Vascularized Lower Respiratory-Physiology-On-A-Chip
Abstract
1. Introduction
2. Lower Respiratory Microenvironment
2.1. Lower Respiratory
2.2. Lower Respiratory Extracellular Matrix Composition and Role
2.2.1. Compositions of Extracellular Matrix and Its Roles
2.2.2. Pulmonary Extracellular Matrix Composition in Disease
2.3. Construction of the Pulmonary Extracellular Matrix Environment in Lower Respiratory-Physiology-On-A-Chip
2.3.1. Natural Polymers
2.3.2. Decellularized Pulmonary Extracellular Matrix
3. Lower Respiratory-Physiology-On-A-Chip
3.1. 2D Lower Respiratory-Physiology-On-A-Chip
3.2. 3D Lower Respiratory-Physiology-On-A-Chip
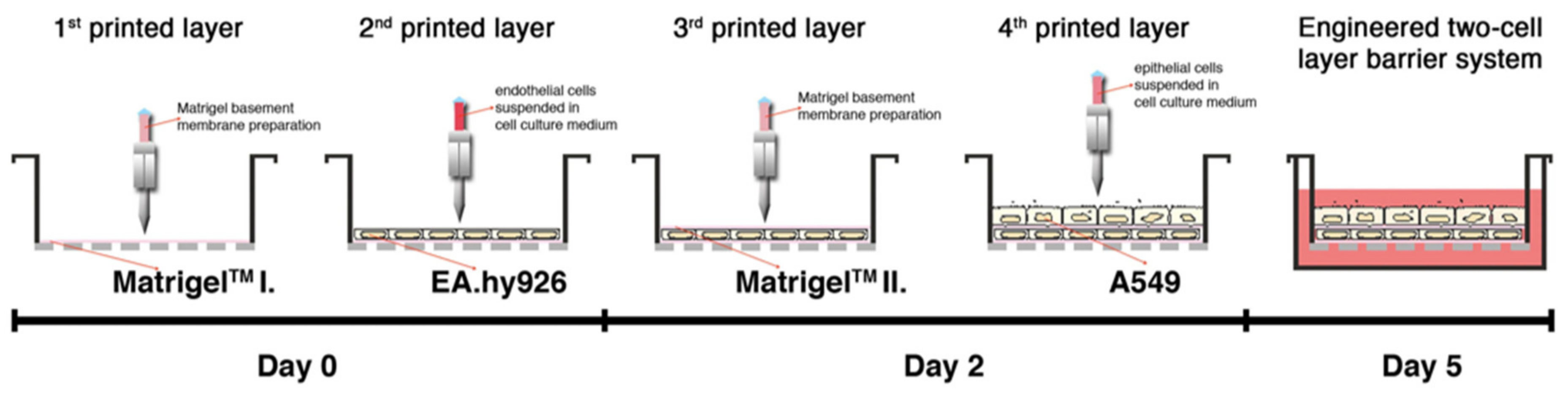
3.2.1. Transwell-Based Air–Liquid Interface Respiratory Model
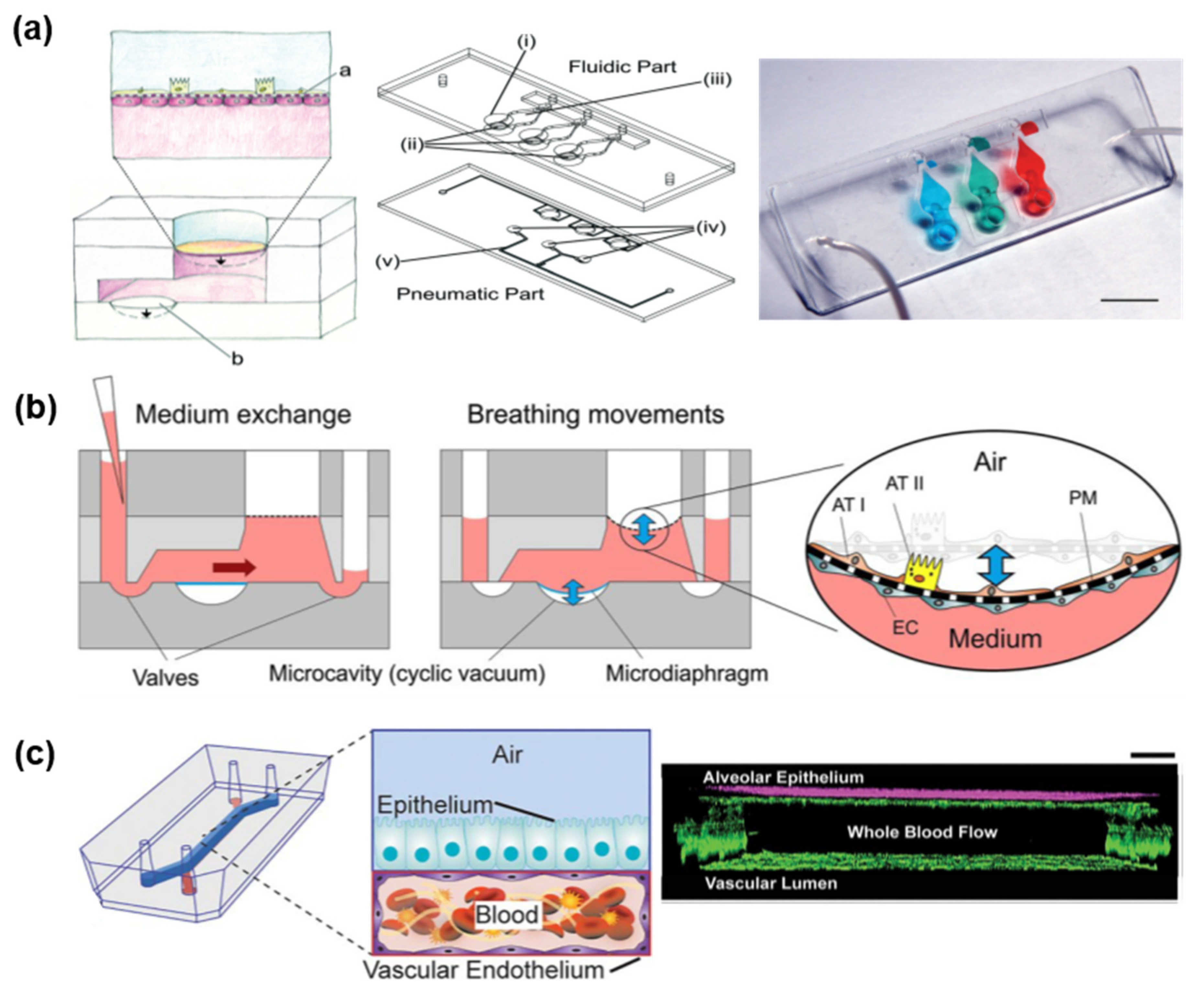
3.2.2. Microfluidic Chip-Based Air–Liquid Interface Respiratory Model
4. Vascularized Respiratory-Physiology-On-A-Chip
4.1. Need for Vascularization
4.2. The Pathophysiological Importance of Pulmonary Vasculature
4.3. Vascularized Respiratory-Physiology-On-A-Chip
4.3.1. Lung-On-A-Chip
4.3.2. Airway-On-A-Chip
5. Application as a Disease Model for Vascularized Lower Respiratory-Physiology-On-A-Chip
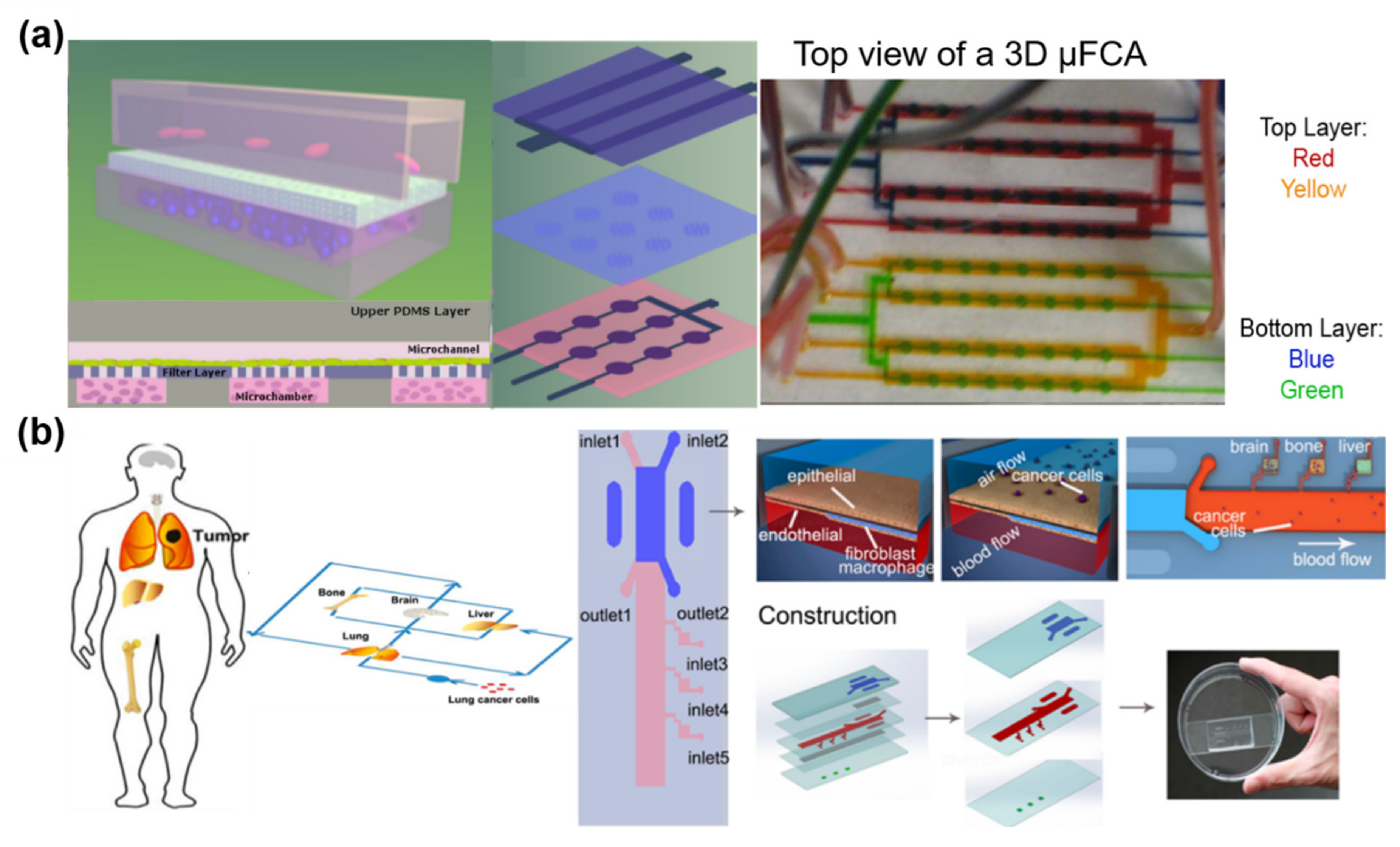
5.1. Cancer
5.2. Asthma, COPD, and PE
6. Future Perspectives and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ferkol, T.; Schraufnagel, D. The Global Burden of Respiratory Disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 4, FSO63. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Pratap, K.; Sinha, J.; Desiraju, K.; Bahal, D.; Kukreti, R. Critical evaluation of challenges and future use of animals in experimentation for biomedical research. Int. J. Immunopathol. Pharmacol. 2016, 29, 551–561. [Google Scholar] [CrossRef]
- Mosig, A.S. Organ-on-chip models: New opportunities for biomedical research. Future Sci. OA 2016, 3, FSO130. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.-H.; Won Kim, S.; Rhie, J.-W.; Shim, J.-H.; Kim, D.-H.; Cho, D.-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Sell, S.; Barnes, C.; Smith, M.; McClure, M.; Madurantakam, P.; Grant, J.; McManus, M.; Bowlin, G. Extracellular matrix regenerated: Tissue engineering via electrospun biomimetic nanofibers. Polym. Int. 2007, 56, 1349–1360. [Google Scholar] [CrossRef]
