EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments
Abstract
Featured Application
Abstract
1. Introduction
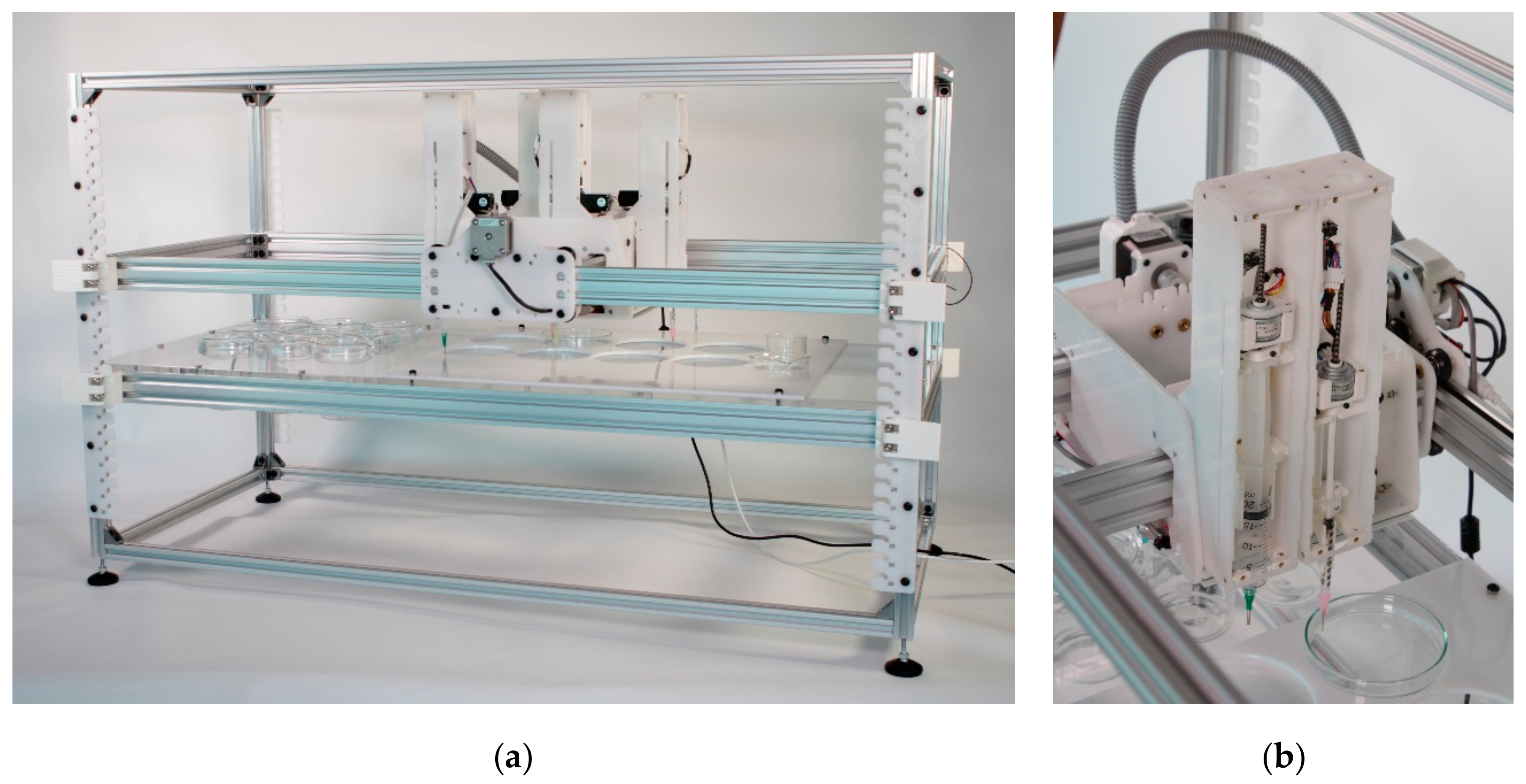
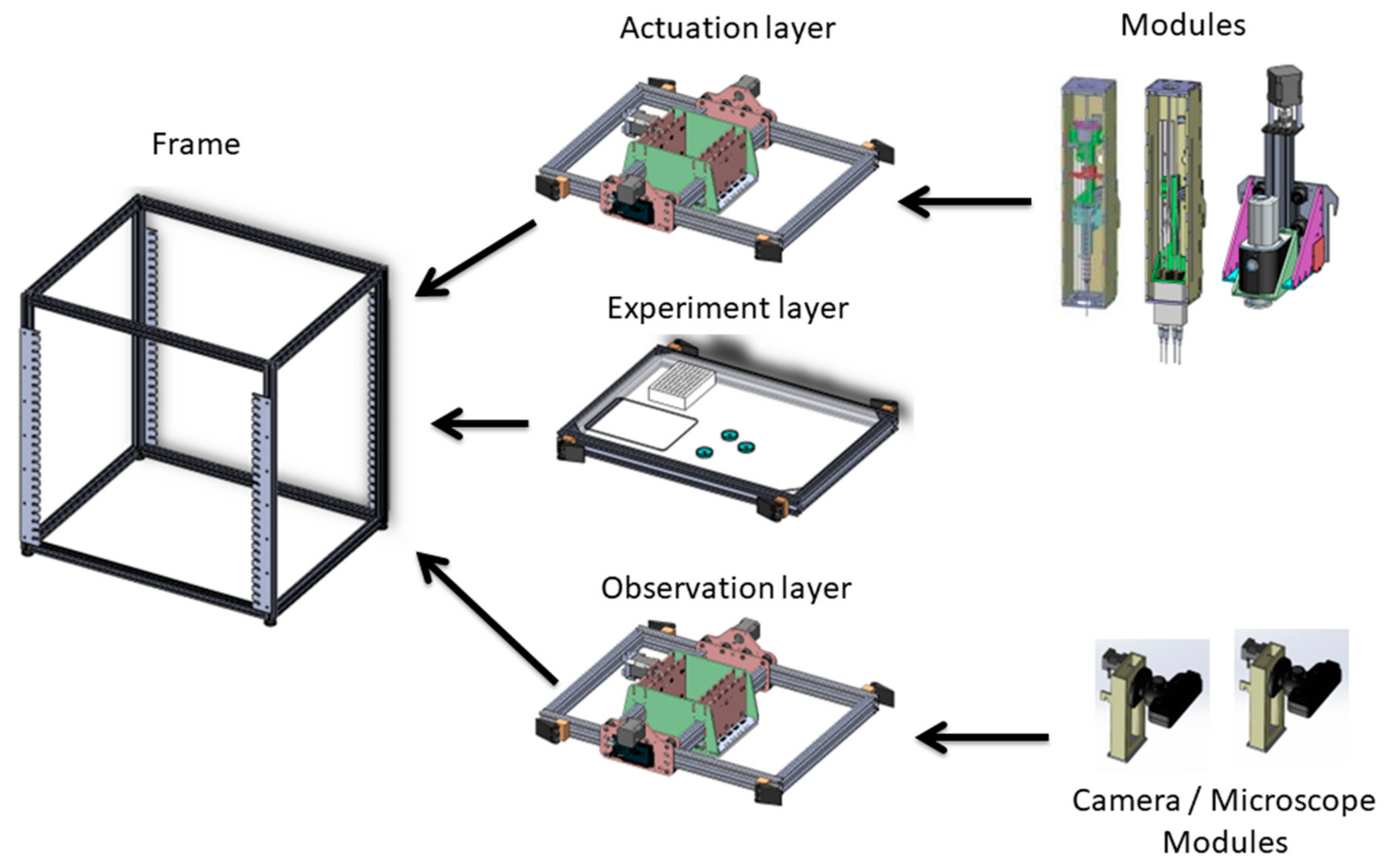
2. EvoBot
3. EvoBot Implementation
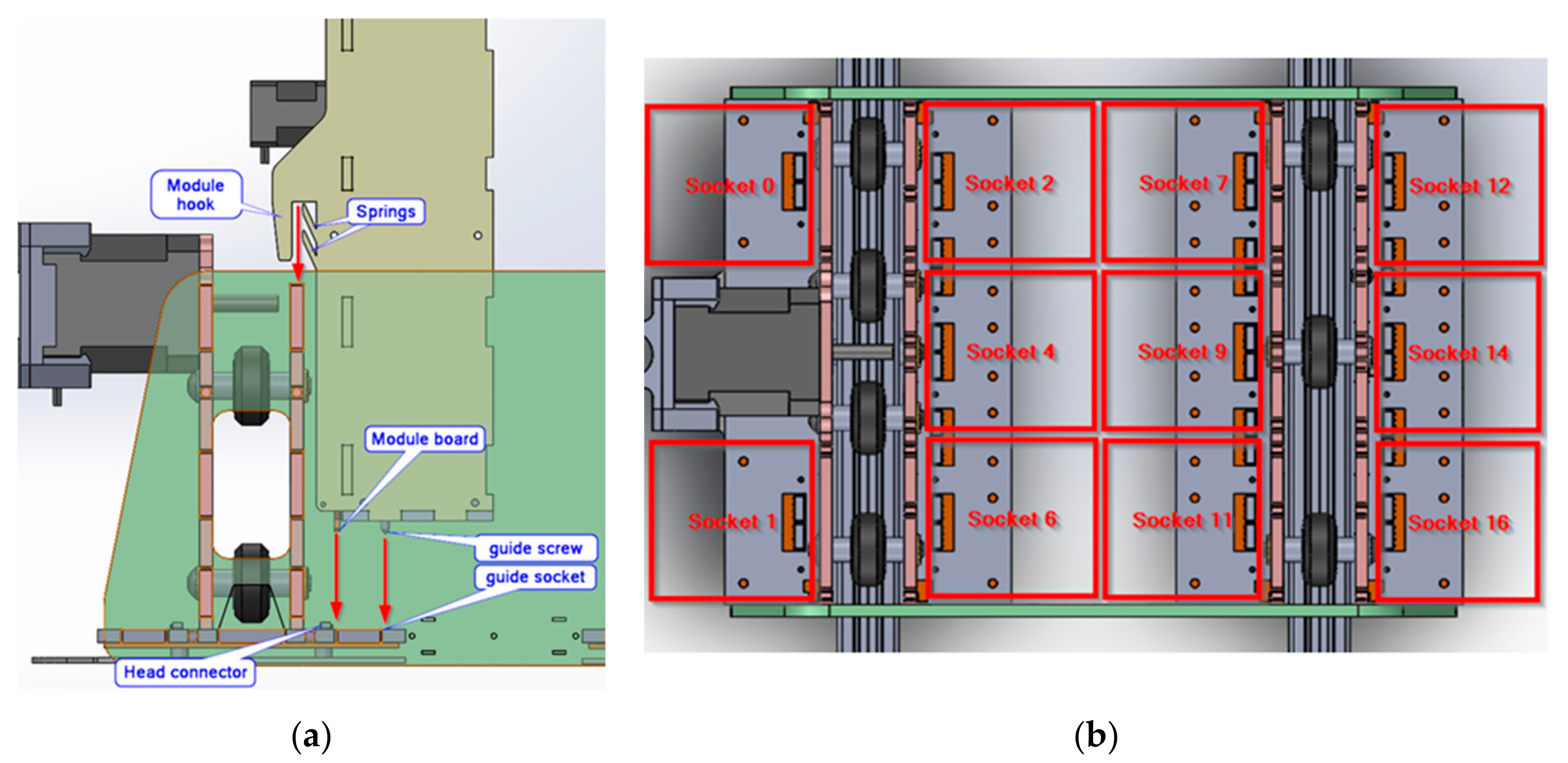
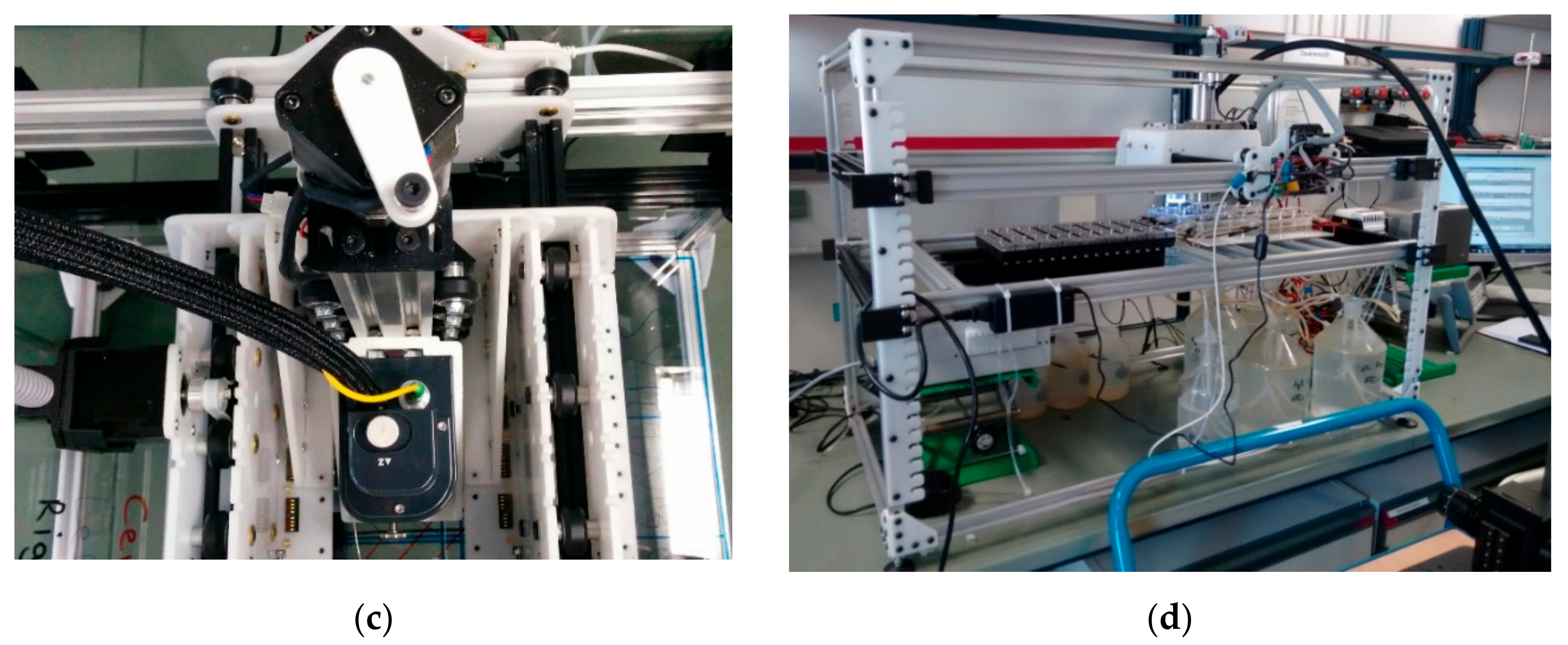
3.1. Mechanics
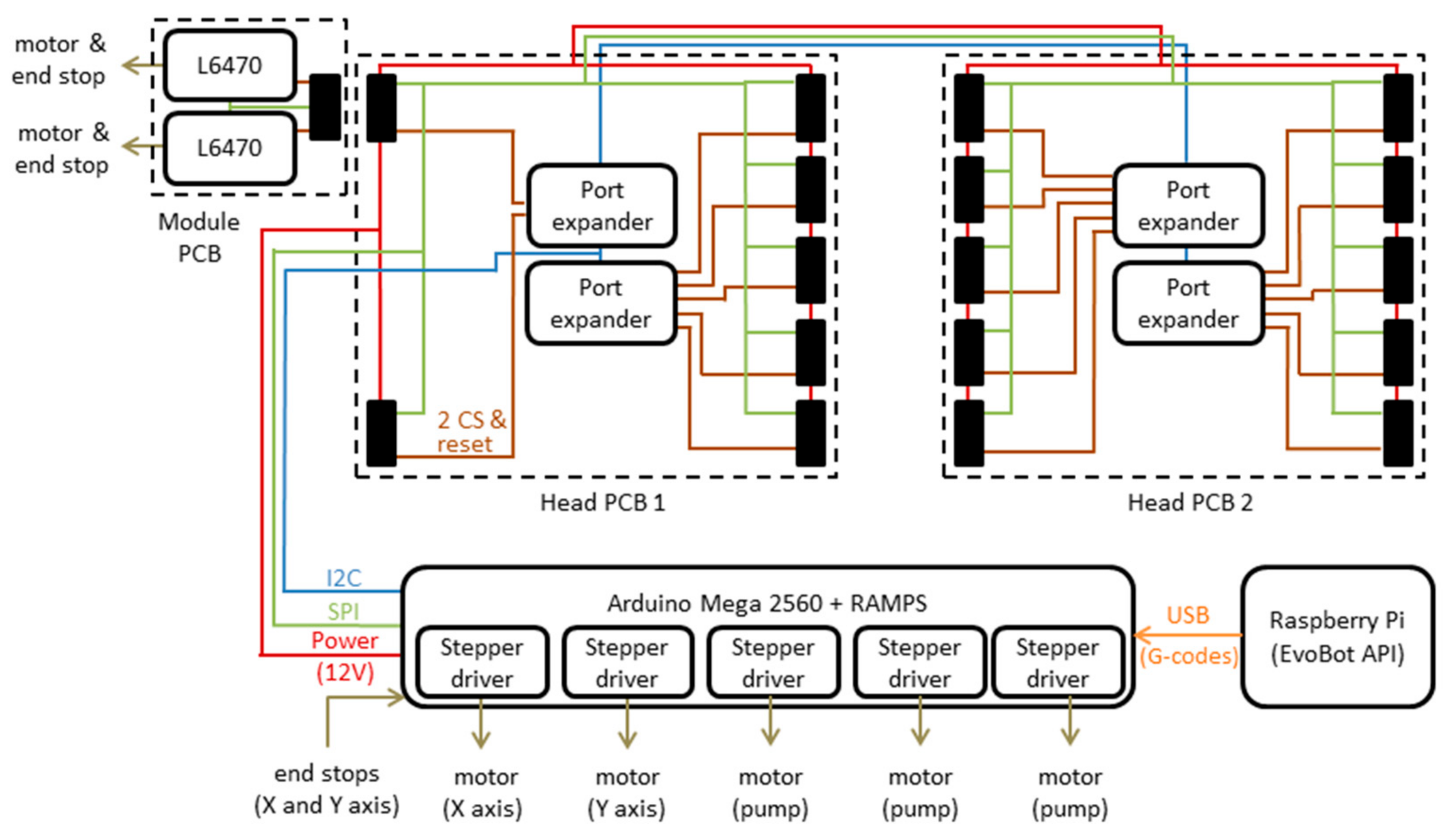
3.2. Electronics
3.3. Software
4. Performance Characteristics
4.1. Mechanical Performance
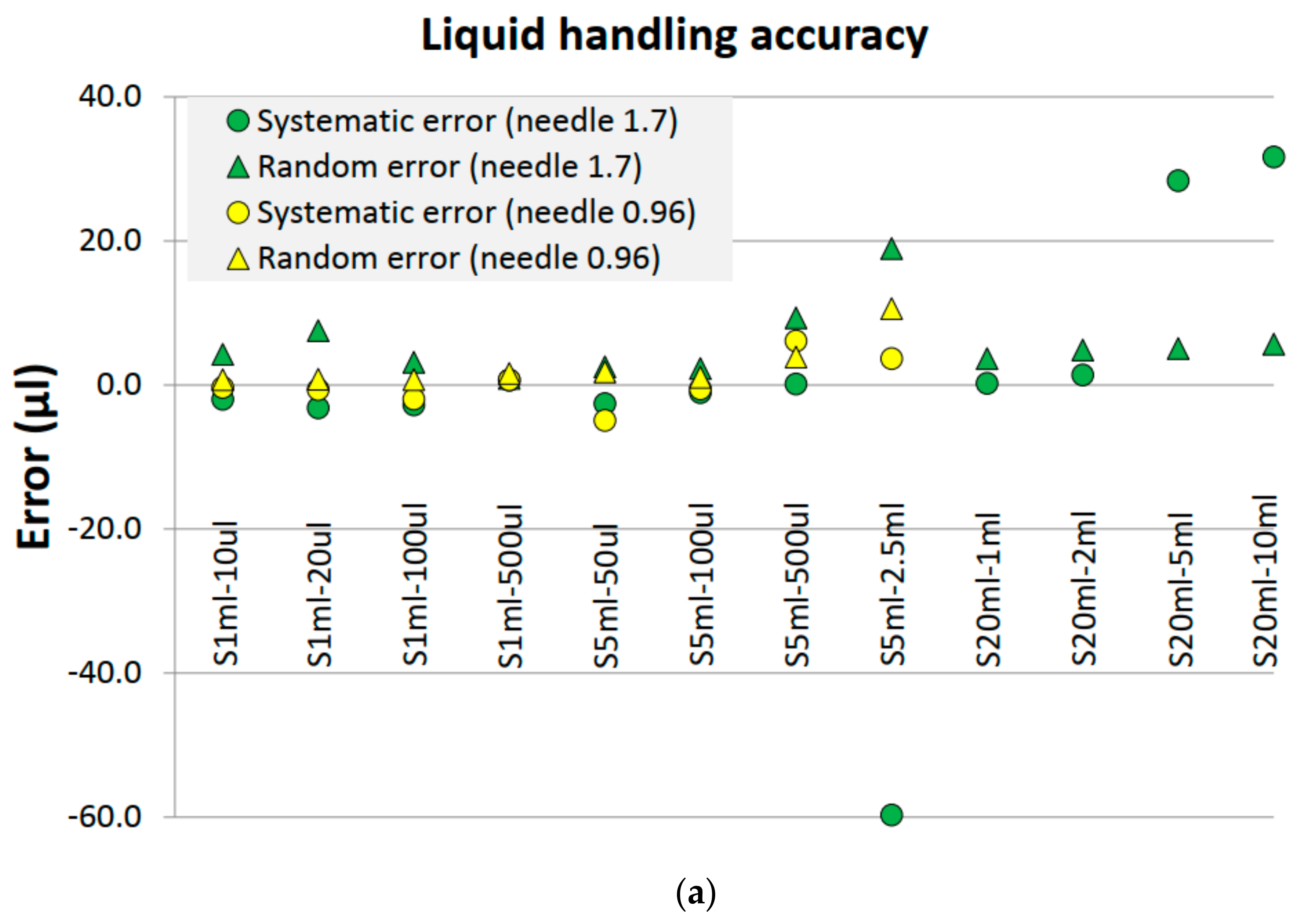
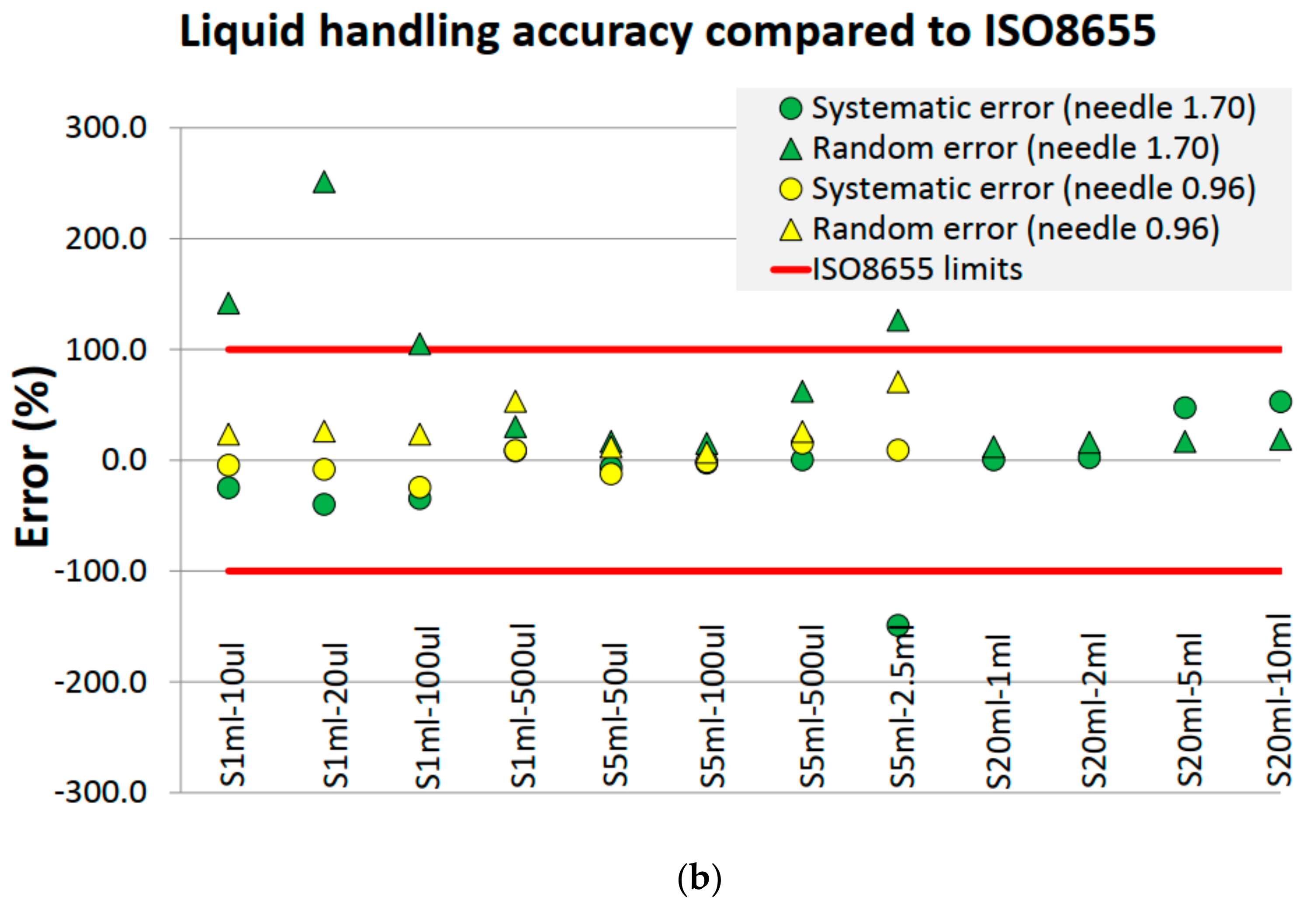
4.2. Liquid Handling Performance
