Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Fe3O4 Nanorings
2.2. Characterization
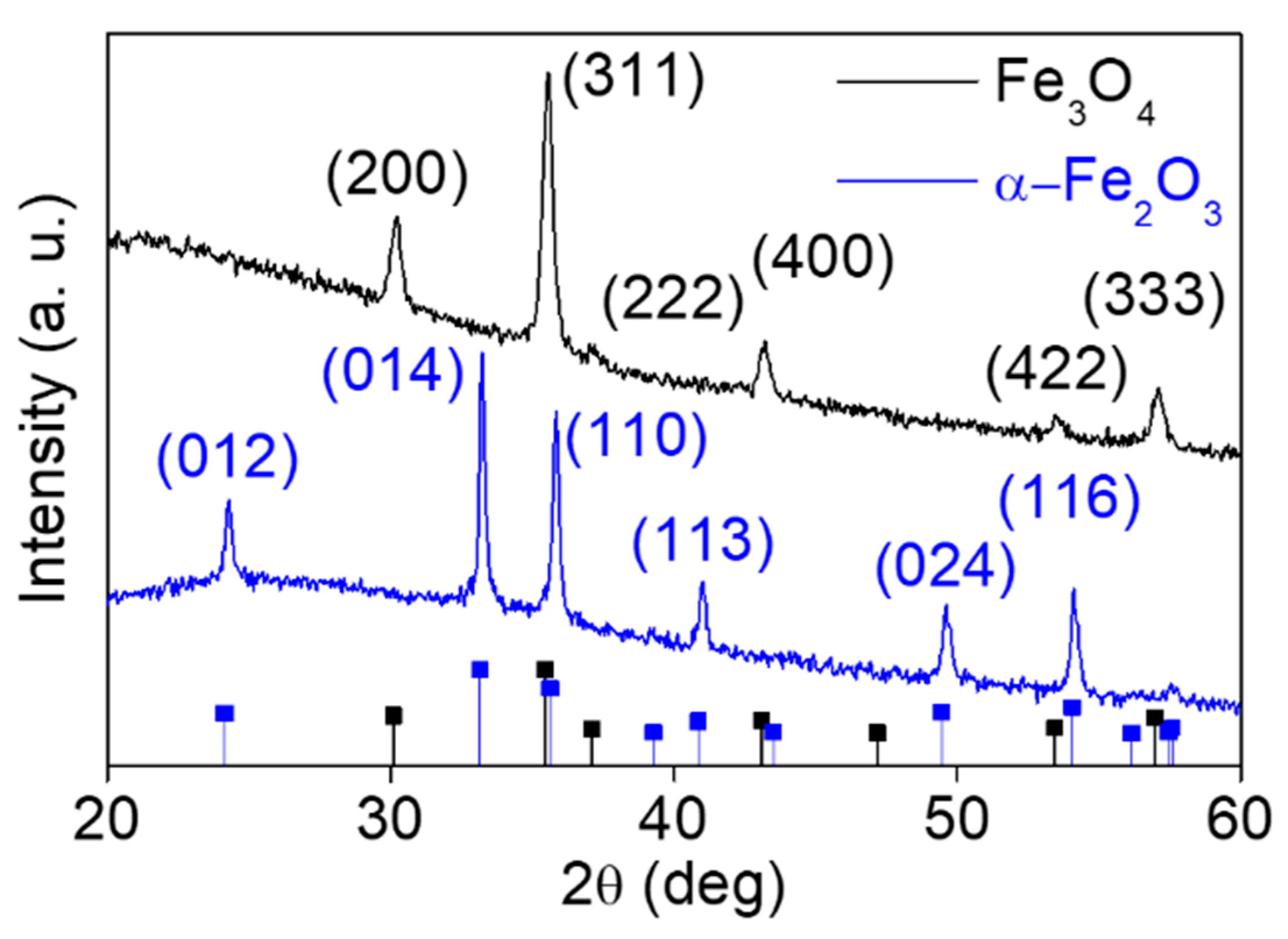
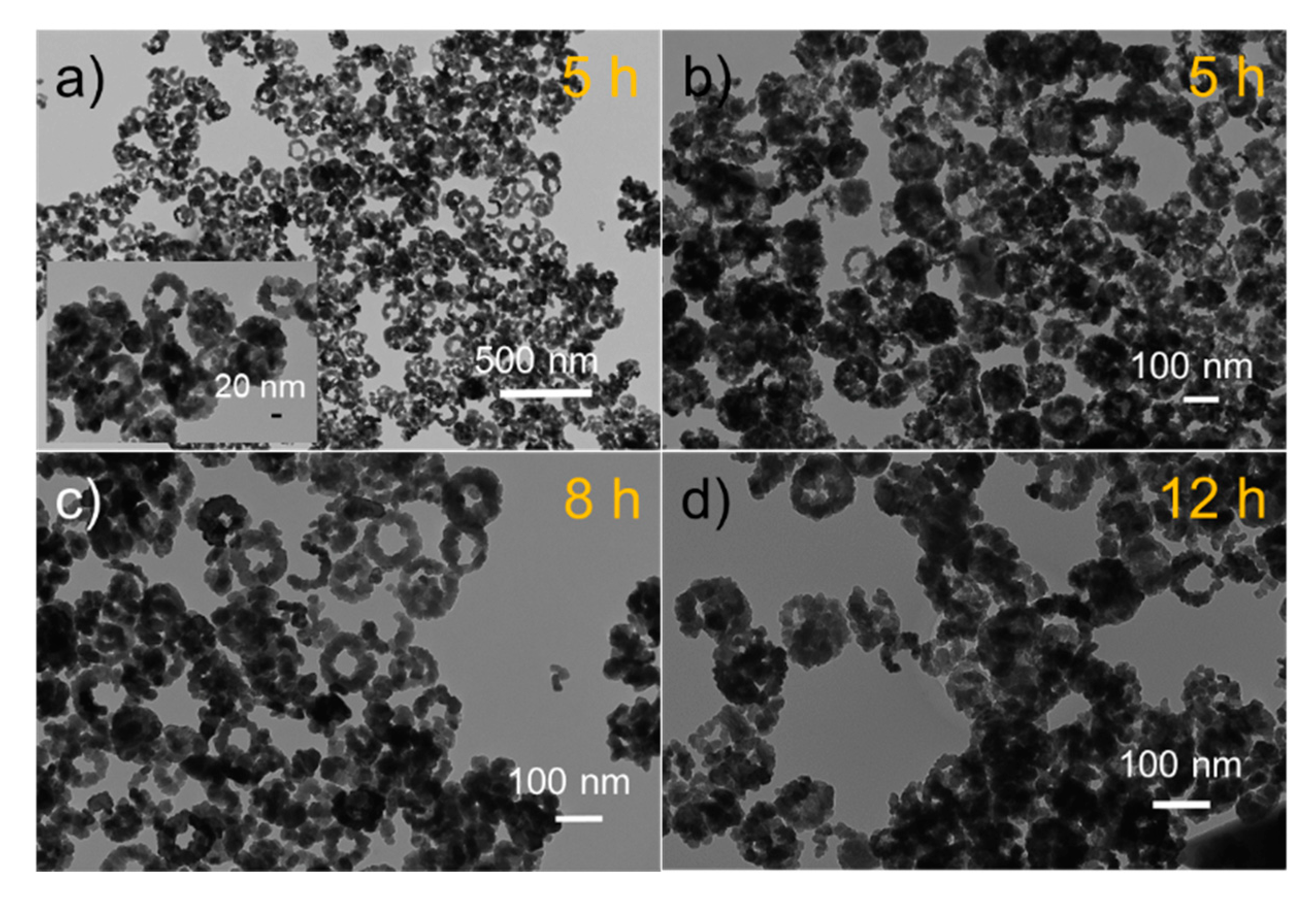
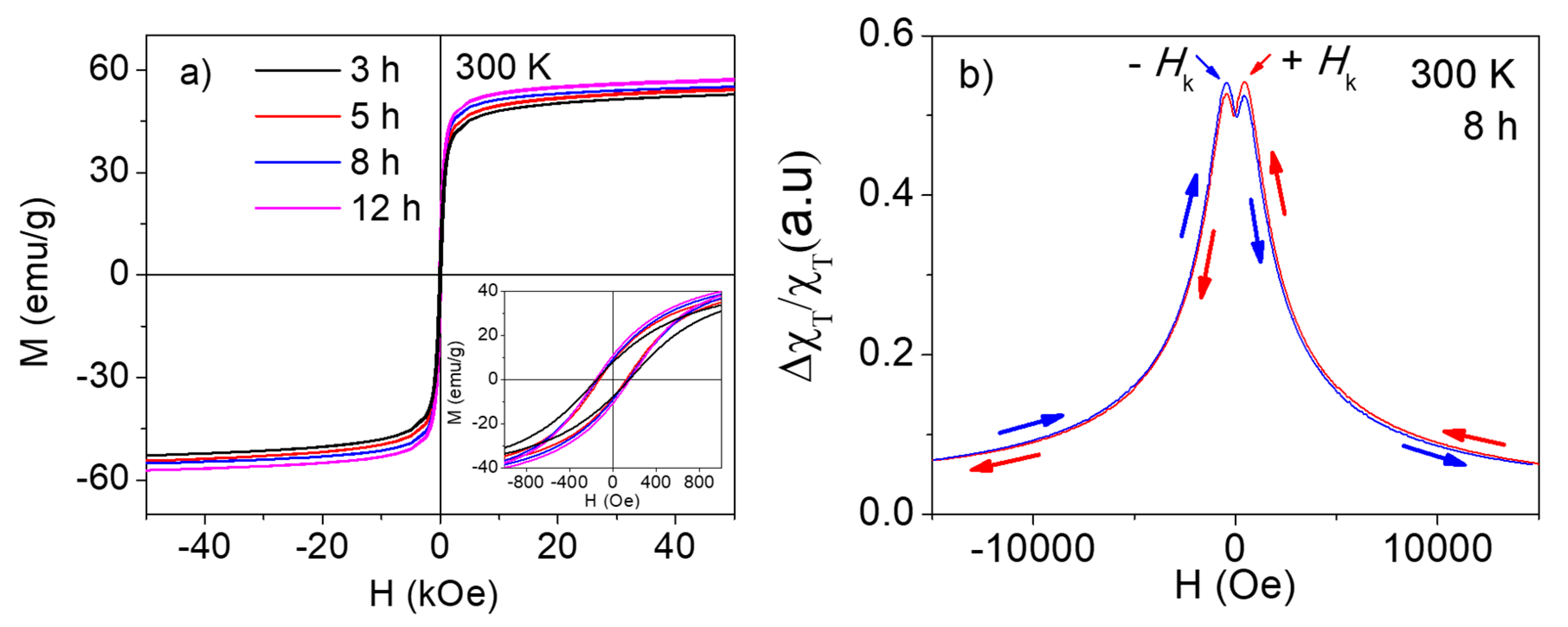
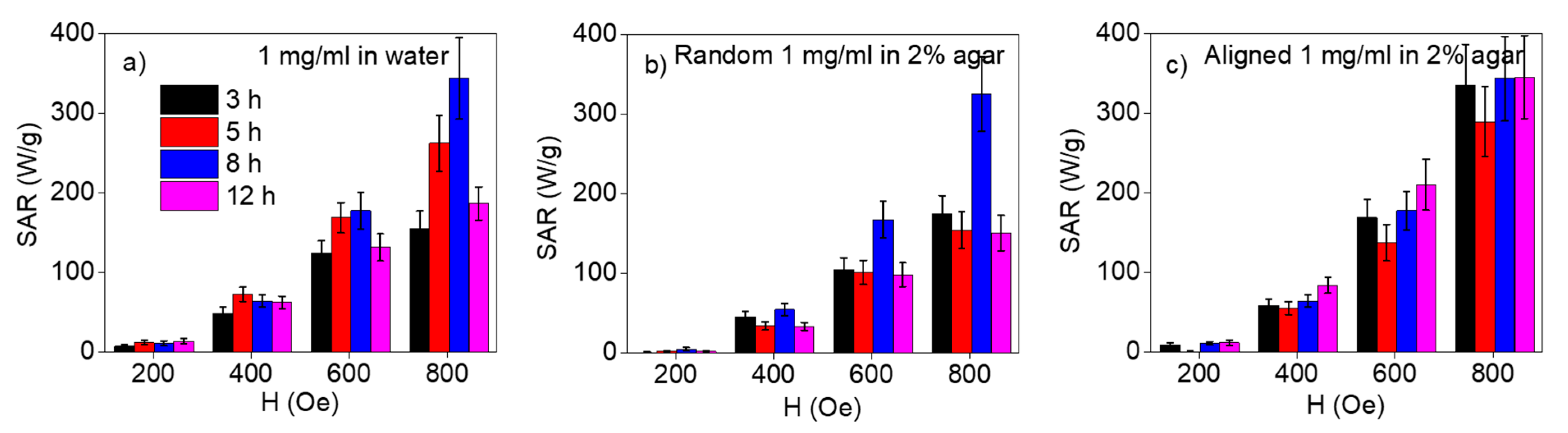
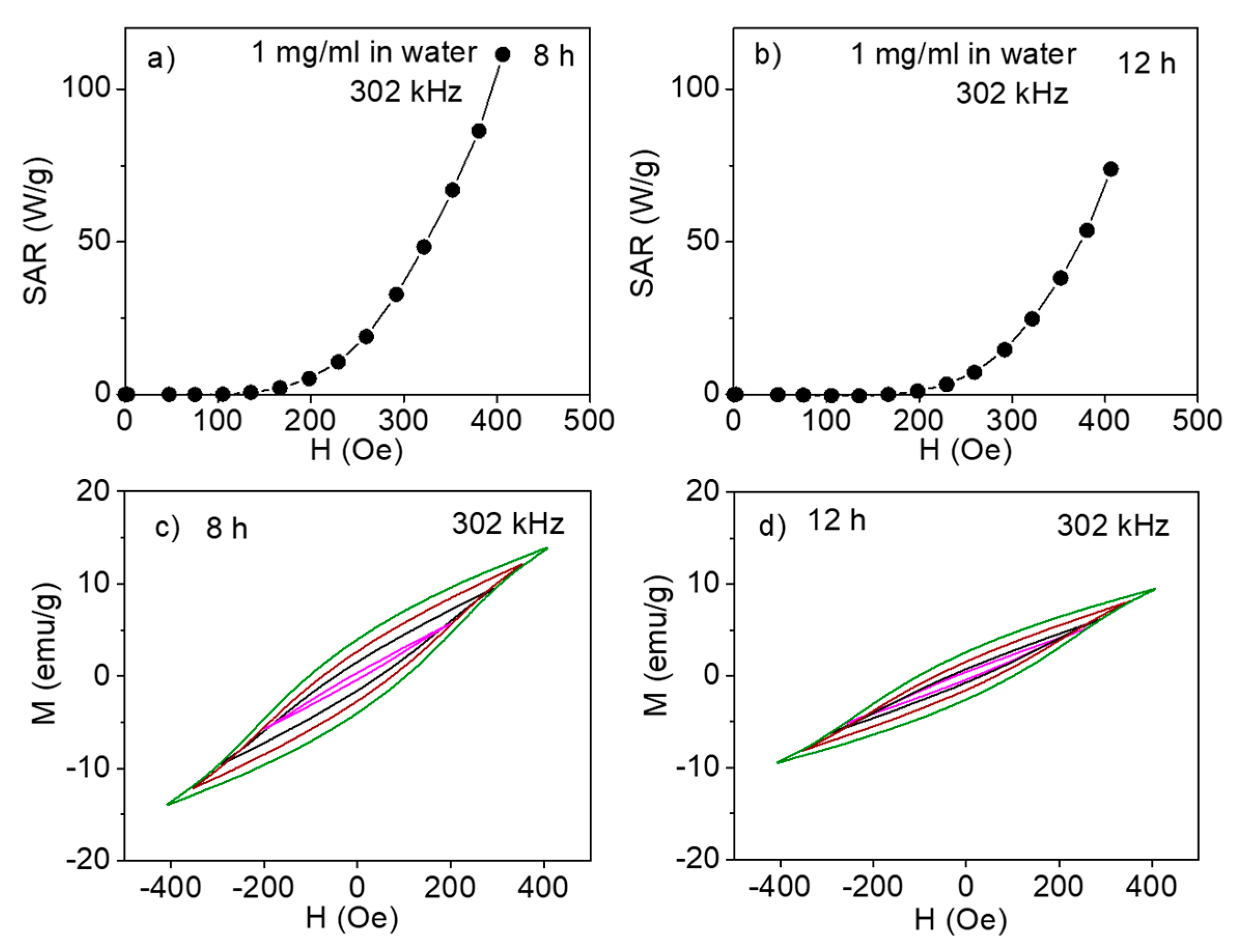
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- O’Handley, R.C. Modern Magnetic Materials Principles and Applications; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Sun, S.H.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G.X. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2003, 126, 273–279. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse Magnetic Single-Crystal Ferrite Microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernandez-Pacheco, R.; Ibarra, M.R.; Santamaria, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic nanoparticles for drug delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Son, S.J.; Reichel, J.; He, B.; Schuchman, M.; Lee, S.B. Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J. Am. Chem. Soc. 2005, 127, 7316–7317. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef]
- Khurshid, H.; Hadjipanayis, C.G.; Chen, H.; Li, W.; Mao, H.; Machaidze, R.; Tzitzios, V.; Hadjipanayis, G.C. Core/shell structured iron/iron-oxide nanoparticles as excellent MRI contrast enhancement agents. J. Magn. Magn. Mater. 2013, 331, 17–20. [Google Scholar] [CrossRef]
