CO2 Capture by Alkaline Carbonation as an Alternative to a Circular Economy
Abstract
1. Introduction
2. Materials and Methods
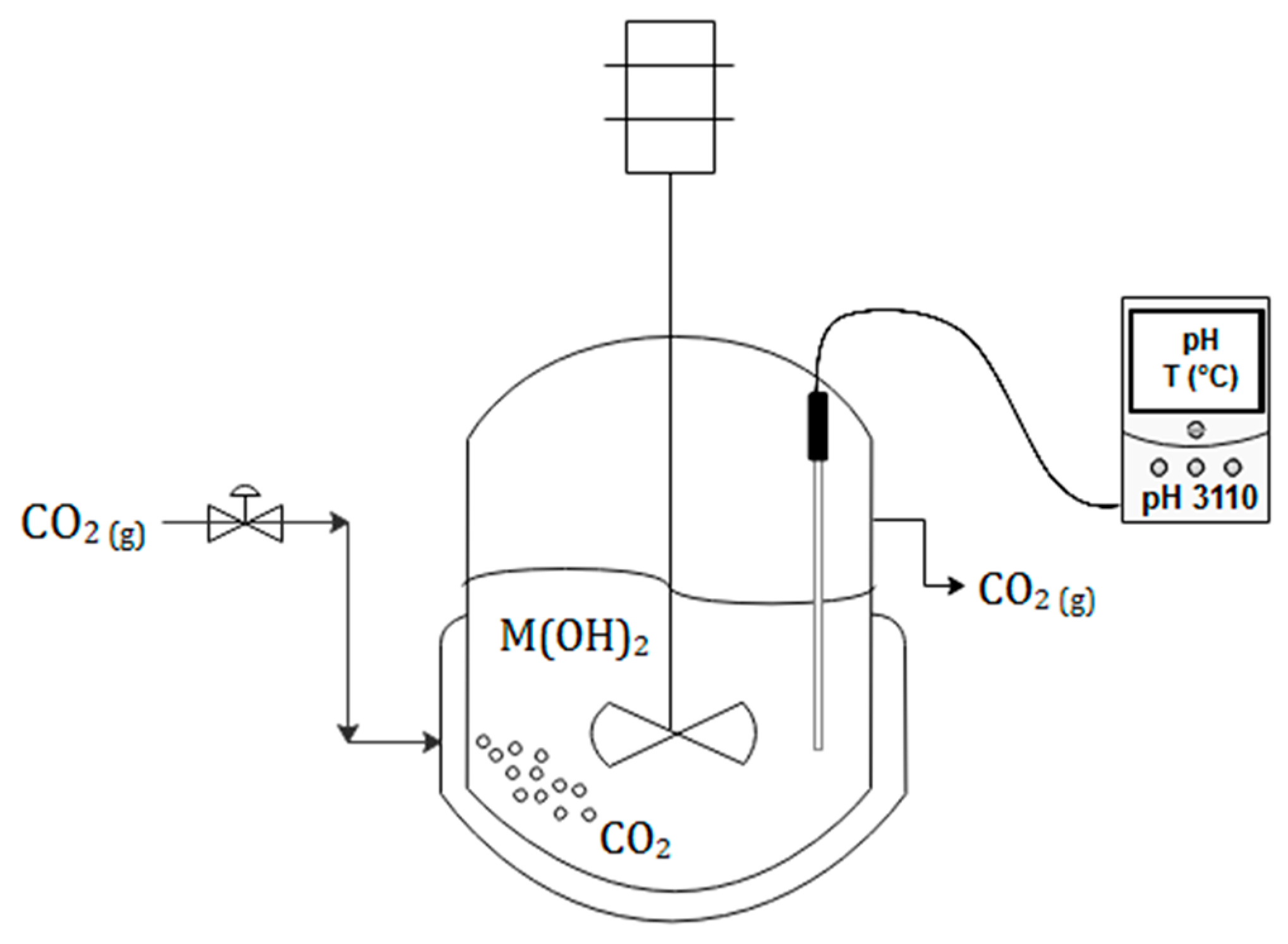
2.1. Reactor/Crystallizer
2.2. Characterization
3. Results