4.3. Computational Performance
5. EvoBot Applications
5.1. Static Liquid Handling
5.2. Static Scanning of Biofilms
5.3. Reactive Nurturing of Microbial Fuel Cells
5.4. D Printing MFC Components
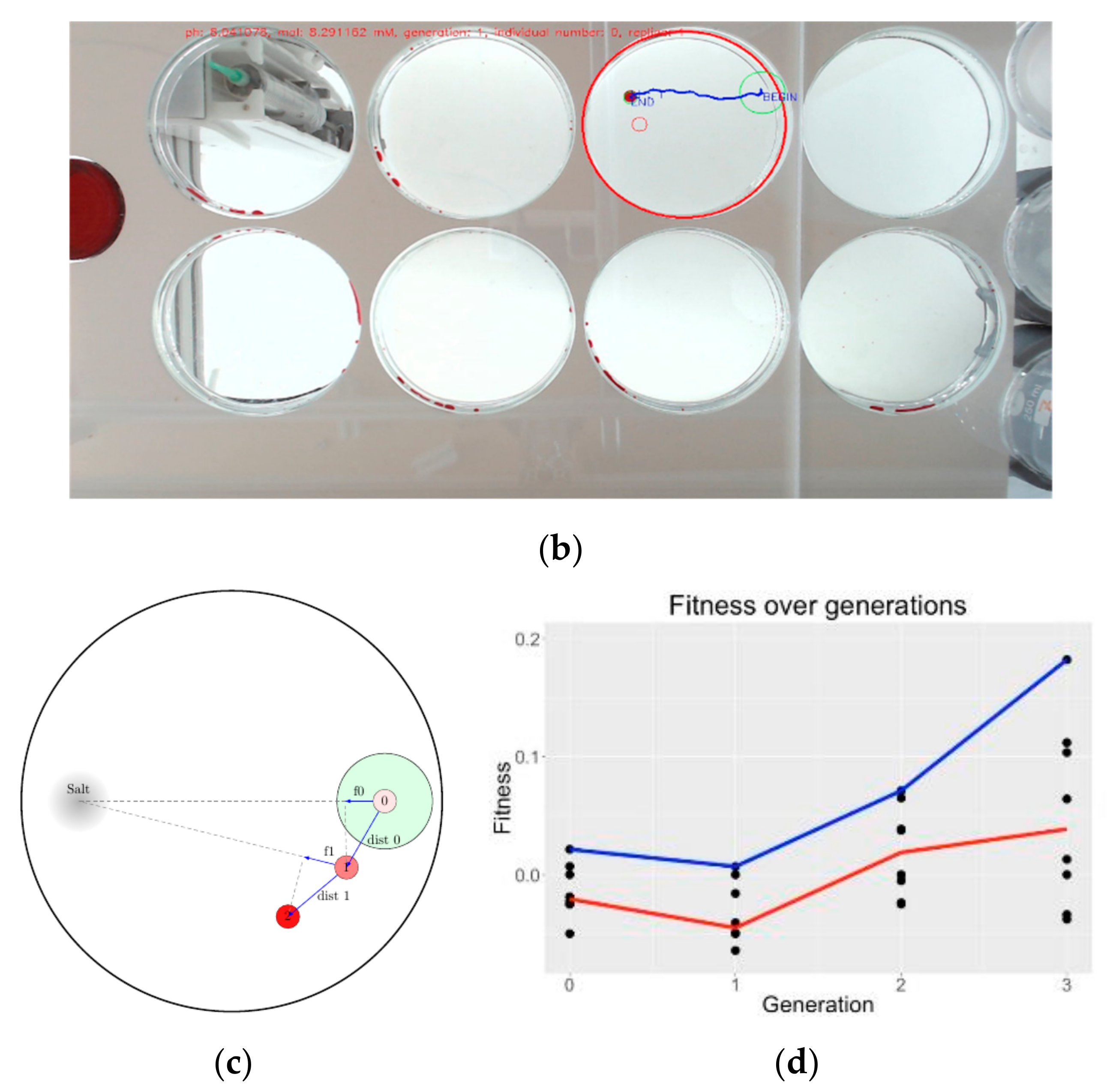
5.5. Computer Vision Based Droplet Experiments
6. Discussion
6.1. Open-Source Hardware
6.2. Cost
6.3. Modularity
6.4. Future Work
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kong, F.; Yuan, L.; Zheng, Y.F.; Chen, W. Automatic liquid handling for life science: A critical review of the current state of the art. J. Lab. Autom. 