- Qiang, Y.; Antony, J.; Sharma, A.; Nutting, J.; Sikes, D.; Meyer, D. Iron/iron oxide core-shell nanoclusters for biomedical applications. J. Nanopart. Res. 2006, 8, 489–496. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; William, W.Y.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Gu, H.; Xu, K.; Xu, C.; Xu, B. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem. Commun. 2006, 9, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Chen, W.; Liu, T.; Zhu, Y.; Jin, P.; Wang, L.; Liu, J.; Wei, Y.; Li, Y. Bifunctional Au-Fe3O4 nanoparticles for protein separation. ACS Nano 2007, 1, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D 2002, 35, R15. [Google Scholar] [CrossRef]
- Sorensen, C.M. Magnetism in Nanoscale Materials in Chemistry; Klabunde, K.J., Ed.; Wiley-Interscience Publication: New York, NY, USA, 2001. [Google Scholar]
- Iwaki, T.; Kakihara, Y. Preparation of high coercivity magnetic FePt nanoparticles by liquid process. J. Appl. Phys. 2003, 94, 6807. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Noh, S.; Na, W.; Jang, J.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Hafeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Das, R.; Rinaldi, M.N.; Alonso, J.; Amghouz, Z.; Gorria, P.; Blanco, J.A.; Phan, M.H.; Srikanth, H. Boosted hyperthermia therapy by combined AC magnetic and photothermal exposures in Ag/Fe3O4 nanoflowers. ACS Appl. Mater. Interfaces 2016, 8, 25162–25169. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Muller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Kumar, C.S.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.H.; Shin, T.H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Sun, X.; Sun, S. Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Alonso, J.; Porshokouh, Z.