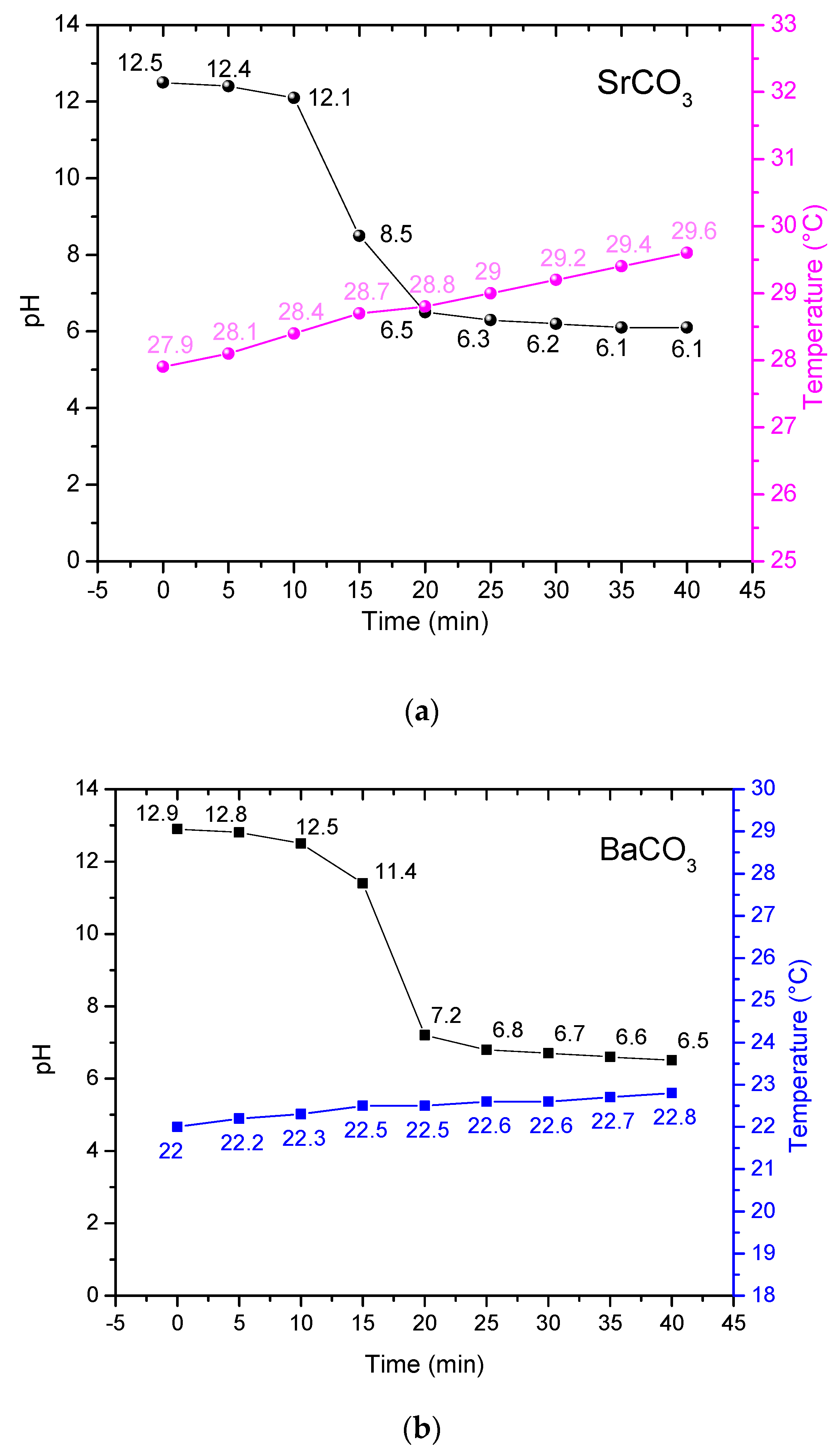
3.1. Trends of Temperature and pH
3.2. Conversion Rate
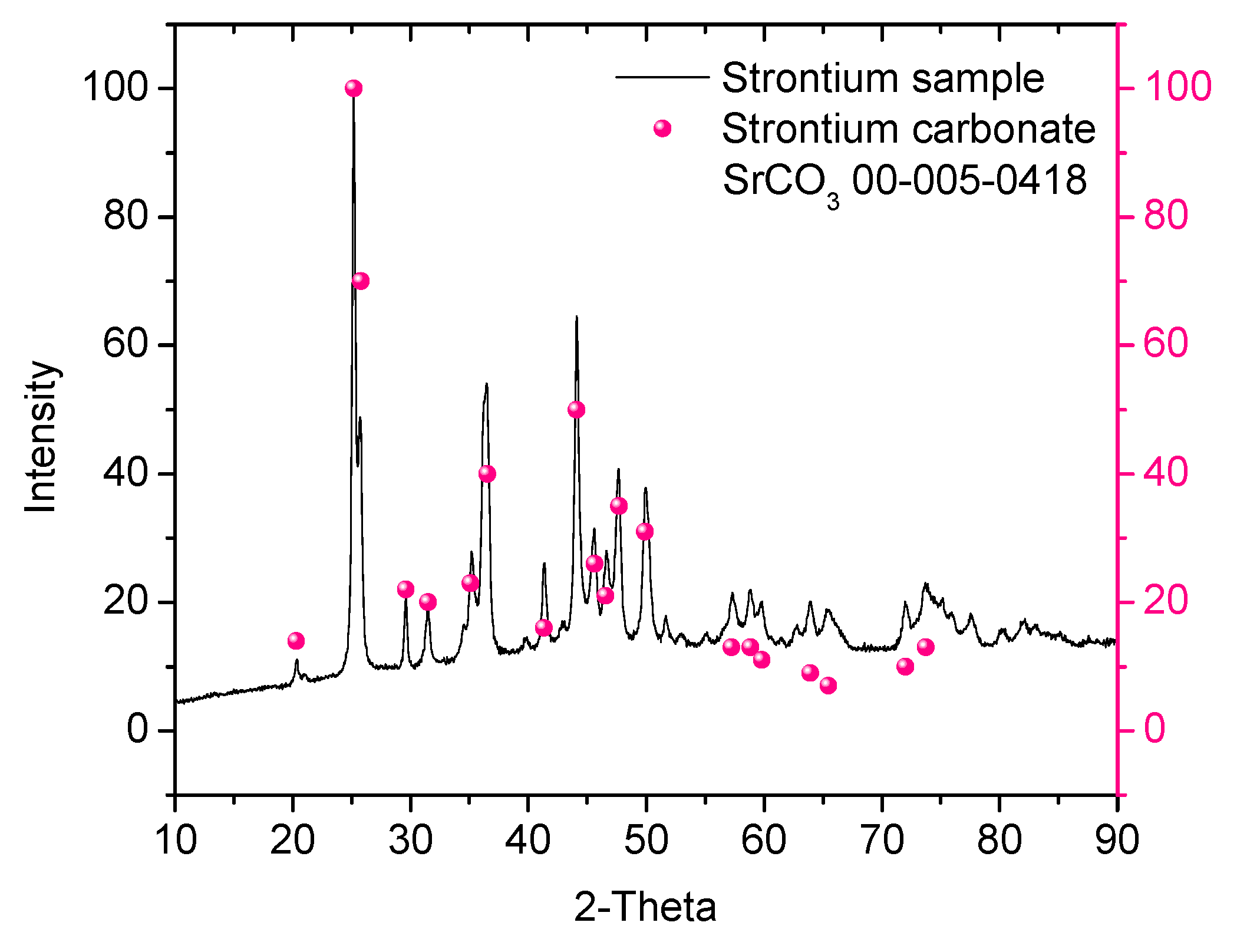
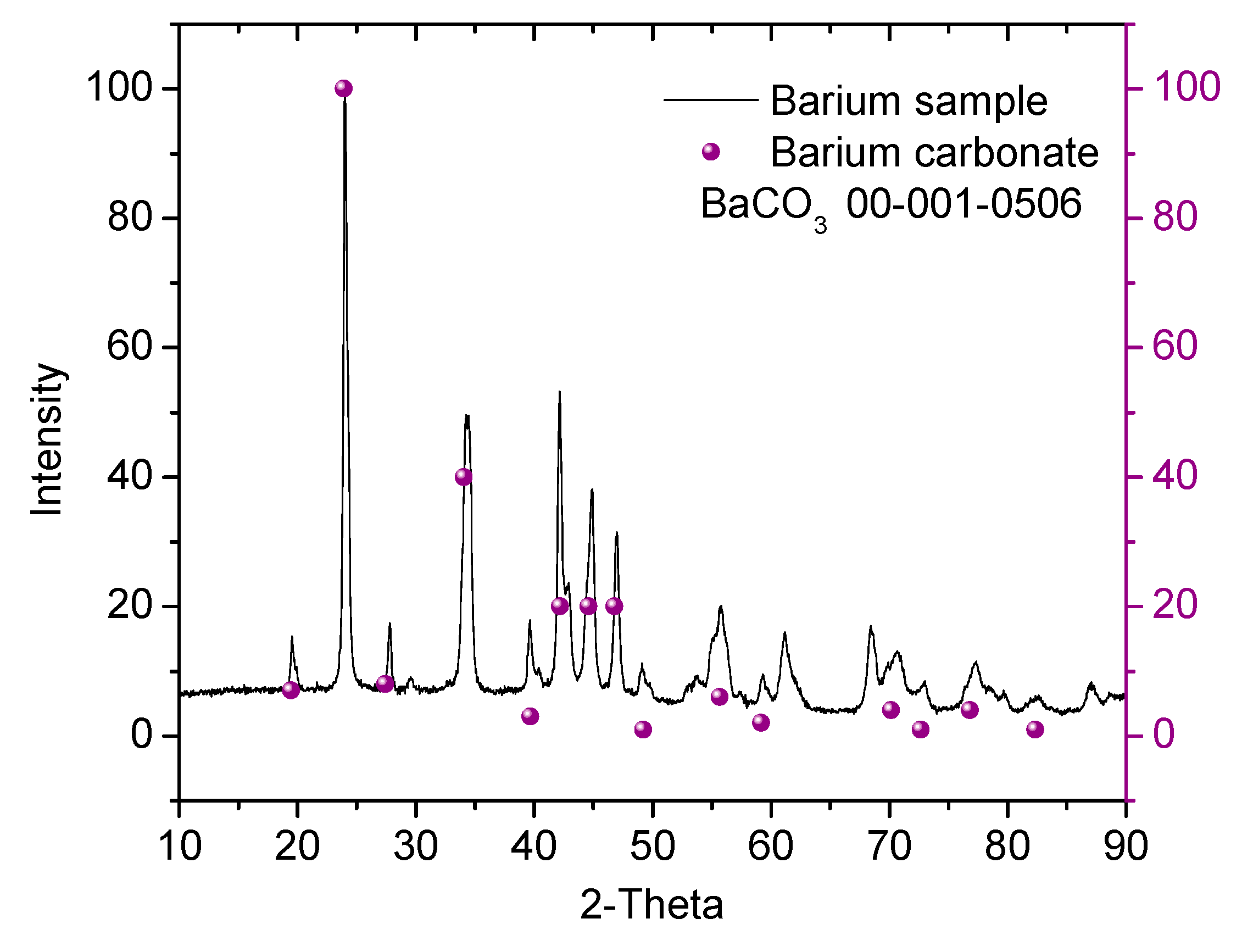
3.3. X-Ray Diffraction
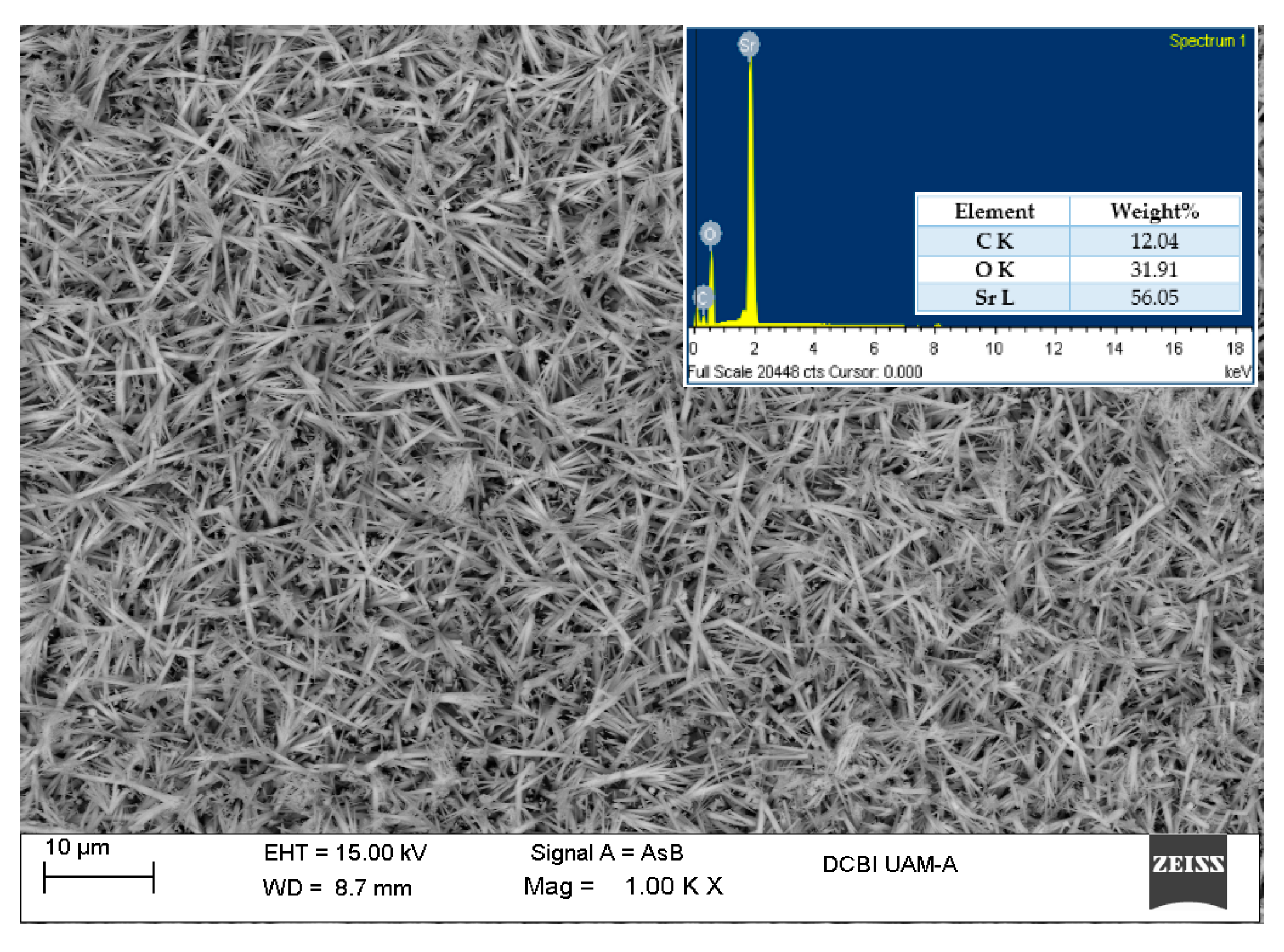
