Synthesis and Characterization of Highly Crystalline Vertically Aligned WSe2 Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Synthesis of WSe2 Nanosheets
2.1.2. Characterization Techniques
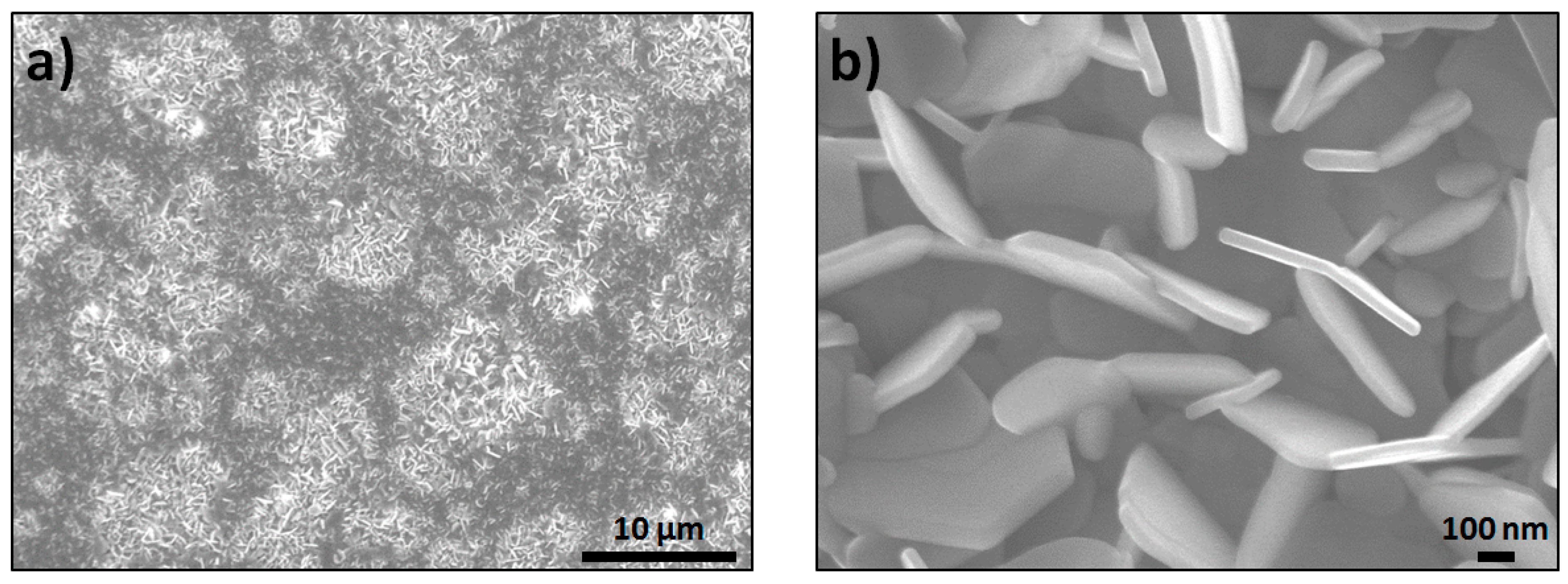
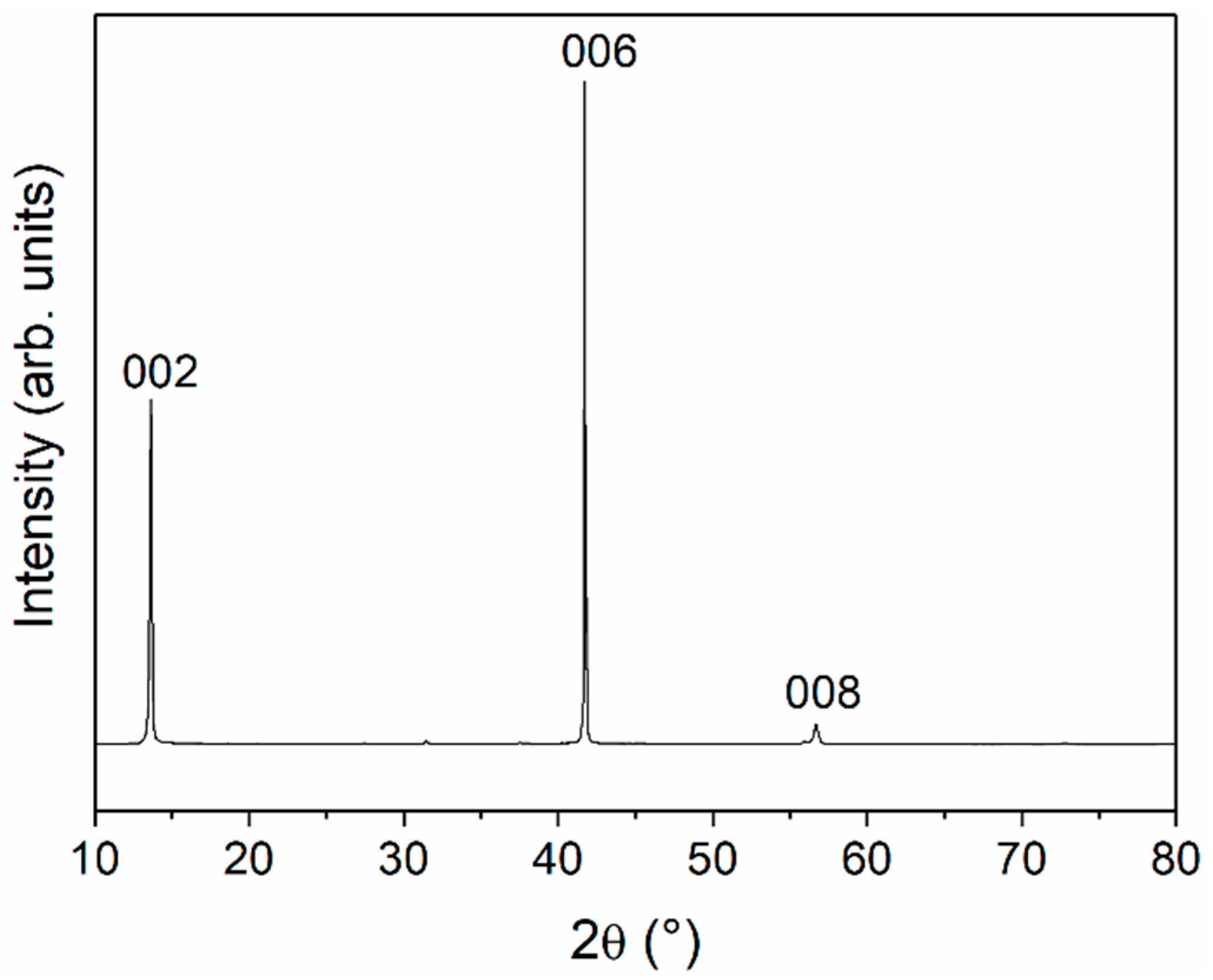
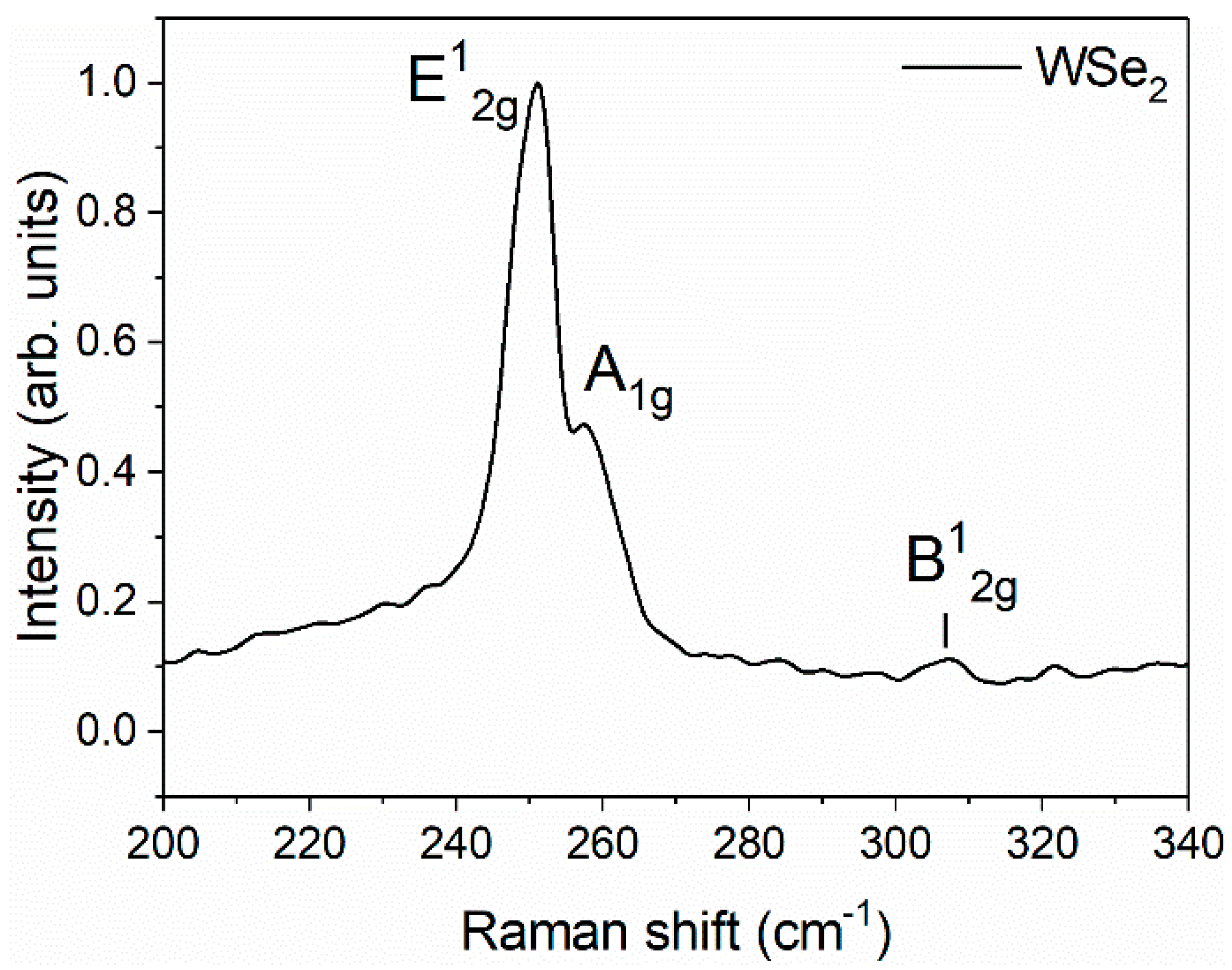
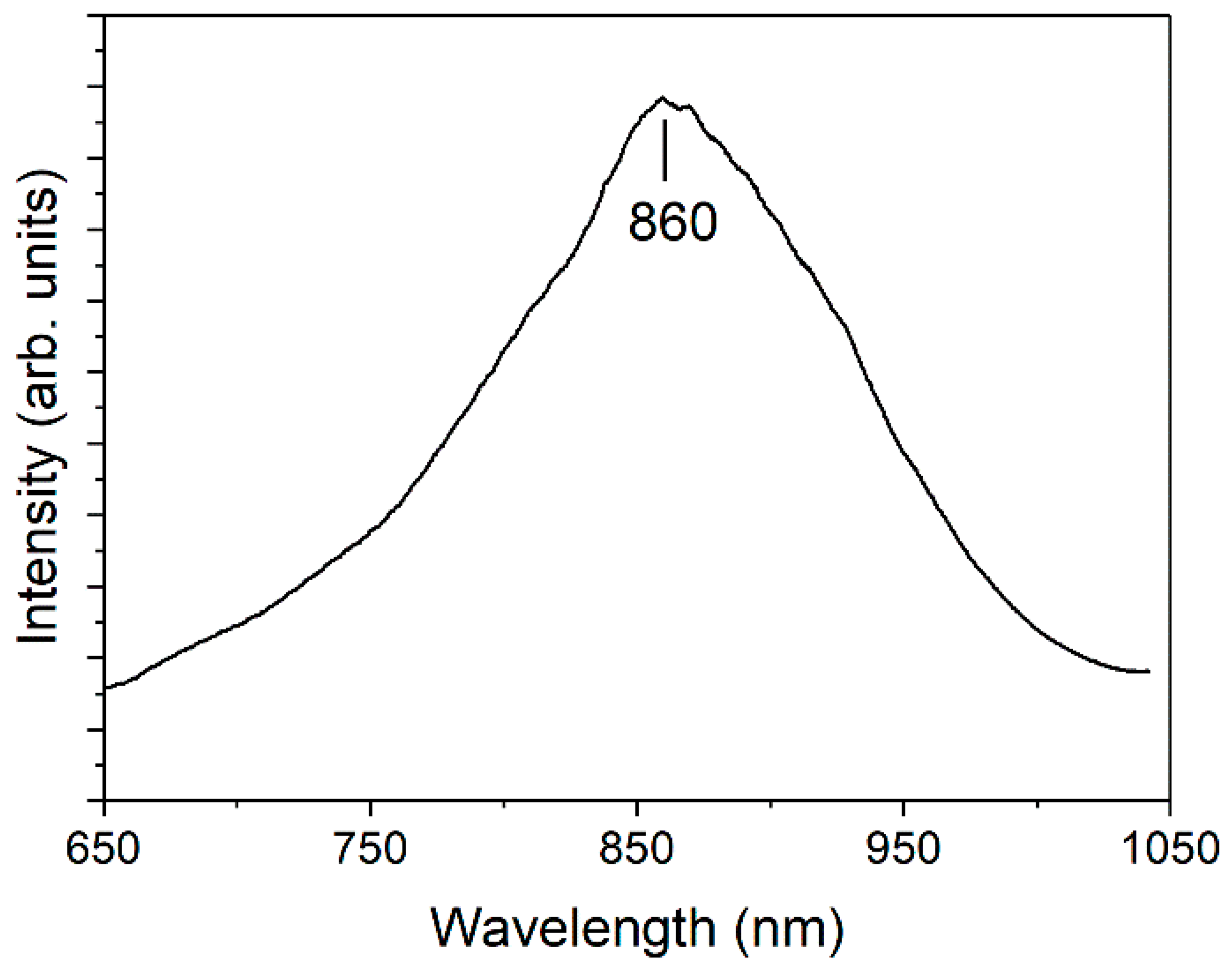
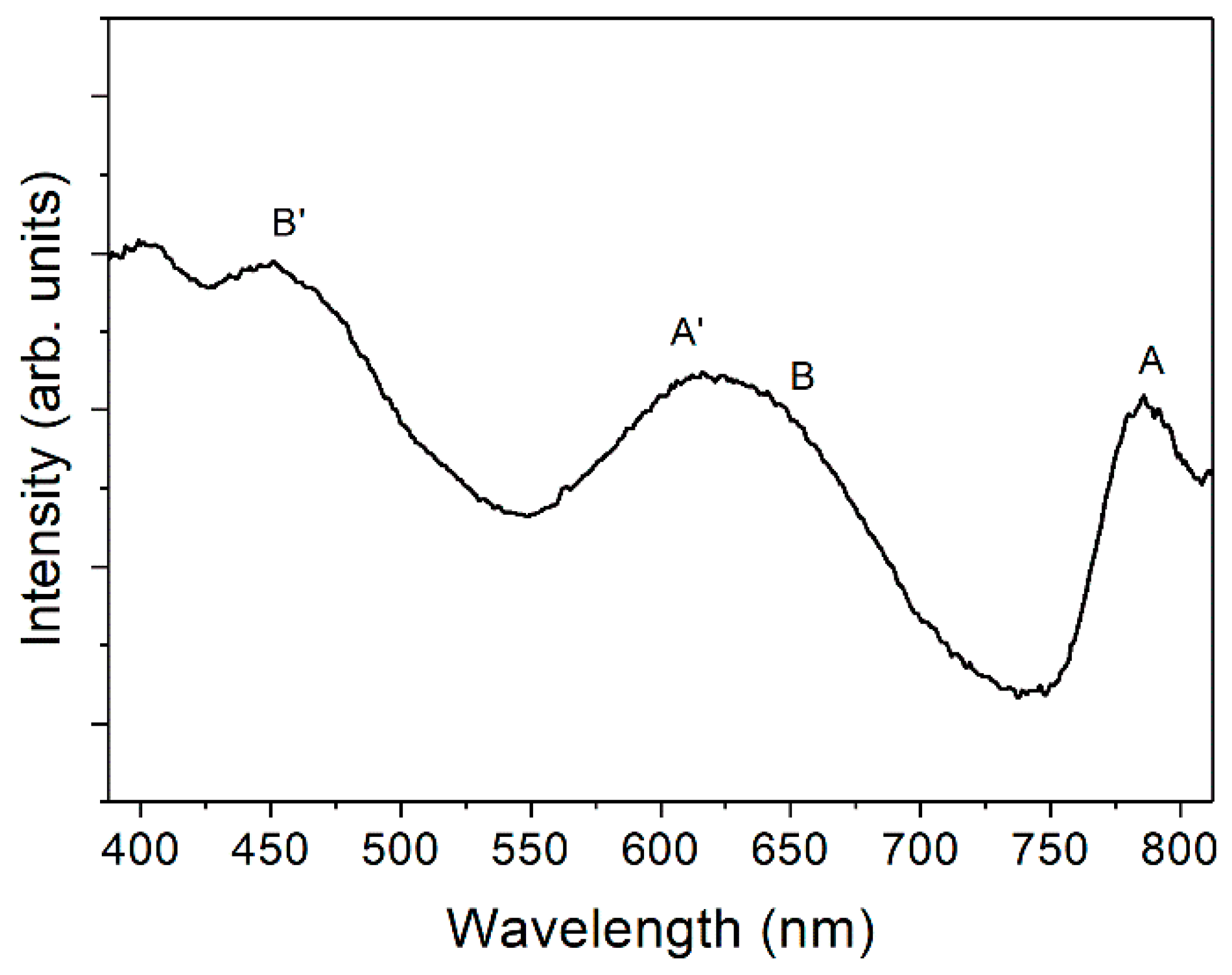
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263. [Google Scholar] [CrossRef]
- Hao, G.; Kou, L.; Lu, D.; Peng, J.; Li, J.; Tang, C.; Zhong, J. Electrostatic properties of two-dimensional WSe2 nanostructures. J. Appl. Phys. 2016, 119, 035301. [Google Scholar] [CrossRef]
- Chiritescu, C.; Cahill, D.G.; Nguyen, N.; Johnson, D.; Bodapati, A.; Keblinski, P.; Zschack, P. Ultralow termal conductivity in disordered, layered WSe2 crystals. Science 2007, 315, 351–353. [Google Scholar] [CrossRef]
- Velazquez, J.M.; Saadi, F.H.; Pieterick, A.P.; Spurgeon, J.M.; Soriaga, M.P.; Brunschwig, B.S.; Lewis, N.S. Synthesis and hydrogen-evolution activity of tungsten selenide thin films deposited on tungsten foils. J. Electroanal. Chem. 2014, 716, 45–48. [Google Scholar] [CrossRef]