2012, 17, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Groschl, M.; Markus, A.; Simone, L. A liquid handling robot for robust and reproducible preparation of standard and quality control samples in bioanalysis. Adv. Robot. Autom. 2017, 6, 160. [Google Scholar] [CrossRef]
- Storch, M.; Haines, M.C.; Baldwin, G.S. DNA-BOT: A low-cost, automated DNA assembly platform for synthetic biology. Unpublished. 2019. preprint available at BioRxiv: 832139. [Google Scholar]
- Ortiz, L.; Pavan, M.; McCarthy, L.; Timmons, J.; Densmore, D.M. Automated robotic liquid handling assembly of modular DNA devices. J. Vis. Exp. 2017, 130, e54703. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.P. Automatic liquid handling for artificial life research. Master’s Thesis, University of Southern Denmark, Odense, Denmark, 2012. [Google Scholar]
- Gutierrez, J.M.P.; Hinkley, T.; Taylor, J.W.; Yanev, K.; Cronin, L. Evolution of oil droplets in a chemorobotic platform. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, M.M.; Parrilla, J.M.; Nicholson, A.; Yanev, K.; Stoy, K. Creating and maintaining chemical artificial life by robotic symbiosis. Artif. Life. 2015, 1, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Amaldass, A.; Yanev, K.; Rasmussen, S. Do it yourself ¨(diy) liquid handling robot for evolutionary search exploration. In Proceedings of the European Conference on Artificial Life, York, UK, 20–24 July 2015. [Google Scholar]
- Zhang, C.; Wijnen, B.; Pearce, J.M. Open-source 3-D platform for low-cost scientific instrument ecosystem. J. Lab. Autom. 2016, 21, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Barthels, F.; Barthels, U.; Schwickert, M.; Schirmeister, T. FINDUS: An Open-Source 3D Printable Liquid-Handling Workstation for Laboratory Automation in Life Sciences. SLAS Technol. Transl. Life Sci. Innov. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Open-Source Lab: How to Build. Your Own Hardware and Reduce Research Costs; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Opentrons Labworks Inc. Introducing Automation to Your Lab. Available online: https://opentrons.com/publications/ultimate-guide-to-choosing-a-pipetting-robot-for-lab-automation.pdf (accessed on 23 January 2020).