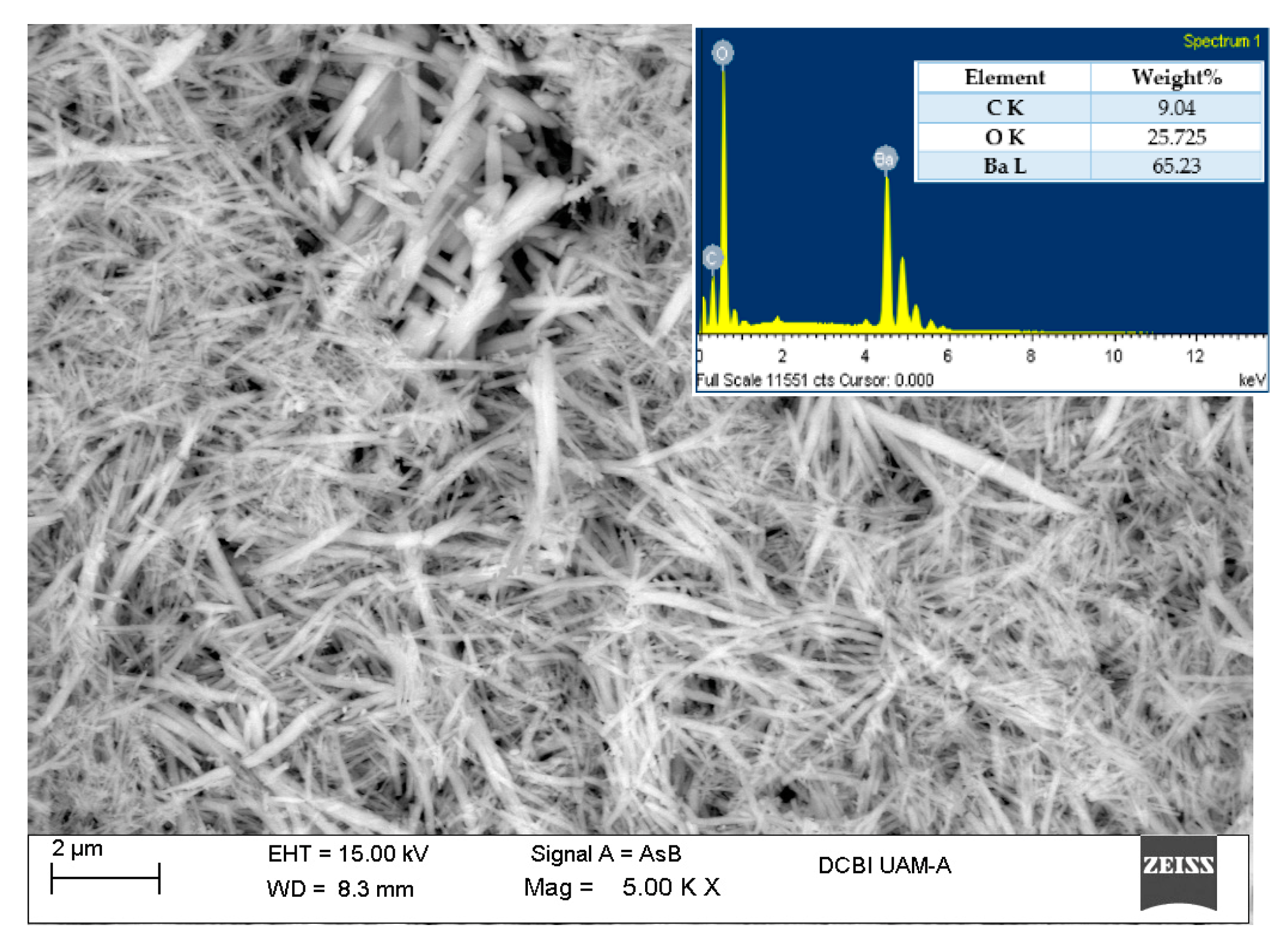
3.4. Scanning Electron Microscopy
3.5. Fourier Transform Infrared Spectroscopy
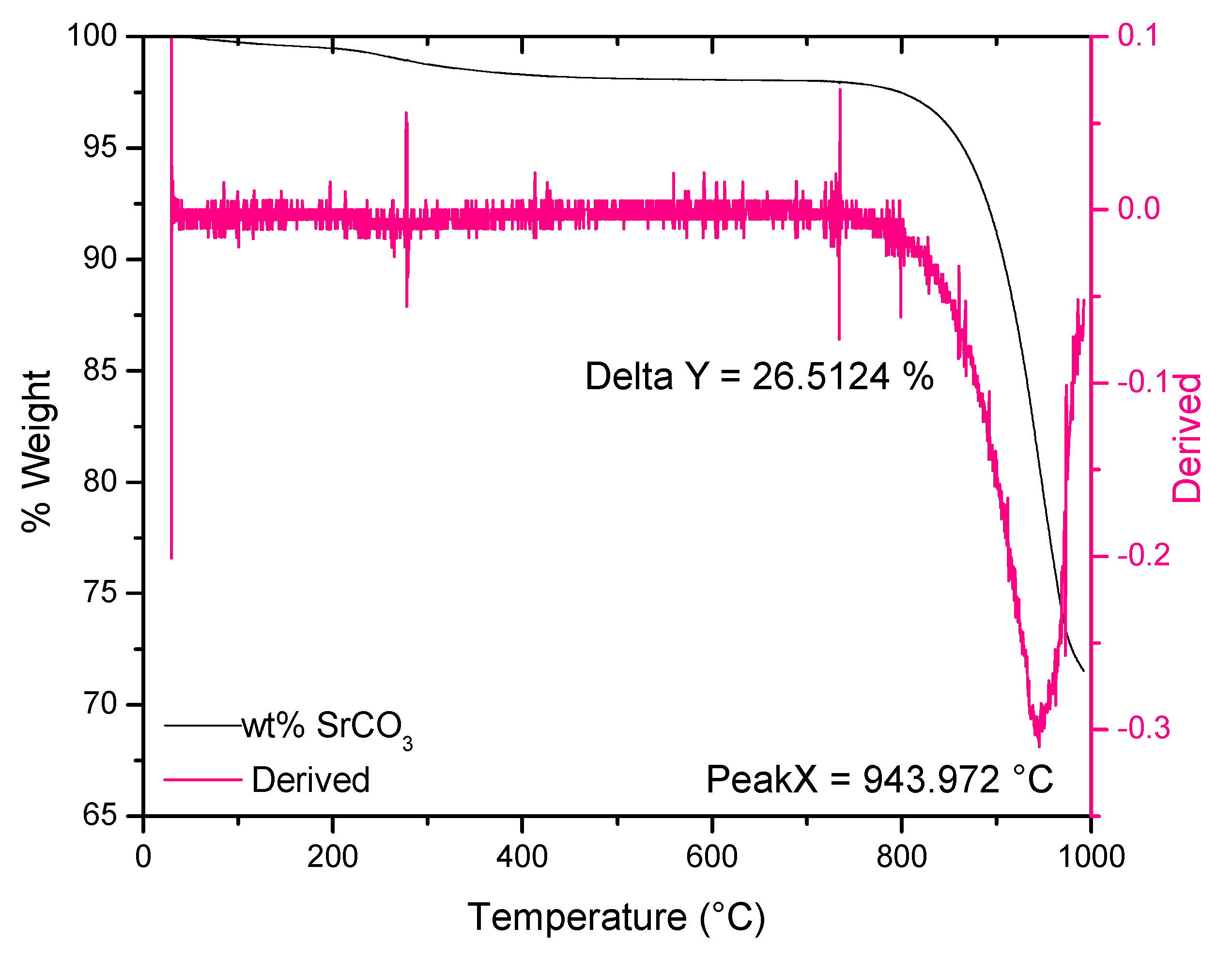
3.6. Thermogravimetric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous Inorganic Membranes for CO2 Capture: Present and Prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Feron, P.; Deng, L.; Favre, E.; Chabanon, E.; Yang, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon captures: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.Y.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar]
- Carbon Sequestration Program Technology Program Plan, National Energy Technology Laboratory. Available online: www.netl.doe.gov (accessed on 17 February 2011).
