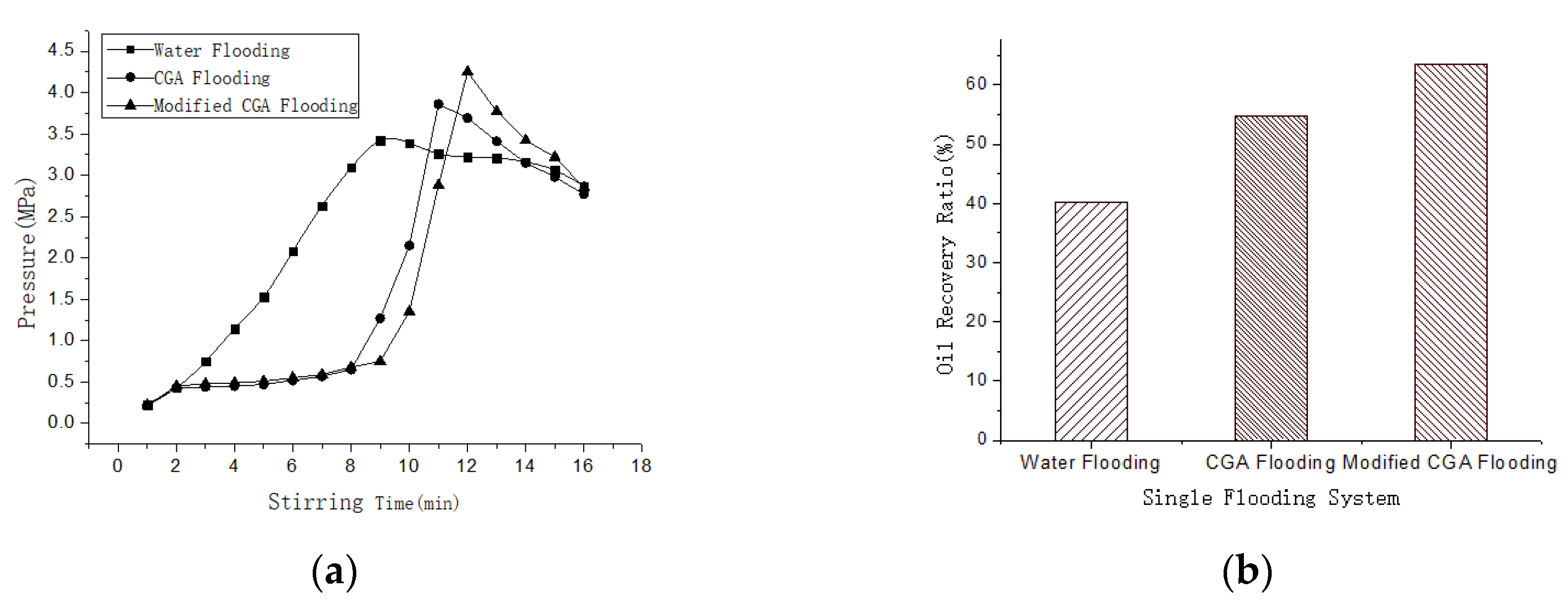
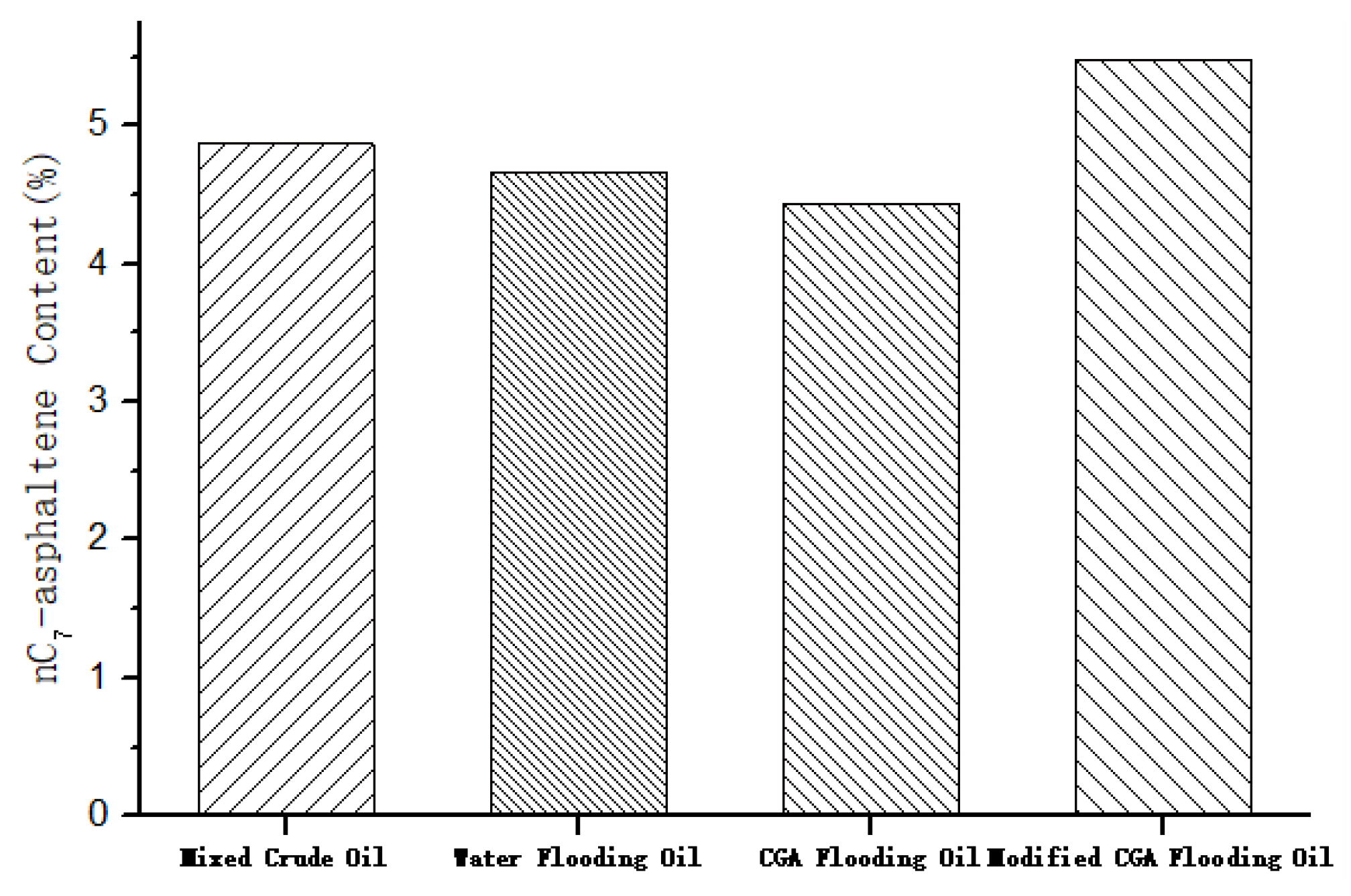
3.5. The Performance of the Modified CGA Foam Flooding
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zou, Y. Oil Production Technology, 1st ed.; Petroleum Industry Press: Beijing, China, 2006. [Google Scholar]
- Zhao, C.; Me, S.; Zhou, S.; Yu, S.; Jiang, J. Discussion on two key questions of ultral low interfacial tension foam system displacement. Pet. Geol. Oilfield Dev. Daqing 2005, 24, 87–89, 92–111. [Google Scholar]
- YU, H.; WAN, X.; Liu, Q.; Chen, W. Pilot Field Test of Foam Compound Flooding. Pet. Geol. Oilfield Dev. Daqing 2003, 20, 108–110, 110–140. [Google Scholar]
- Lu, X.; Chen, H.; Luan, Z. Study on the mechanism of nitrogen hot-water foam flooding. J. Xi’an Pet. Inst. (Nat. Sci. Ed.) 2003, 18, 44, 49–52. [Google Scholar]
- Dong, X.; Kang, W. Application of Surfactants in Oilfield, 1st ed.; Petroleum Industry Press: Beijing, China, 2005. [Google Scholar]
- Long, D.; Zhu, W.; Lou, Z.; Yue, X. Clean production technlogy of oil industry-the characteristics of foam flood and the mechanism of oil recovery. Petrochem. Des. 2007, 24, 47–50. [Google Scholar]
- Sebba, F. Biliquid foams-a preliminary report. J. Colloid Interf. Sci. 1972, 40, 468–474. [Google Scholar] [CrossRef]
