The Clinical and Economic Value of Triclosan-Coated Surgical Sutures in Abdominal Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.1.1. Criteria for Considering Studies for Literature Review
2.1.2. Search Methods for Identification of Studies
2.1.3. Study Selection
2.1.4. Data Extraction and Management
2.2. Budget Impact Model
2.2.1. Model Inputs
2.2.2. Scenario and Sensitivity Analyses
3. Results
3.1. Clinical Results
3.2. Economic Results
3.2.1. Base Case Analyses
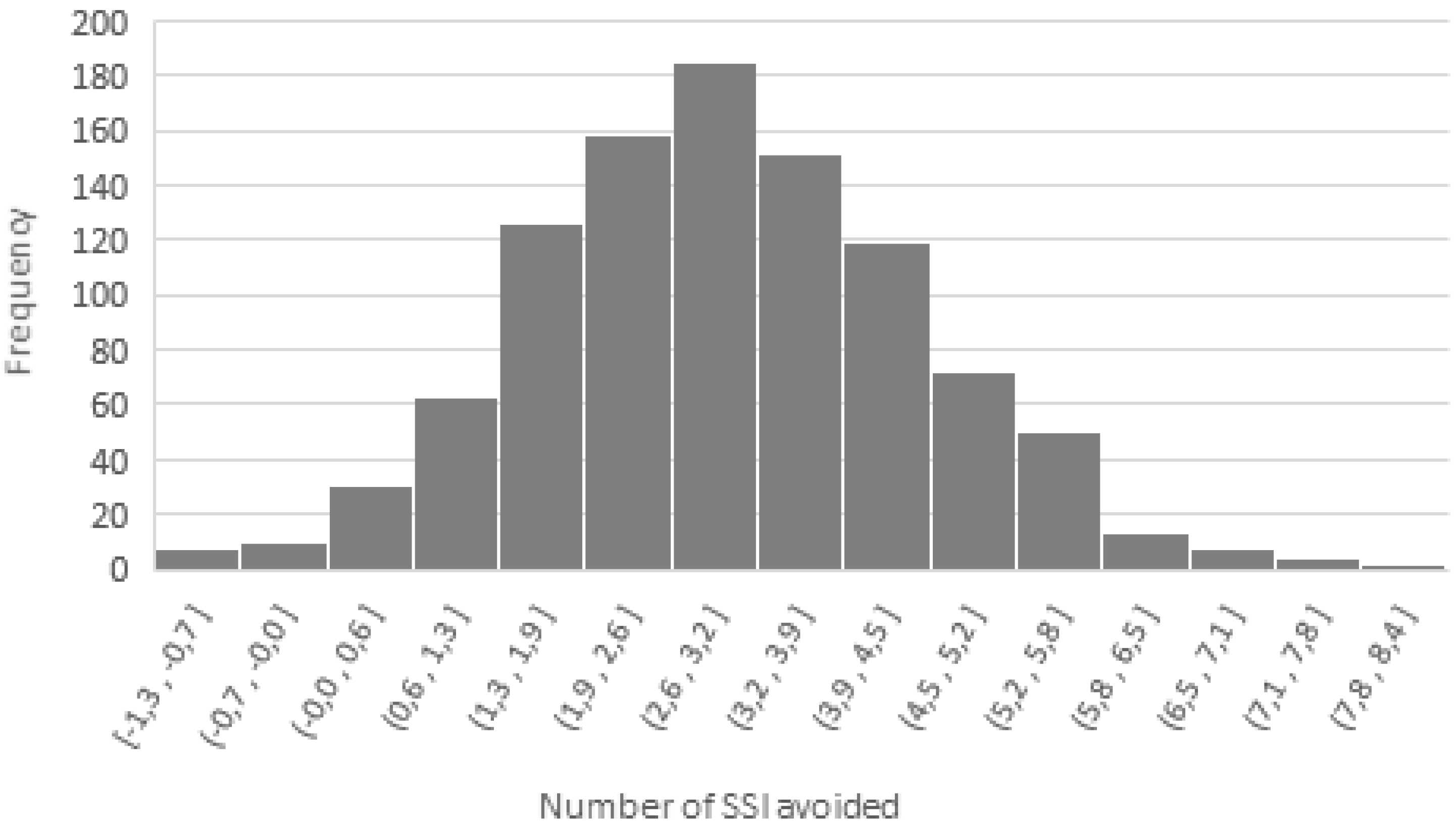
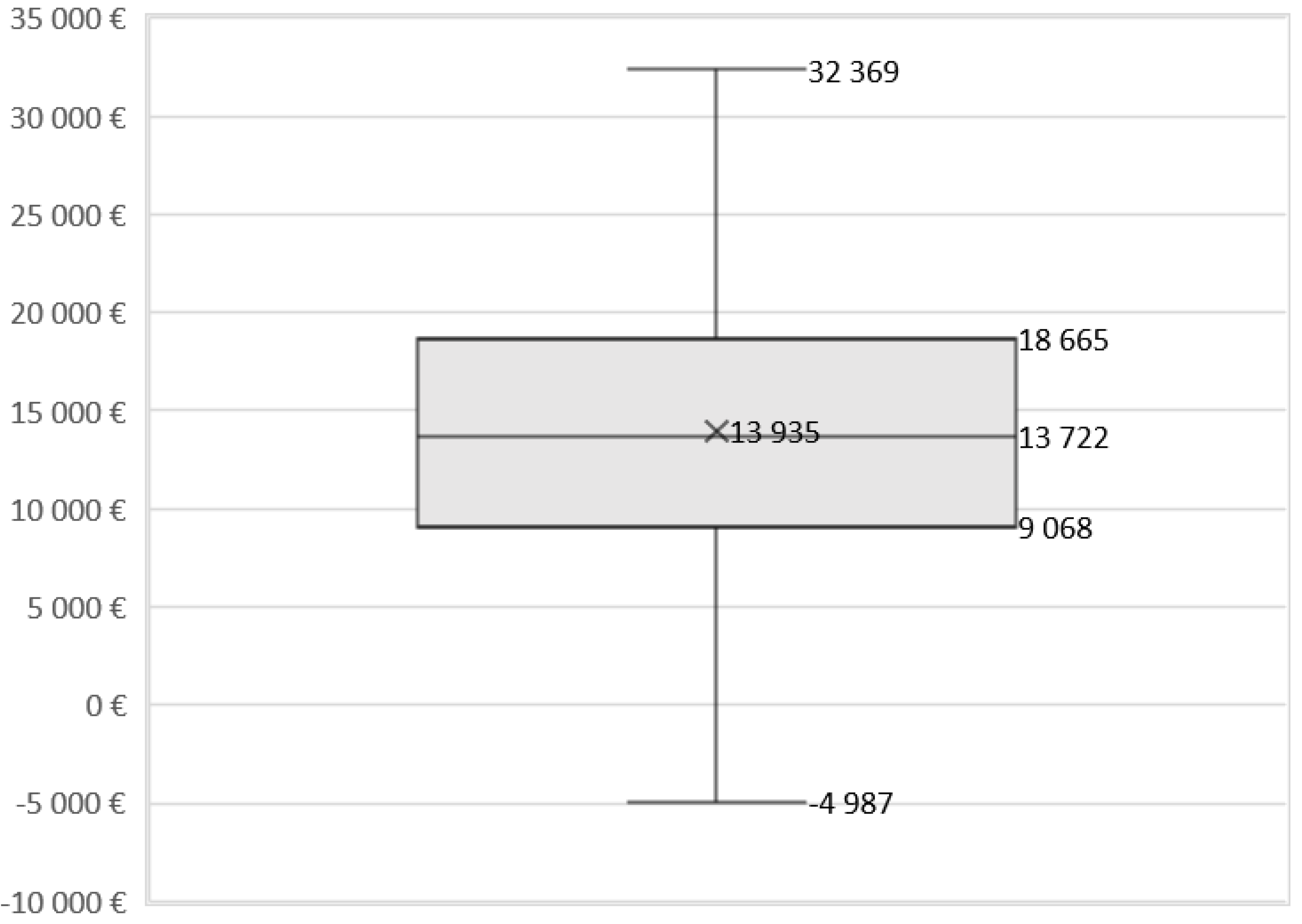
3.2.2. Scenario and Sensitivity Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Publication Year | N. Studies/ N. Pts | Results for all Surgery and Study Type | Reason for Exclusion |
|---|---|---|---|---|
| Chang et al. | 2012 | 7 RCTs 836 pts | OR = 0.77 95% CI: 0.40–1.51 p = 0.45 | All surgery types No abdominal surgery results |
| Sajid et al. | 2013 | 7 RCTs 1631 pts | RR = 0.61 95% CI: 0.37–0.99 p = 0.04 | All surgery types No abdominal surgery results |
| Edmiston et al. | 2013 | 13 RCTs 3568 pts | RR = 0.734 95% CI: 0.590–0.913 p = 0.005 | All surgery types No abdominal surgery results |
| Wu et al. | 2017 | 18 13 RCTs 5 non-RCTs 7458 pts | RCTs: OR = 0.72 95% CI: 0.59–0.88 p = 0.001 Non-RCTs: OR = 0.58 95% CI: 0.40–0.83 p = 0.003 | All surgery types No abdominal surgery results |
| de Jonge et al. | 2017 | 21 RCTs 6462 pts | RR = 0.72 95% CI: 0.60–0.86 P < 0.001 | All surgery types No abdominal surgery results |