- Wang, H.; Kong, D.; Johanes, P.; Cha, J.; Zheng, G.; Yan, K.; Liu, N.; Cui, Y. MoS2 and WSe2 nanofilms with vertically aligned moleculer layers on curved and rough surfaces. ACS Nano 2013, 13, 3426–3433. [Google Scholar]
- Bozheyev, F.; Friedrich, D.; Nie, M.; Rengachari, M.; Ellmer, K. Preparation of highly (001)-oriented photoactive tungstene diselenide (WSe2) by an amorphous solid-liquid-crystalline solid (aSLcS) rapid-crystallization process. Phys. Status Solidi A 2014, 211, 2013–2019. [Google Scholar] [CrossRef]
- Jana, M.J.; Rao, C.N.R. Two dimensional analogues of graphene: Transition metal dichalgenides. Philos. Trans. R. Soc. A 2016, 374, 20150318. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chuang, S.; Chang, T.C.; Takei, K.; Takahashi, T.; Javey, A. High-performance single layered WSe2 p-FETs with chemically doped contacts. Nano Lett. 2012, 12, 3788–3792. [Google Scholar] [CrossRef] [PubMed]
- Baugher, B.W.H.; Churchill, H.O.H.; Yang, Y.; Jarillo-Herrero, P. Optoelectronic devices based on ellectrically tunable p-n diodes in a monolayer dichalcogenide. Nat. Nanotechnol. 2014, 9, 262–267. [Google Scholar] [CrossRef]
- Ross, J.S.; Klement, P.; Jones, A.M.; Ghimire, N.J.; Yan, J.; Mandrus, D.G.; Taniguchi, T.; Watanabe, K.; Kitamura, K.; Yao, W.; et al. Electrically tunable excitonic light-emitting diodes based on monolayer WSe2 p-n junctions. Nat. Nanotechnol. 2014, 9, 268–272. [Google Scholar] [CrossRef]