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; Garcia, J.A.; Liamazares, J.L.S.; Srikanth, H. Tunable High Aspect Ratio Iron Oxide Nanorods for Enhanced Hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Das, R.; Cardarelli, J.A.; Phan, M.H.; Srikanth, H. Magnetically tunable iron oxide nanotubes for multifunctional biomedical applications. J. Alloy. Compd. 2019, 789, 323–329. [Google Scholar] [CrossRef]
- Robles, J.; Das, R.; Glassell, M.; Phan, M.H.; Srikanth, H. Exchange-coupled Fe3O4/CoFe2O4 nanoparticles for advanced magnetic hyperthermia. AIP Adv. 2018, 8, 056719. [Google Scholar] [CrossRef]
- Nemati, Z.; Das, R.; Alonso, J.; Clements, E.; Phan, M.H.; Srikanth, H. Iron oxide nanospheres and nanocubes for magnetic hyperthermia therapy: A comparative study. J. Electron. Mater. 2017, 46, 3764–3769. [Google Scholar] [CrossRef]
- Glöckl, G.; Glockl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Matter. 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Jordan, A. MagForce Nanotherapy: With tumor specific nanoparticles against cancer. VDI Ber. 2005, 1920, 111. [Google Scholar]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Sykes, E.A.; Dai, Q.; Tsoi, K.M.; Hwang, D.M.; Chan, W.C.W. Nanoparticle exposure in animals can be visualized in the skin and analysed via skin biopsy. Nat. Commun. 2014, 5, 3796. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic vortex nanorings: A new class of hyperthermia agent for highly efficient in vivo regression of tumors. Adv. Mater. 2015, 27, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-M.; Yi, J.-B.; Yang, Y.; Kho, K.