- Choi, B.-H.; Choi, Y.S.; Kang, D.G.; Kim, B.J.; Song, Y.H.; Cha, H.J. Cell behavior on extracellular matrix mimic materials based on mussel adhesive protein fused with functional peptides. Biomaterials 2010, 31, 8980–8988. [Google Scholar] [CrossRef]
- Green, J.J.; Elisseeff, J.H. Mimicking biological functionality with polymers for biomedical applications. Nature 2016, 540, 386–394. [Google Scholar] [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From Three-Dimensional Cell Culture to Organs-on-Chips. Trends Cell Biol. 2011, 21, 745–754. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Aguilar-Aguila-Isaías, M.A.; García-Reyes, I.E.; Hernández-Antonio, A.; Ahmed, I.; Sharma, A.; Parra-Saldívar, R.; Iqbal, H.M.N. Organs-on-a-Chip Module: A Review from the Development and Applications Perspective. Micromachines 2018, 9, 536. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-H.; Punde, T.H.; Shih, P.-C.; Fu, C.-Y.; Wang, T.-P.; Hsu, L.; Chang, H.-Y.; Liu, C.-H. A capillary-endothelium-mimetic microfluidic chip for the study of immune responses. Sens. Actuators B Chem. 2015, 209, 470–477. [Google Scholar] [CrossRef]
- Sebastian, B.; Dittrich, P.S. Microfluidics to Mimic Blood Flow in Health and Disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar] [CrossRef]
- Michna, R.; Gadde, M.; Ozkan, A.; DeWitt, M.; Rylander, M. Vascularized microfluidic platforms to mimic the tumor microenvironment. Biotechnol. Bioeng. 2018, 115, 2793–2806. [Google Scholar] [CrossRef]
- Tu, J.; Inthavong, K.; Ahmadi, G. Computational Fluid and Particle Dynamics in the Human Respiratory System; Biological and Medical Physics, Biomedical Engineering; Springer: Berlin, Germany, 2013; ISBN 978-94-007-4487-5. [Google Scholar]
- Breeze, R.; Turk, M. Cellular structure, function and organization in the lower respiratory tract. Environ. Health Perspect. 1984, 55, 3–24. [Google Scholar] [CrossRef]