- Pospischil, A.; Furchi, M.M.; Mueller, T. Solar-energy conversion and light emission in a atomic monolayer p-n diodes. Nat. Nanotechnol. 2014, 9, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Jäger-Waldau, A.; Bucher, E. WSe2 thin films prepared by soft selenization. Thin Solid Films 1991, 200, 157–164. [Google Scholar] [CrossRef]
- Patel, A.A.; Rathod, J.R.; Patel, H.S.; Patel, K.D.; Pathak, V.M. Structural and optical characterization of tungstene diselenide crystals grown by DVT technique. Adv. Mater. Res. 2013, 665, 53–57. [Google Scholar] [CrossRef]
- Li, H.; Gao, D.; Li, K.; Pang, M.; Xie, S.; Liu, R.; Zou, J. Texture control and growth mechanism of WSe2 film prepared by rapid selenization of W film. Appl. Surf. Sci. 2017, 394, 142–148. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Wang, Y.; Yin, Z.; Cong, C.; He, Q.; Wang, L.; Ding, F.; Yu, T.; Zhang, H. Mechanical exfoliation and characterization of single and few-layer nanosheets of WSe2, TaS2 and TaSe2. Small 2013, 9, 1974–1981. [Google Scholar] [CrossRef]
- Magda, G.Z.; Peto, J.; Dobrik, G.; Hwang, C.; Biró, L.P.; Tapasztó, L. Exfoliation of large-area metal chalcogenide single layers. Sci. Rep. 2015, 5, 14714. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Wang, Y.; Laleyan, D.A.; Wu, Y.; Ouyang, B.; Ou, P.; Song, J.; Mi, Z. Wafer-scale synthesis of monolayer WSe2: A multi-functional photocatalyst for efficient overall pure water splitting. Nano Energy 2018, 51, 54–60. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Fominski, V.Y.; Gnedovets, A.G.; Romanov, R.I. Experimental and numerical study of the chemical composition of WSex thin films obtained by pulsed laser deposition in vacuum and in a buffer gas atmosphere. Appl. Surf. Sci. 2012, 258, 7000–7007. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Chen, J.-S.; Niu, H.-L.; Mao, C.-J.; Song, J.-M. Synthesis of ultrathin WSe2 nanosheets and their high-performance catalysis for conversion of amines to imines. Nanoscale 2018, 10, 20266–20271. [Google Scholar] [CrossRef]
- Jung, W.; Lee, S.; Yoo, D.; Jeong, D.; Miro, P.; Kuc, A.; Heine, T.; Cheon, J. Colloidal synthesis of single-layer MSe2 (M = Mo, W) nanosheets via anisotropic solution-phase growth approach. J. Am. Chem. Soc. 2015, 137, 7266–7269. [Google Scholar] [CrossRef]
- Jebaraj Devadasan, J.; Sanjeviraja, C.; Jayachandran, M. Electrosynthesis and characterization of n-WSe2 thin films. Mater. Chem. Phys. 2002, 77, 397–401. [Google Scholar] [CrossRef]