-W.; Tan, H.-R.; Shen, Z.-X.; Ding, J.; Sun, X.-W.; Olivo, M.C.; Feng, Y.-P. Single-crystalline MFe2O4 nanotubes/nanorings synthesized by thermal transformation process for biological applications. ACS Nano 2009, 3, 2798–2808. [Google Scholar] [CrossRef]
- Chaves-O’Flynn, G.D.; Kent, A.D.; Stein, D.L. Micromagnetic study of magnetization reversal in ferromagnetic nanorings. Phys. Rev. B 2009, 79, 184421. [Google Scholar] [CrossRef]
- Zhu, F.Q.; Chern, G.W.; Tchernyshyov, O.; Zhu, X.C.; Zhu, J.G.; Chien, C.L. Magnetic bistability and controllable reversal of asymmetric ferromagnetic nanorings. Phys. Rev. Lett. 2006, 96, 027205. [Google Scholar] [CrossRef]
- Castano, F.J.; Ross, C.A.; Frandsen, C.; Eilez, A.; Gil, D.; Smith, H.I.; Redjdal, M.; Humphrey, F.B. Metastable states in magnetic nanorings. Phys. Rev. B 2003, 67, 184425. [Google Scholar] [CrossRef]
- Li, S.P.; Peyrade, D.; Natali, M.; Lebib, A.; Chen, Y.; Ebels, U.; Buda, L.D.; Ounadjela, K. Flux Closure Structures in Cobalt Rings. Phys. Rev. Lett. 2001, 86, 1102. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.-L.; Yi, J.-B.; Yang, Y.; Fan, H.-M.; Ding, J. Stable vortex magnetite nanorings colloid: Micromagnetic simulation and experimental demonstration. J. Appl. Phys. 2012, 111, 044303. [Google Scholar] [CrossRef]
- Fan, H.M.; You, G.J.; Li, Y.; Zheng, Z.; Tan, H.R.; Shen, Z.X.; Tang, S.H.; Feng, Y.P. Shape controlled synthesis of single-crystalline Fe2O3 hollow nanocrystals and their tunable optical properties. J. Phys. Chem. C 2009, 113, 9928–9935. [Google Scholar] [CrossRef]