- Hogan, B.; Tata, P.R. Cellular organization and biology of the respiratory system. Nat. Cell Biol. 2019. [Google Scholar] [CrossRef]
- Bérubé, K.; Prytherch, Z.; Job, C.; Hughes, T. Human primary bronchial lung cell constructs: The new respiratory models. Toxicology 2010, 278, 311–318. [Google Scholar] [CrossRef]
- Raghu, G.; Striker, L.J.; Hudson, L.D.; Striker, G.E. Extracellular matrix in normal and fibrotic human lungs. Am. Rev. Respir. Dis. 1985, 131, 281–289. [Google Scholar]
- McGowan, S.E. Extracellular matrix and the regulation of lung development and repair. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1992, 6, 2895–2904. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A.; Kaminski, N. Idiopathic Pulmonary Fibrosis: Aberrant Recapitulation of Developmental Programs? PLoS Med. 2008, 5, 0050062. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Ritzenthaler, J.D.; Fenton, M.J.; Roser, S.; Schuyler, W. Transcriptional regulation of the human interleukin 1beta gene by fibronectin: role of protein kinase C and activator protein 1 (AP-1). Cytokine 2000, 12, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Schaefer, L.; Iozzo, R.V. Instructive roles of extracellular matrix on autophagy. Am. J. Pathol. 2014, 184, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Tschumperlin, D.J. Matrix, mesenchyme, and mechanotransduction. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S24–S29. [Google Scholar] [CrossRef]
- Zhou, Y.; Horowitz, J.C.; Naba, A.; Ambalavanan, N.; Atabai, K.; Balestrini, J.; Bitterman, P.B.; Corley, R.A.; Ding, B.-S.; Engler, A.J.; et al. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol. J. 2018, 73, 77–104. [Google Scholar] [CrossRef]
- Annoni, R.; Lanças, T.; Yukimatsu Tanigawa, R.; de Medeiros Matsushita, M.; de Morais Fernezlian, S.; Bruno, A.; Fernando Ferraz da Silva, L.; Roughley, P.J.; Battaglia, S.; Dolhnikoff, M.; et al. Extracellular matrix composition in COPD. Eur. Respir. J. 2012, 40, 1362–1373. [Google Scholar] [CrossRef]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for In Vitro Investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Hoshiba, T.; Yamaoka, T. CHAPTER 1 Extracellular Matrix Scaffolds for Tissue Engineering and Biological Research. RSC 2019. [Google Scholar]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab. Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Benam, K.H.; Villenave, R.; Lucchesi, C.; Varone, A.; Hubeau, C.; Lee, H.-H.; Alves, S.E.; Salmon, M.; Ferrante, T.C.; Weaver, J.C.; et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat. Methods 2016, 13, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Benam, K.H.; Novak, R.; Nawroth, J.; Hirano-Kobayashi, M.; Ferrante, T.C.; Choe, Y.; Prantil-Baun, R.; Weaver, J.C.; Bahinski, A.; Parker, K.K.; et al. Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst. 2016, 3, 456–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Chow, C.-W.; Young, E.W.K. Microfluidic lung airway-on-a-chip with arrayable suspended gels for studying epithelial and smooth muscle cell interactions. Lab. Chip 2018, 18, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Jiang, L.; Qin, J. A 3D human lung-on-a-chip model for nanotoxicity testing. Toxicol. Res. 2018, 7, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Douville, N.J.; Zamankhan, P.; Tung, Y.-C.; Li, R.; Vaughan, B.L.; Tai, C.-F.; White, J.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Combination of fluid and solid mechanical stresses contribute to cell death and detachment in a microfluidic alveolar model. Lab. Chip 2011, 11, 609–619. [Google Scholar] [CrossRef]
- Taylor, P.M.; Cass, A.E.G.; Yacoub, M.H. Extracellular matrix scaffolds for tissue engineering heart valves. Prog. Pediatr. Cardiol. 2006, 21, 219–225. [Google Scholar] [CrossRef]
- Berkholtz, C.B.; Lai, B.E.; Woodruff, T.K.; Shea, L.D. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem. Cell Biol. 2006, 126, 583–592. [Google Scholar] [CrossRef]
- Buzza, M.S.; Zamurs, L.; Sun, J.; Bird, C.H.; Smith, A.I.; Trapani, J.A.; Froelich, C.J.; Nice, E.C.; Bird, P.I. Extracellular Matrix Remodeling by Human Granzyme B via Cleavage of Vitronectin, Fibronectin, and Laminin. J. Biol. Chem. 2005, 280, 23549–23558. [Google Scholar] [CrossRef]