- Sebba, F. The behavior of minute oil droplets encasulated in a water film. Colloid Polym. Sci. 1979, 257, 392–396. [Google Scholar] [CrossRef]
- Sebba, F. Foams and Biliquid Foam-Aphrons; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Sebba, F. Preparation and properties of polyaphrons (biliquid foams). Chem. Industry 1984, 367–372. [Google Scholar]
- Bergeron, V.; Sebba, F. An unusual gel without a gelling agent. Langmuir 1987, 3, 857–858. [Google Scholar] [CrossRef]
- Bjorndalen, H.N.; Jossy, W.E.; Alvarez, J.M.; Kuru, E. A laboratory investigation of the factors controlling the filtration loss when drilling with colloidal gas aphron (CGA) fluids. J. Petrol. Sci. Eng. 2014, 117, 1–7. [Google Scholar] [CrossRef]
- Bjorndalen, N.; Kuru, E. Physico-Chemical Characterization of Aphron-Based Drilling Fluids. J. Can. Pet. Technol. 2008, 47, 7. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L. Optimization of Characteristics for Colloidal Gas Aphruns. Environ. Sci. Technol. 2006, 29, 37–39. [Google Scholar]
- Zhao, G.; Zhu, B. Measurement of surface tension of liquid - introduction of drop volume. Chem. Bull. 1981, 6, 21–26. [Google Scholar]



| Item | Oil Displacement System | Void Volume (mL) | Water Phase Permeability (µm2) | Porosity (%) | Oil Saturation (%) |
|---|---|---|---|---|---|