- Liu, X.-L.; Yong, Y.; Wu, J.-P.; Zhang, Y.-F.; Fan, H.-M.; Ding, J. Novel magnetic vortex nanorings/nanodiscs: Synthesis and theranostic applications. Chin. Phys. B 2015, 24, 127505. [Google Scholar] [CrossRef]
- Jia, C.-J.; Sun, L.-D.; Luo, F.; Han, X.-D.; Heyderman, L.J.; Yan, Z.-G.; Yan, C.-H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-Scale Synthesis of Single-Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [PubMed]
- Garaio, E.; Collantes, J.M.; Plazaola, F.; Garcia, J.A.; Castellanos-Rubio, I. A multifrequency eletromagnetic applicator with an integrated AC magnetometer for magnetic hyperthermia experiments. Meas. Sci. Technol. 2014, 25, 115702. [Google Scholar] [CrossRef]
- Luo, W.; Nagel, S.R.; Rosenbaum, T.F.; Rosensweig, R.E. Dipole interactions with random anisotropy in a frozen ferrofluid. Phys. Rev. Lett. 1991, 67, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef]
- Frey, N.A.; Srinath, S.; Srikanth, H.; Varela, M.; Pennycook, S.; Miao, G.; Gupta, A. Magnetic anisotropy in epitaxial CrO2 and CrO2/Cr2O3 bilayer thin films. Phys. Rev. B 2006, 74, 24420. [Google Scholar] [CrossRef]
- Frey, N.A.; Srinath, S.; Srikanth, H.; Wang, C.; Sun, S. Static and dynamic magnetic properties of composite Au-Fe3O4 nanoparticles. IEEE Trans. Magn. 2007, 46, 3094. [Google Scholar] [CrossRef]
- Frey, N.A.; Phan, M.H.; Srikanth, H.; Srinath, S.; Wang, C.; Sun, S. Interparticle interactions in coupled Au–Fe3O4 nanoparticles. J. Appl. Phys. 2009, 105, 07B502. [Google Scholar] [CrossRef]
- Srikanth, H.; Wiggins, J.; Rees, H. Radio-frequency impedance measurements using a tunnel-diode oscillator technique. Rev. Sci. Instrum. 1999, 70, 3097–3101. [Google Scholar] [CrossRef]