- Balestrini, J.L.; Niklason, L.E. Extracellular matrix as a driver for lung regeneration. Ann. Biomed. Eng. 2015, 43, 568–576. [Google Scholar] [CrossRef]
- Calle, E.A.; Mendez, J.J.; Ghaedi, M.; Leiby, K.L.; Bove, P.F.; Herzog, E.L.; Sundaram, S.; Niklason, L.E. Fate of distal lung epithelium cultured in a decellularized lung extracellular matrix. Tissue Eng. Part A 2015, 21, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch. Med. Sci. AMS 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Frega, M. Neuronal Network Dynamics in 2D and 3D in vitro Neuroengineered Systems; Springer: Berlin, Germany, 2016; ISBN 978-3-319-30237-9. [Google Scholar]
- Lin, H.; Li, H.; Cho, H.-J.; Bian, S.; Roh, H.-J.; Lee, M.-K.; Kim, J.S.; Chung, S.-J.; Shim, C.-K.; Kim, D.-D. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J. Pharm. Sci. 2007, 96, 341–350. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Starner, T.D.; Scheetz, T.E.; Traver, G.L.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G.; McCray, P.B.; Zabner, J. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L25–L31. [Google Scholar] [CrossRef]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of Normal Human Bronchial Epithelial (NHBE) Cell 3D Cultures for in vitro Lung Model Studies. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Eenjes, E.; Mertens, T.C.J.; Buscop-van Kempen, M.J.; van Wijck, Y.; Taube, C.; Rottier, R.J.; Hiemstra, P.S. A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Firth, A.L.; Dargitz, C.T.; Qualls, S.J.; Menon, T.; Wright, R.; Singer, O.; Gage, F.H.; Khanna, A.; Verma, I.M. Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, E1723–E1730. [Google Scholar] [CrossRef]
- Schruf, E.; Schroeder, V.; Le, H.Q.; Schönberger, T.; Raedel, D.; Stewart, E.L.; Fundel-Clemens, K.; Bluhmki, T.; Weigle, S.; Schuler, M.; et al. Recapitulating idiopathic pulmonary fibrosis related alveolar epithelial dysfunction in an iPSC-derived air-liquid interface model. bioRxiv 2019, 830109. Available online: https://www.biorxiv.org/content/10.1101/830109v1 (accessed on 30 January 2020).