- Olajire, A.A. CO2 capture and separation technologies for end-of-pipe applications—A review. Energy 2010, 35, 2610–2628. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chang, E.; Chiang, P.C. CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049. [Google Scholar] [PubMed]
- Bruhn, T.; Naims, H.; Olfe-Krutlein, B. Separating the debate on CO2 utilization from carbon capture and storage. Environ. Sci. Policy 2016, 60, 38–43. [Google Scholar] [CrossRef]
- Japan CCS Company has achieved its target of 300,000 tonnes of CO2 injection at the Tomakomai CCS Demonstration Project; Future Energy Publishing: London, UK, 2019.
- EPA, Overview of Greenhouse Gases. Carbon Dioxide Emissions. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases-carbon-dioxide (accessed on 15 August 2018).
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Petrol. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Ho, Y.; Jin, K.; Young, L.; Meehye, L.; Suk, C. Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem. Eng. J. 2012, 183, 77–87. [Google Scholar]
- Goldberg, P.; Chen, Z.-Y.; O’Connor, W.; Walters, R.; Ziock, H. CO2 Mineral Sequestration Studies in US. In Proceedings of the National Conference on Carbon Sequestration, Washington, DC, USA; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2001. [Google Scholar]
- Manahan, S.E. Environmental Chemistry and the Five Spheres of the Environment. In Envirommental Chemistry, 7th ed.; CRC Press Taylor & Francis Group: New York, NY, USA, 2017; pp. 1–23. [Google Scholar]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Manan, M.; Hamidi, H.; Mohamad, A. Carbon Dioxide Mineral Carbonation Through pH-swing Process: A Review. Energy Procedia 2014, 61, 2783–2786. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Saffari, M.; Milani, D.; Montoya, A.; Valix, M.; Abbas, A. Sustainable transformation of fly ash industrial waste into a construction cement blend via CO2 carbonation. J. Clean. Prod. 2017, 156, 660–669. [Google Scholar] [CrossRef]
- Huang, C.K.; Kerr, P.F. Infrared Study of the Carbonate Minerals. Am. Mineral. 1960, 45, 311–324. [Google Scholar]
- Soong, Y.; Fauth, D.L.; Howard, B.H.; Jones, J.R.; Harrison, D.K.; Goodman, A.L.; Gray, M.L.; Frommell, E.A. CO2 sequestration with brine solution and fly ashes. Energy Convers. Manag. 2006, 47, 1676–1685. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Maitra, S.; Chakrabarty, N.; Pramanik, J. Decomposition kinetics of alkaline earth carbonates by integral approximation method. Cerámica 2008, 54, 268–272. [Google Scholar] [CrossRef]
- Hamer, F.; Hamer, J. The Potter’s Dictionary of Materials and Techniques, 5th ed.; University of Pennsylvania Press: Philadelphia, PA, USA, 2004. [Google Scholar]
- Ropp, R. Alkaline earth oxy-carbon compounds. Encyclopedia of the Alkaline Earth Compounds; Elsevier: London, UK, 2012; pp. 368–370. [Google Scholar]