- Aharoni, A.; Frei, E.H.; Shtrikman, S.; Treves, D. The reversible susceptibility tensor of the Stoner-Wolfarth model. Bull. Res. Counc. Isr. Sect. F 1957, 6A, 215–238. [Google Scholar]
- Chandra, S.; Das, R.; Kalappattil, V.; Eggers, T.; Harnagea, C.; Nechache, R.; Phan, M.H.; Rosei, F.; Srikanth, H. Epitaxial magnetite nanorods with enhanced room temperature magnetic anisotropy. Nanoscale 2017, 9, 7858–7867. [Google Scholar] [CrossRef] [PubMed]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo, F.O.; Marciello, M.; Morales, M.D.P.; Baldomir, D.; Martinez-Boubeta, C. Multiplying magnetic hyperthermia response by nanoparticle assembling. J. Phys. Chem. C. 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Sangnier, A.P.; Preveral, S.; Curcio, A.; Silva, A.K.A.; Lefèvre, C.T.; Pignol, D.; Lalatonne, Y.; Wilhelm, C. Targeted thermal therapy with genetically engineered magnetite magnetosomes@ RGD: Photothermia is far more efficient than magnetic hyperthermia. J. Control. Release 2018, 279, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Boubeta, C.M.; Simeonidis, K.; Serantes, D.; Conde-Leborán, I.; Kazakis, I.; Stefanou, G.; Peña, L.; Galceran, R.; Balcells, L.; Monty, C.; et al. Adjustable Hyperthermia Response of Self-Assembled Ferromagnetic Fe-MgO Core–Shell Nanoparticles by Tuning Dipole–Dipole Interactions. Adv. Funct. Mater. 2012, 22, 3737–3744. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.H.; Srikanth, H. Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J. Phys. Chem. C 2018, 122, 42367–42381. [Google Scholar] [CrossRef]