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R.; et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; Broekman, W.; Daniel, N.M.; van der Vlugt, L.E.P.M.; van Schadewijk, A.; Taube, C.; Hiemstra, P.S. Cigarette Smoke Modulates Repair and Innate Immunity following Injury to Airway Epithelial Cells. PloS ONE 2016, 11, e0166255. [Google Scholar] [CrossRef] [PubMed]
- Nalayanda, D.D.; Puleo, C.; Fulton, W.B.; Sharpe, L.M.; Wang, T.-H.; Abdullah, F. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed. Microdevices 2009, 11, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Fujioka, H.; Tung, Y.-C.; Futai, N.; Paine, R.; Grotberg, J.B.; Takayama, S. Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proc. Natl. Acad. Sci. USA 2007, 104, 18886–18891. [Google Scholar] [CrossRef] [PubMed]
- Punde, T.H.; Wu, W.-H.; Lien, P.-C.; Chang, Y.-L.; Kuo, P.-H.; Chang, M.D.-T.; Lee, K.-Y.; Huang, C.-D.; Kuo, H.-P.; Chan, Y.-F.; et al. A biologically inspired lung-on-a-chip device for the study of protein-induced lung inflammation. Integr. Biol. Quant. Biosci. Nano Macro 2015, 7, 162–169. [Google Scholar] [CrossRef]
- Dong, H.; Zheng, L.; Duan, X.; Zhao, W.; Chen, J.; Liu, S.; Sui, G. Cytotoxicity analysis of ambient fine particle in BEAS-2B cells on an air-liquid interface (ALI) microfluidics system. Sci. Total Environ. 2019, 677, 108–119. [Google Scholar] [CrossRef]
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Cukierman, E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [Google Scholar] [CrossRef]
- Ghaemmaghami, A.M.; Hancock, M.J.; Harrington, H.; Kaji, H.; Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 2012, 17, 173–181. [Google Scholar] [CrossRef]
- Chung, M.; Ahn, J.; Son, K.; Kim, S.; Jeon, N.L. Biomimetic Model of Tumor Microenvironment on Microfluidic Platform. Adv. Healthc. Mater. 2017, 6, 1700196. [Google Scholar] [CrossRef]
- Li, M.; Ku, D.N.; Forest, C.R. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab. Chip 2012, 12, 1355–1362. [Google Scholar] [CrossRef]
- Blanco, I.; Piccari, L.; Barberà, J.A. Pulmonary vasculature in COPD: The silent component. Respirology 2016, 21, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Barberà, J.A.; Peinado, V.I.; Santos, S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 21, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F.; Cool, C.D. Pulmonary vascular involvement in chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 28s–32s. [Google Scholar] [CrossRef] [PubMed]
- Meyrick, B. Structure function correlates in the pulmonary vasculature during acute lung injury and chronic pulmonary hypertension. Toxicol. Pathol. 1991, 19, 447–457. [Google Scholar] [CrossRef]
- Hassoun, P.M.; Mouthon, L.; Barberà, J.A.; Eddahibi, S.; Flores, S.C.; Grimminger, F.; Jones, P.L.; Maitland, M.L.; Michelakis, E.D.; Morrell, N.W.; et al. Inflammation, Growth Factors, and Pulmonary Vascular Remodeling. J. Am. Coll. Cardiol. 2009, 54, S10–S19. [Google Scholar] [CrossRef]
- Coker, R.K.; Laurent, G.J. Pulmonary fibrosis: cytokines in the balance. Eur. Respir. J. 1998, 11, 1218–1221. [Google Scholar] [CrossRef]
- Gonzales, J.N.; Verin, A.D. Pulmonary Vascular Endothelial Cells. In Endothelial Dysfunction; InTech Open: London, UK, 2018; ISBN 978-1-78984-254-8. [Google Scholar]