- Huang, J.K.; Pu, J.; Hsu, C.L.; Chiu, M.H.; Juang, Z.Y.; Chang, Y.H.; Chang, W.H.; Iwasa, Y.; Takenobu, T.; Li, L.J. Large-area synthesis of highly crystalline WSe2 monolayers and device applications. ACS Nano 2013, 8, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Boscher, N.D.; Carmalt, C.J.; Parkin, I.P. Atmospheric pressure chemical vapor deposition of WSe2 thin films on glass-highly hydrophobic sticky surfaces. J. Mater. Chem. 2006, 16, 122–127. [Google Scholar] [CrossRef]
- Liu, B.; Fathi, M.; Chen, L.; Abbas, A.; Ma, Y.; Zhou, C. Chemical vapor deposition growth of monolayer WSe2 with tunable device characteristics and growth mechanism study. ACS Nano 2015, 9, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, B.; Liu, Y.; Tang, W.; Chang, C.T.; Li, L.; Zheng, J.; Gao, L.; Zheng, Y.; Shin, H.S.; et al. Chemical vapor deposition of large-sized hexagonal WSe2 crystals on dielectric substrates. Adv. Mater. 2015, 27, 6722–6727. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, M.; Wang, L.; Chen, Y.; Xing, Z.; Zhang, T.; Liu, Z.; Zuo, J.; Nan, F.; Mendes, R.G.; et al. Ultrafast self-limited growth of strictly monolayer WSe2 crystals. Small 2016, 12, 5741–5749. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Abbas, A.N.; Ma, Y.; Fang, X.; Liu, Y.; Zhou, C. Screw-dislocation-driven growth of two-dimensional few-layer and pyramid-like WSe2 by sulfur-assisted chemical vapor deposition. ACS Nano 2014, 8, 11543–11551. [Google Scholar] [CrossRef]
- Deokar, G.; Vignaud, D.; Arenal, R.; Louette, P.; Colomer, J.F. Synthesis and characterization of MoS2 nanosheets. Nanotechnology 2016, 27, 075604. [Google Scholar] [CrossRef]
- Salitra, G.; Hodes, G.; Klein, E.; Tenne, R. Highly oriented WSe2 thin films prepared by selenization of evaporated WO3. Thin Solid Films 1994, 245, 180–185. [Google Scholar] [CrossRef]
- Tonndorf, P.; Schmidt, R.; Böttger, P.; Zhang, X.; Börner, J.; Liebig, A.; Albrecht, M.; Kloc, C.; Gordan, O.; Zahn, D.R.T.; et al. Photoluminescenc emission and Raman response of monolayer MoS2, MoSe2 and WSe2. Opt. Express 2013, 21, 1908–4916. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yu, T.; Yang, G.; Chen, H.; Zhang, C.; Feng, Q.; Ma, J.; Liu, W.; Xu, H.; et al. Accurate identification of layer number for few-layer WS2 and WSe2 via spectroscopic study. Nanotechnology 2018, 29, 124001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ghorannevis, Z.; Chu, L.; Toh, M.; Kloc, C.; Tan, P.H.; Eda, G. Evolution of electronic structure in