- Yim, M.; Shen, W.-M.; Salemi, B.; Rus, D.; Moll, M.; Lipson, H.; Klavins, E.; Chirikjian, G.S. Modular self-reconfigurable robot systems. IEEE Robot. Autom. Mag. 2007, 14, 43–52. [Google Scholar] [CrossRef]
- Faina, A.; Nejatimoharrami, F.; Stoy, K.; Taylor, B.; Theodosiou, P.; Ieropoulos, I. Evobot: An open-source, modular liquid handling robot for nurturing microbial fuel cells. In Proceedings of the International Conference on the Synthesis and Simulation of Living Systems (ALIFE), Cancún, Mexico, 4–8 July 2016. [Google Scholar]
- Openbuilds, V-slot. Available online: http://openbuildspartstore.com/ (accessed on 23 January 2020).
- Blauert, F.; Wagner, M.; Horn, H. Optimization of biofilm structure by means of an evolutionary platform. In Proceedings of the Workshop on Exploiting Synergies between Biology and Artificial Life Technologies: Tools, Possibilities, and Examples at ALIFE Conference, New York, NY, USA, 30 July–2 August 2014. [Google Scholar]
- Theodosiou, P.; Faina, A.; Nejatimoharrami, F.; Stoy, K.; Greenman, J.; Melhuish, C.; Ieropoulos, I. EvoBot: Towards a Robot-Chemostat for Culturing and Maintaining Microbial Fuel Cells (MFCs). In Proceedings of the Conference on Biomimetic and Biohybrid Systems (Living Machines), Stanford, CA, USA, 26–28 July 2017. [Google Scholar]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Theodosiou, P.; Greenman, J.; Ieropoulos, I. 3D-Printable Cathode Electrode for Monolithically Printed Microbial Fuel Cells (MFCs). In Proceedings of the 233rd Electrochemical Society (ECS) meeting, Seattle, WA, USA, 13–17 May 2018. [Google Scholar]
- Theodosiou, P.; Greenman, J.; Ieropoulos, I. Towards monolithically printed MFCs: Development of a 3D-printable membrane electrode assembly (mea). Int. J. Hydrog. Energy 2019, 44, 4450–4462. [Google Scholar] [CrossRef]
- Nejatimoharrami, F.; Faina, A.; Cejkova, J.; Hanczyc, M.; Stoy, K. Robotic automation to augment quality of artificial chemical life experiments. In Proceedings of the International Conference on the Synthesis and Simulation of Living Systems (ALIFE), Cancún, Mexico, 4–8 July 2016. [Google Scholar]
- Holler, S.; Porcelli, C.; Ieropoulos, I.A.; Hanczyc, M. Transport of live cells under sterile conditions using a chemotactic droplet. Sci. Rep. 2018, 8, 8408. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Müller, S.D.; Koumoutsakos, P. Reducing the time complexity of the derandomized evolution strategy with covariance matrix adaptation (CMA-ES). Evol. Comput. 2003, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fortin, F.A.; Rainville, F.M.D.; Gardner, M.A.; Parizeau, M.; Gagné, C. DEAP: Evolutionary algorithms made easy. J. Mach. Learn. Res. 2012, 13, 2171–2175. [Google Scholar]