| Sandpack cylinder 1 | Water flooding | 64.7 | 2.15 | 29.6 | 88.3 |
| Sandpack cylinder 2 | CGA flooding | 64.5 | 2.14 | 29.5 | 88.2 |
| Sandpack cylinder 3 | Modified CGA flooding | 64.6 | 2.13 | 29.6 | 88.3 |
| Parameter | Water Flooding | CGA Flooding | Modified CGA Flooding |
|---|---|---|---|
| Oil recovery ratio (%) | 42.1 | 10.7 | 16.4 |
| Parameter | CGA-Mixed Crude Oil System | Modified CGA-Mixed Crude Oil System |
|---|---|---|
| Interfacial tension (mN∙m−1) | 3.29 | 2.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.; Sun, X.; Li, X. Preparation of Modified Colloidal Gas Aphrons and Analysis of the Oil Displacement Effect. Appl. Sci. 2020, 10, 927. https://doi.org/10.3390/app10030927
You S, Sun X, Li X. Preparation of Modified Colloidal Gas Aphrons and Analysis of the Oil Displacement Effect. Applied Sciences. 2020; 10(3):927. https://doi.org/10.3390/app10030927
Chicago/Turabian StyleYou, Shaohua, Xiaofei Sun, and Xiaoyu Li. 2020. "Preparation of Modified Colloidal Gas Aphrons and Analysis of the Oil Displacement Effect" Applied Sciences 10, no. 3: 927. https://doi.org/10.3390/app10030927
APA StyleYou, S., Sun, X., & Li, X. (2020). Preparation of Modified Colloidal Gas Aphrons and Analysis of the Oil Displacement Effect. Applied Sciences, 10(3), 927. https://doi.org/10.3390/app10030927