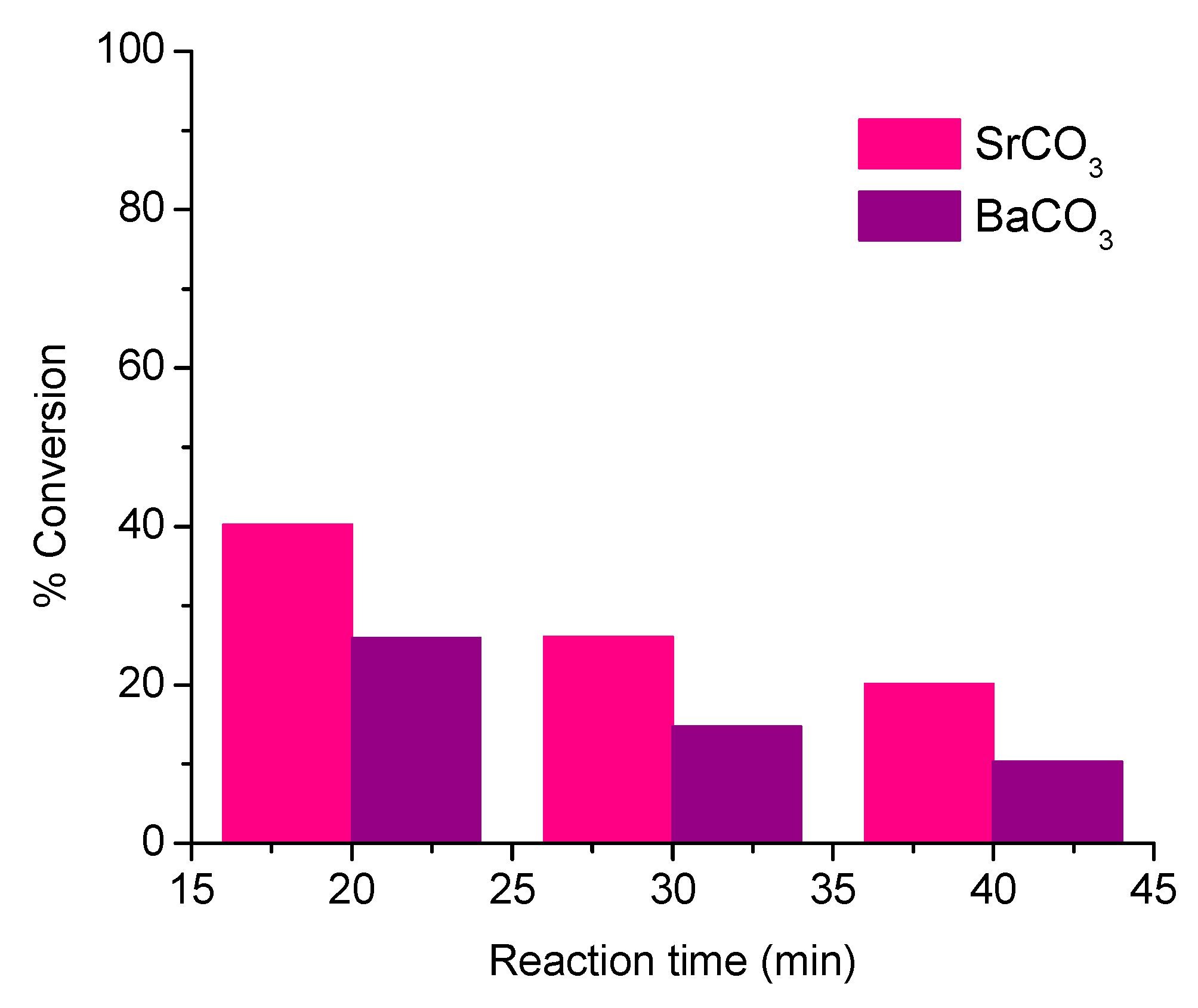
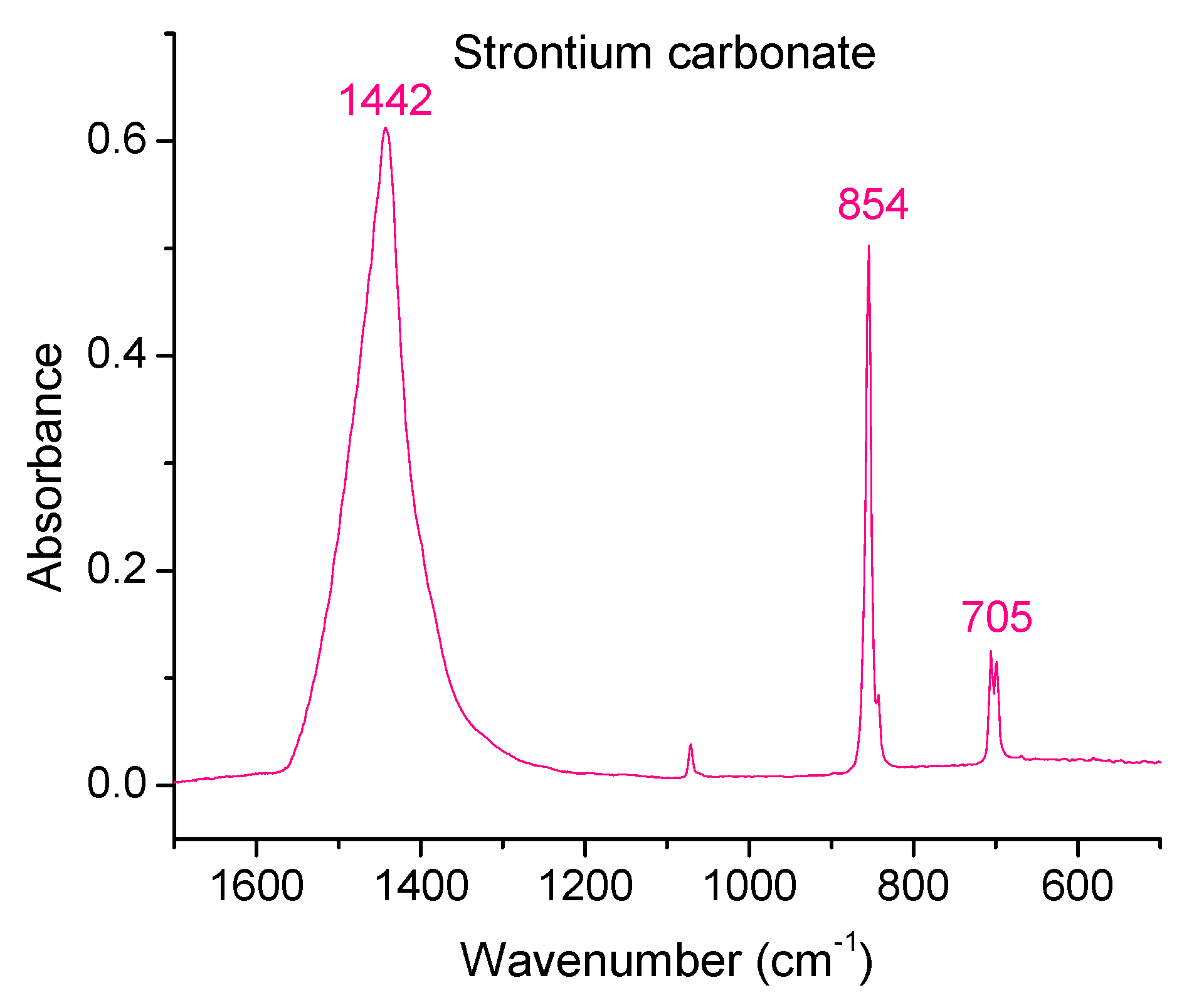
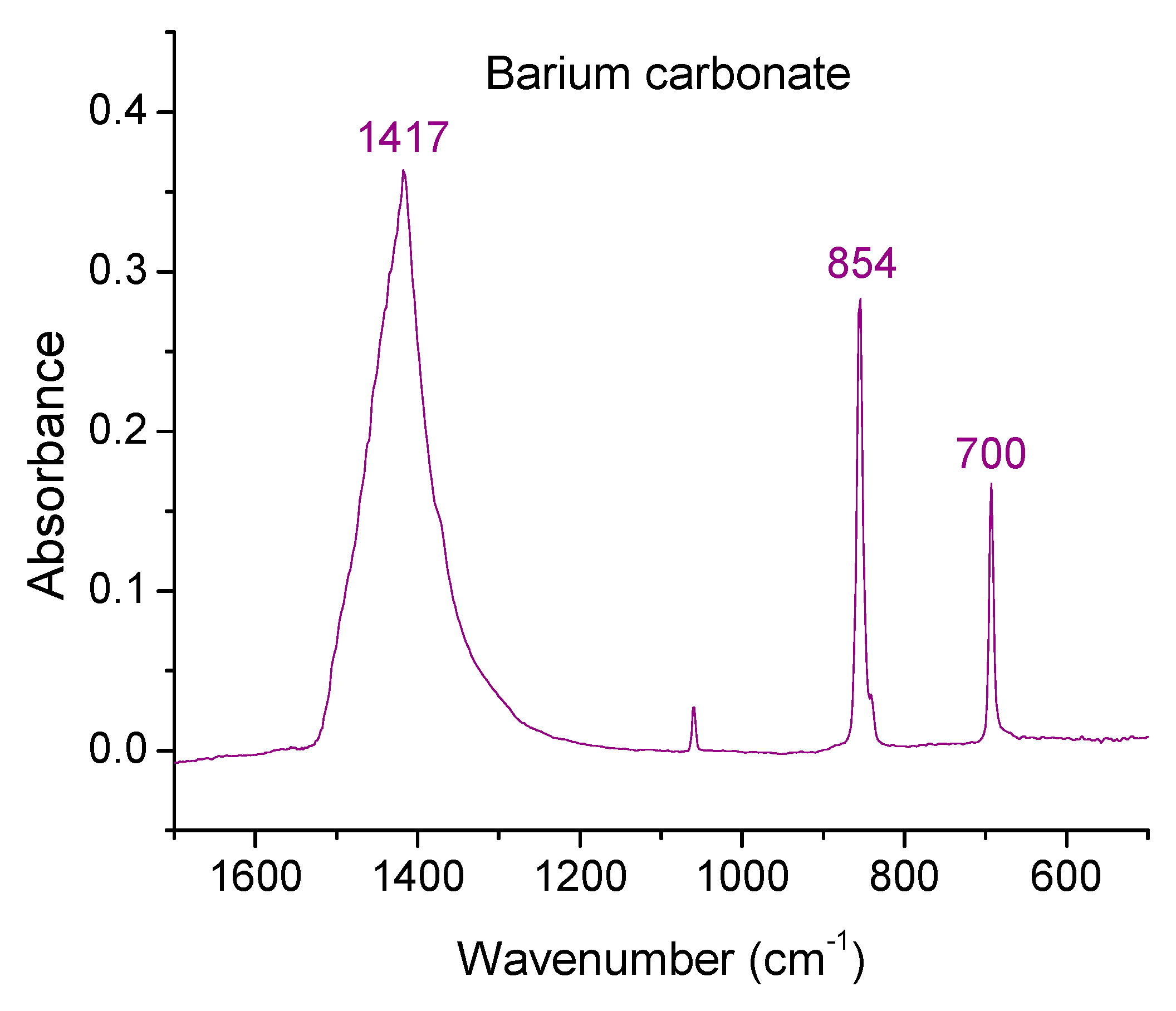
| Vibration Type | Range (cm−1) | Mode |
|---|---|---|
| Symmetric stretching | 1065 | ν1 |
| Bending out of plane | 880–850 | ν2 |
| Bending in-plane | 720–680 | ν4 |
| Asymmetric stretching | 1450–1410 | ν3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa Cruz-Navarro, D.; Mugica-Álvarez, V.; Gutiérrez-Arzaluz, M.; Torres-Rodríguez, M. CO2 Capture by Alkaline Carbonation as an Alternative to a Circular Economy. Appl. Sci. 2020, 10, 863. https://doi.org/10.3390/app10030863
Santa Cruz-Navarro D, Mugica-Álvarez V, Gutiérrez-Arzaluz M, Torres-Rodríguez M. CO2 Capture by Alkaline Carbonation as an Alternative to a Circular Economy. Applied Sciences. 2020; 10(3):863. https://doi.org/10.3390/app10030863
Chicago/Turabian StyleSanta Cruz-Navarro, Dalia, Violeta Mugica-Álvarez, Mirella Gutiérrez-Arzaluz, and Miguel Torres-Rodríguez. 2020. "CO2 Capture by Alkaline Carbonation as an Alternative to a Circular Economy" Applied Sciences 10, no. 3: 863. https://doi.org/10.3390/app10030863
APA StyleSanta Cruz-Navarro, D., Mugica-Álvarez, V., Gutiérrez-Arzaluz, M., & Torres-Rodríguez, M. (2020). CO2 Capture by Alkaline Carbonation as an Alternative to a Circular Economy. Applied Sciences, 10(3), 863. https://doi.org/10.3390/app10030863