| Reaction Time (h) | Crystallite Size (nm) | Outer Diameter (nm) | Inner to Outer Diameter Ratio (β) | MS (emu/g) | HC (Oe) | SAR in Water at 800 Oe (W/g) |
|---|---|---|---|---|---|---|
| 3 | 15 | 100 | 0.5 | 52 | 150 | 155 |
| 5 | 15 | 110 | 0.55 | 54 | 130 | 262 |
| 8 | 17 | 110 | 0.5 | 55 | 140 | 342 |
| 12 | 18 | 105 | 0.5 | 57 | 155 | 186 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.; Witanachchi, C.; Nemati, Z.; Kalappattil, V.; Rodrigo, I.; García, J.Á.; Garaio, E.; Alonso, J.; Lam, V.D.; Le, A.-T.; et al. Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings. Appl. Sci. 2020, 10, 787. https://doi.org/10.3390/app10030787
Das R, Witanachchi C, Nemati Z, Kalappattil V, Rodrigo I, García JÁ, Garaio E, Alonso J, Lam VD, Le A-T, et al. Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings. Applied Sciences. 2020; 10(3):787. https://doi.org/10.3390/app10030787
Chicago/Turabian StyleDas, Raja, Chiran Witanachchi, Zohreh Nemati, Vijaysankar Kalappattil, Irati Rodrigo, José Ángel García, Eneko Garaio, Javier Alonso, Vu Dinh Lam, Anh-Tuan Le, and et al. 2020. "Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings" Applied Sciences 10, no. 3: 787. https://doi.org/10.3390/app10030787
APA StyleDas, R., Witanachchi, C., Nemati, Z., Kalappattil, V., Rodrigo, I., García, J. Á., Garaio, E., Alonso, J., Lam, V. D., Le, A.-T., Phan, M.-H., & Srikanth, H. (2020). Magnetic Vortex and Hyperthermia Suppression in Multigrain Iron Oxide Nanorings. Applied Sciences, 10(3), 787. https://doi.org/10.3390/app10030787