- Baden, T.; Chagas, A.M.; Gage, C.; Marzullo, T.; Prieto-Godino, L.L.; Euler, T. Open labware: 3-d printing your own lab equipment. PLOS Biol. 2015, 13, e1002086. [Google Scholar] [CrossRef] [PubMed]
- Machiraju, R. Flexible robotic platforms that allow scientists to remain scientists. BioCoder 2015, 8, 29–36. [Google Scholar]
- May, M. A DIY approach to automating your lab. Nature 2019, 569, 587. [Google Scholar] [CrossRef] [PubMed]
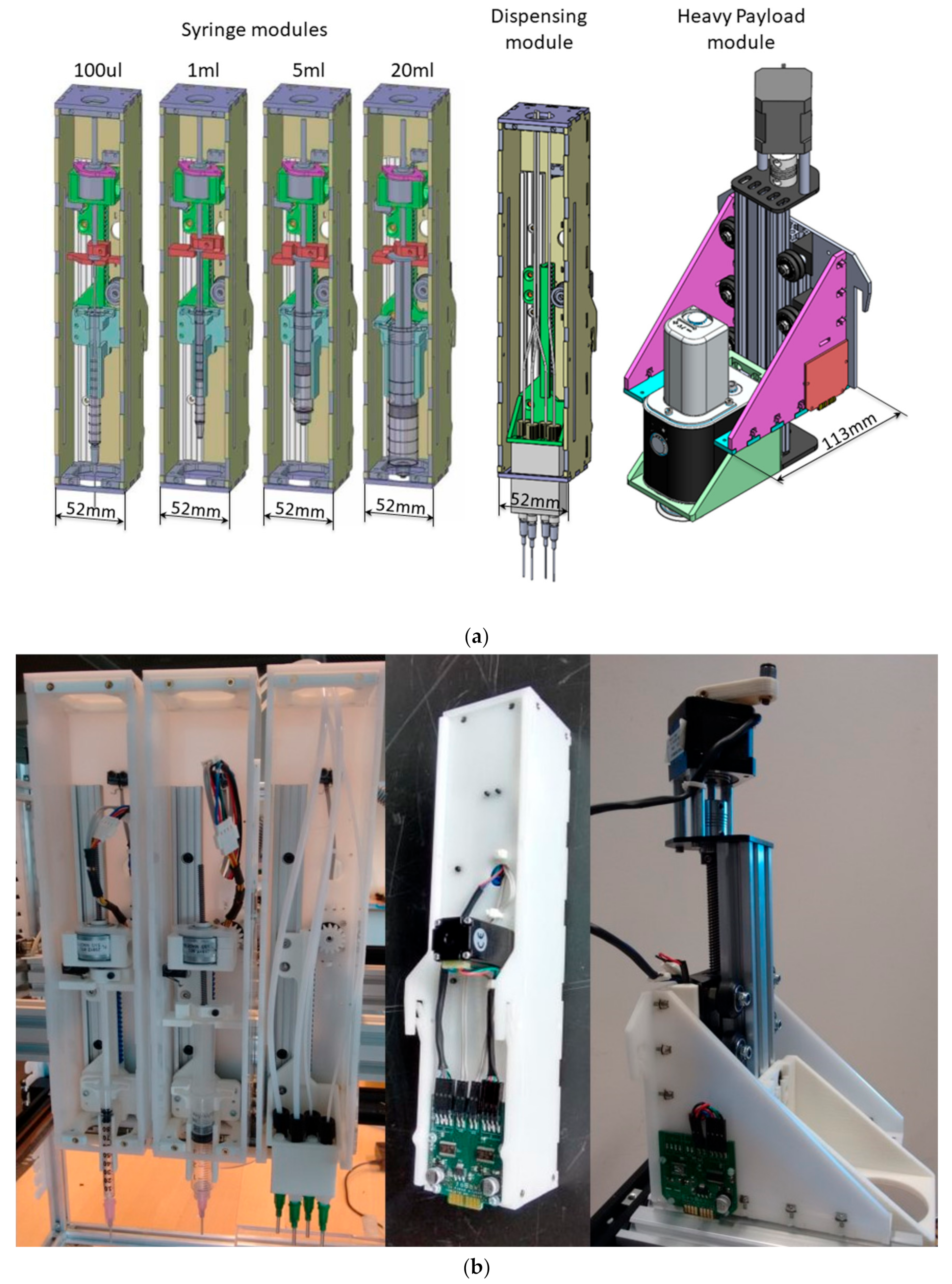




| Property | Actuation Layer | Syringe and Dispensing Module | Heavy Payload Module |
|---|---|---|---|
| Size (mm) | 600 × 400 * | 51 × 52 × 256 | 113 × 143 × 328 |
| Stroke (mm) | 300 × 200 * | Vertical axis: 78 Plunger axis: 60 † | 100 |
| Repeatability (mm) | (X and Y axis) < 0.1 | Vertical axis: <0.1 Plunger axis: - †,‡ | not tested |
| Speed (mm/s) | (X and Y axis) 180 | Vertical axis: 235 Plunger axis: 8 † | 8 |
| Acceleration (mm/s2) | (X and Y axis) 3000 | Vertical axis: 235 Plunger axis: 4 † | 4 |
| Payload (N) | - | 3 | >15 |
| Elements Used | |||||
|---|---|---|---|---|---|
| Application | Head | Syringe | Heavy Payload | Camera | Customisation |
| Static liquid handling | X | X | None | ||
| 3D scanning | X | OCT | Heavy payload added | ||
| Feeding MFCs | X | X | Voltage sensing added | ||
| 3D printing MFCs | X | X | Paste extruder | Heavy payload added | |
| Droplets experiments | X | X | X | Camera module added | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faiña, A.; Nejati, B.; Stoy, K. EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments. Appl. Sci. 2020, 10, 814. https://doi.org/10.3390/app10030814
Faiña A, Nejati B, Stoy K. EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments. Applied Sciences. 2020; 10(3):814. https://doi.org/10.3390/app10030814
Chicago/Turabian StyleFaiña, Andres, Brian Nejati, and Kasper Stoy. 2020. "EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments" Applied Sciences 10, no. 3: 814. https://doi.org/10.3390/app10030814
APA StyleFaiña, A., Nejati, B., & Stoy, K. (2020). EvoBot: An Open-Source, Modular, Liquid Handling Robot for Scientific Experiments. Applied Sciences, 10(3), 814. https://doi.org/10.3390/app10030814