- Erzurum, S.; Rounds, S.I.; Stevens, T.; Aldred, M.; Aliotta, J.; Archer, S.L.; Asosingh, K.; Balaban, R.; Bauer, N.; Bhattacharya, J.; et al. Strategic Plan for Lung Vascular Research. Am. J. Respir. Crit. Care Med. 2010, 182, 1554–1562. [Google Scholar] [CrossRef]
- Stucki, J.D.; Hobi, N.; Galimov, A.; Stucki, A.O.; Schneider-Daum, N.; Lehr, C.-M.; Huwer, H.; Frick, M.; Funke-Chambour, M.; Geiser, T.; et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Jain, A.; Barrile, R.; van der Meer, A.D.; Mammoto, A.; Mammoto, T.; De Ceunynck, K.; Aisiku, O.; Otieno, M.A.; Louden, C.S.; Hamilton, G.A.; et al. Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics. Clin. Pharmacol. Ther. 2018, 103, 332–340. [Google Scholar] [CrossRef]
- Horváth, L.; Umehara, Y.; Jud, C.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci. Rep. 2015, 5, 7974. [Google Scholar] [CrossRef]
- Park, J.Y.; Ryu, H.; Lee, B.; Ha, D.-H.; Ahn, M.; Kim, S.; Kim, J.Y.; Jeon, N.L.; Cho, D.-W. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018, 11, 015002. [Google Scholar] [CrossRef]
- Dereli-Korkut, Z.; Akaydin, H.D.; Ahmed, A.H.R.; Jiang, X.; Wang, S. Three dimensional microfluidic cell arrays for ex vivo drug screening with mimicked vascular flow. Anal. Chem. 2014, 86, 2997–3004. [Google Scholar] [CrossRef]
- Xu, Z.; Li, E.; Guo, Z.; Yu, R.; Hao, H.; Xu, Y.; Sun, Z.; Li, X.; Lyu, J.; Wang, Q. Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2016, 8, 25840–25847. [Google Scholar] [CrossRef]
- Huh, D.; Leslie, D.C.; Matthews, B.D.; Fraser, J.P.; Jurek, S.; Hamilton, G.A.; Thorneloe, K.S.; McAlexander, M.A.; Ingber, D.E. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci. Transl. Med. 2012, 4, 159ra147. [Google Scholar] [CrossRef]
| ECM Glycoproteins | Collagens | Proteoglycans | |
|---|---|---|---|
| 5430419D17RIK | LGI3 | COL10A1 | ACAN |
| ABI3BP | LTBP1; LTBP2; LTBP3; LTBP4 | COL11A1; 11A2 | ASPN |
| ADIPOQ | MATN1; MATN2; MATN4 | COL12A1 | BGN |
| AEBP1 | MFAP2; MFAP4; MFAP5 | COL13A1 | CHAD |
| AGRN | MFGE8 | COL14A1 | DCN |
| AW551984 | MGP | COL15A1 | FMOD |
| BMPER | MMRN1; MMRN2 | COL16A1 | HAPLN1 |
| CILP | NDNF | COL17A1 | HAPLN3 |
| CILP2 | NID1; NID2 | COL18A1 | HAPLN4 |
| COLQ | NPNT | COL19A1 | HSPG2 |
| COMP | NTN1; NTN3; NTN4 | COL1A1; 1A2 | IMPG1 |
| CRISPLD2 | PAPLN | COL22A1 | LUM |
| DPT | PCOLCE; PCOLCE2 | COL23A1 | OGN |
| ECM1; ECM2 | POSTN | COL24A1 | PODN |
| EFEMP1; EFEMP2 | PXDN | COL25A1 | PRELP |
| EGFEM1 | RELN | COL27A1 | PRG2 |
| ELN | SBSPON | COL28A1 | PRG3 |
| EMID1 | SLIT3 | COL2A1 | VCAN |
| EMILIN1; EMILIN2 | SNED1 | COL3A1 | |
| FBLN1; FBLN2; FBLN5 | SPARC; SPARCL1 | COL4A1; 2; 3; 4; 5; 6 | |
| FBN1; FBN2 | SPON1 | COL5A1; 5A2; 5A3 | |
| FGA; FGB; FGG | SRPX; SPRX2 | COL6A1; 2; 3; 4; 5; 6 | |
| FGL2 | SVEP1 | COL7A1 | |
| FN1 | TGFBI | COL8A1; A2 | |
| FRAS1 | THBS1; THBS2; THBS3 | COL9A1; 9A2; 9A3 | |
| GLDN | THSD4 | ||
| HMCN1; HMCN2 | TINAG; TINAGL1 | ||
| IGFALS | TNC; TNXB | ||
| IGFBP6; IGFBP7 | VTN | ||
| IGSF10 | VWA1; 3A; 5A; 5B1; A9 | ||
| KCP | VWF | ||
| LAMA1; A2; A3; A4; A5; B1; B2; B3; C1; C2; C3 | WISP2 | ||
| Polymer Composition | Cell Populations | Features | Ref. |