atomically thin sheets of WS2 and WSe2. ACS Nano 2012, 7, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Beal, A.R.; Knights, J.C.; Liang, W.Y. Transmission spectra of some transition metal dichalcogenides. II. Group VIA: Trigonal prismatic coordination. J. Phys. C Solid State Phys. 1972, 5, 3540. [Google Scholar] [CrossRef]
- Sokolikova, M.S.; Sherrell, P.C.; Palczynski, P.; Bemmer, V.L.; Mattevi, C. Direct solution-phase synthesis of 1T’ WSe2 nanosheets. Nat. Commun. 2019, 10, 712. [Google Scholar] [CrossRef]
- Wu, P.C.; Yang, C.L.; Du, Y.; Lai, C.H. Scalable epitaxial growth of WSe2 thin films pn SiO2/Si vai a self-assembled PtSe2 buffer layer. Sci. Rep. 2019, 9, 8017. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Calandra, M. Chemically exfoliated single-layer MoS2: Stability, lattice dynamics, and catalytic adsorption from first principles. Phys. Rev. B 2013, 88, 245428. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single- and few-layers MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, B.; Zhang, A.; Chen, L.; Fathi, M.; Shen, C.; Abbas, A.N.; Ge, M.; Mecklenburg, M.; Zhou, C. Reversible semiconducting-to-metallic phase transition in chemical vapor deposition grown monolayer WSe2 and applications for devices. ACS Nano 2015, 9, 7383–7391. [Google Scholar] [CrossRef]