|---|---|---|---|
| Collagen I | Primary human airway epithelial cells, human lung microvascular endothelial cells, neutrophils Primary human airway epithelial cells obtained from healthy donors or COPD patients | Porous membrane sandwiched microchip, secretion of the inflammatory cytokines | [33] |
| Porous membrane sandwiched microchip, smoke-induced pathological microenvironment | [34] | ||
| Collagen I/Fibronectin | Human alveolar epithelial cells, endothelium cells, neutrophils | Porous membrane sandwiched microchip, alveolar-capillary barrier | [32] |
| Collagen I/Matrigel | Calu-3, human bronchial smooth muscle cells | Multilayer PMMA chip | [35] |
| Collagen I/Matrigel/Chitosan | Human alveolar epithelial cells, HUVECs | Multichannel microfluidic chip, perfusion culture | [36] |
| Collagen I/Fibronectin/Gelatin | Bronchial epithelial cells, primary human pulmonary alveolar epithelial cells, HUVECs | Porous membrane sandwiched microchip, mechanical stress induced by respiration movements | [31] |
| Fibronectin | A549, primary murine alveolar epithelial cells | Porous membrane sandwiched microchip, surface tension forces | [37] |
| Coating Material | Cell Population | Features | Ref. |
|---|---|---|---|
| Fibronectin or collagen | Human alveolar epithelial cells and microvascular endothelial cells | Stretchable microfluidic system | [32] |
| Fibronectin or gelatin and collagen I | Bronchial epithelial cells and primary human pulmonary alveolar epithelial cells, primary HUVECs | Stretchable microfluidic system | [31] |
| Fibronectin and collagen IV/ I | Bronchial epithelial 16HBE14o- cells, primary human alveolar epithelial cells, primary human lung microvascular endothelial cells | Stretchable microfluidic system | [72] |
| Fibronectin and collagen I | Human umbilical vein endothelial cells from pooled donors, primary alveolar epithelial cells containing type I and II cells | Microfluidic system | [73] |
| Coating Material | Cell Population | Features | Ref. |
|---|---|---|---|
| Collagen I | Primary human small airway epithelial cells and primary human lung microvascular endothelial cells | Microfluidic system | [33] |
| Decellularized extracellular matrix bioink derived from porcine tracheal mucosa | Human dermal microvascular endothelial cells and primary human tracheal epithelial cells and lung fibroblasts | 3D printing | [75] |
| Matrigel | human alveolar epithelial type II cell line A549 and the EA.hy926 hybrid human cell line | 3D printing | [74] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.; Choi, Y.-m.; Jang, J. Vascularized Lower Respiratory-Physiology-On-A-Chip. Appl. Sci. 2020, 10, 900. https://doi.org/10.3390/app10030900
Nam H, Choi Y-m, Jang J. Vascularized Lower Respiratory-Physiology-On-A-Chip. Applied Sciences. 2020; 10(3):900. https://doi.org/10.3390/app10030900
Chicago/Turabian StyleNam, Hyoryung, Yoo-mi Choi, and Jinah Jang. 2020. "Vascularized Lower Respiratory-Physiology-On-A-Chip" Applied Sciences 10, no. 3: 900. https://doi.org/10.3390/app10030900
APA StyleNam, H., Choi, Y.-m., & Jang, J. (2020). Vascularized Lower Respiratory-Physiology-On-A-Chip. Applied Sciences, 10(3), 900. https://doi.org/10.3390/app10030900