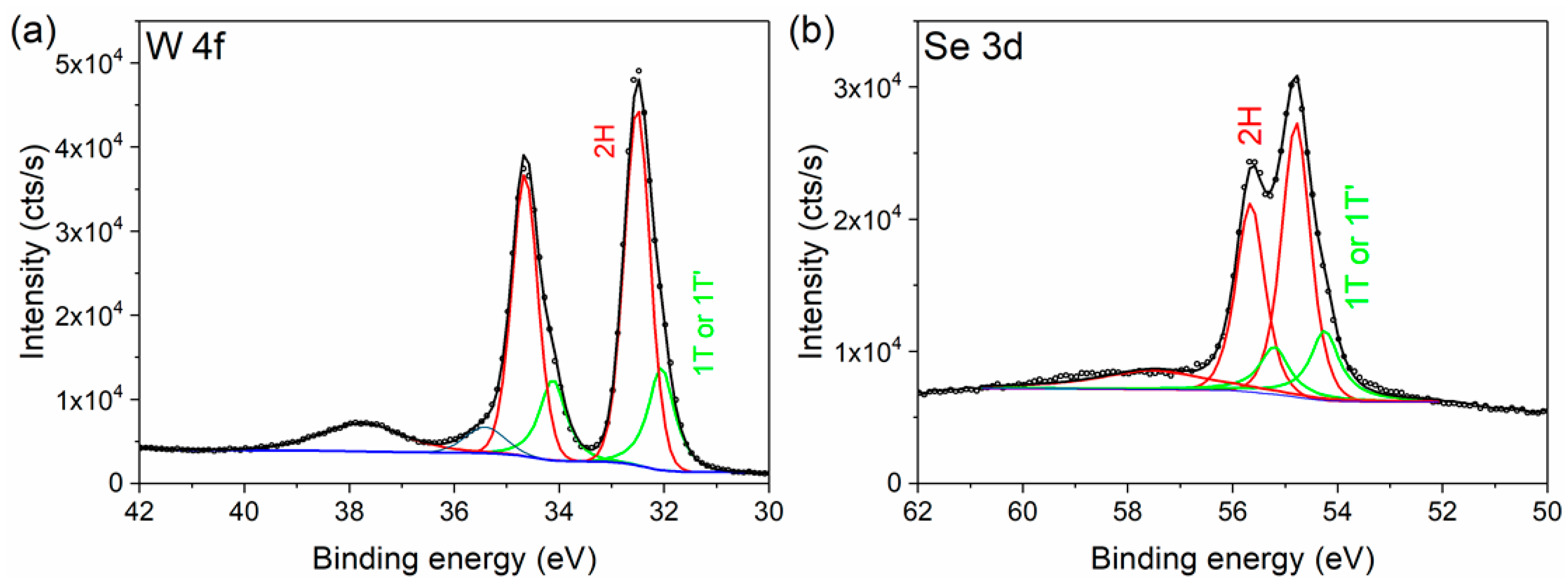
| W 4f Core Level | Se 3d Core Level | |||
|---|---|---|---|---|
| 2H Phase | 1T or 1T’ Phase | 2H Phase | 1T or 1T’ Phase | |
| % Area | 74.3 | 25.7 | 75.0 | 25.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Castillo, A.; Haye, E.; Acosta, S.; Bittencourt, C.; Colomer, J.-F. Synthesis and Characterization of Highly Crystalline Vertically Aligned WSe2 Nanosheets. Appl. Sci. 2020, 10, 874. https://doi.org/10.3390/app10030874
Sierra-Castillo A, Haye E, Acosta S, Bittencourt C, Colomer J-F. Synthesis and Characterization of Highly Crystalline Vertically Aligned WSe2 Nanosheets. Applied Sciences. 2020; 10(3):874. https://doi.org/10.3390/app10030874
Chicago/Turabian StyleSierra-Castillo, Ayrton, Emile Haye, Selene Acosta, Carla Bittencourt, and J.-F. Colomer. 2020. "Synthesis and Characterization of Highly Crystalline Vertically Aligned WSe2 Nanosheets" Applied Sciences 10, no. 3: 874. https://doi.org/10.3390/app10030874
APA StyleSierra-Castillo, A., Haye, E., Acosta, S., Bittencourt, C., & Colomer, J.-F. (2020). Synthesis and Characterization of Highly Crystalline Vertically Aligned WSe2 Nanosheets. Applied Sciences, 10(3), 874. https://doi.org/10.3390/app10030874