| Leaper et al. | 2017 | 34 20 RCTs 14 non-RCTs NR pts | OR = 0.61 95% CI: 0.52–0.73 P < 0.001 | All surgery types No abdominal surgery results No RCTs results |
| Hunger et al. | 2018 | 6 3 RCTs 3 non-RCTs 5188 pts | RCTs: RR = 0.67 95% CI: 0.48–0.94 p = 0.02 Non-RCTs: OR = 0.4 95% CI: 0.3–0.54 P < 0.001 | All surgery types No abdominal surgery results |
References
- European Centre for Disease Prevention and Control. Surveillance of Surgical Site Infections and Prevention Indicators in European Hospitals: HAI-Net SSI Protocol; Version 2.2; Tommi Kärki: Carl Suetens, Stockholm, May 2017; ISBN 978-92-9498-060-1. [Google Scholar]
- Preventing Surgical Site Infections: Implementation Approaches for Evidence-Based Recommendations. World Health Organization: Geneva, 2018. Licence: CC BYNC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/273154/9789241514385-eng.pdf (accessed on 1 August 2019).
- Aga, E.; Keinan-Boker, L.; Eithan, A.; Mais, T.; Rabinovich, A.; Nassar, F. Surgical site infections after abdominal surgery: Incidence and risk factors. A prospective cohort study. Infect. Dis. 2015, 47, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, R.; Protic, M.; Gluhovic, A.; Potic, Z.; Milosevic, Z.; Stojadinovic, A. Prospective clinical trial of factors predicting the early development of incisional hernia after midline laparotomy. J. Am. Coll. Surg. 2010, 210, 210–219. [Google Scholar] [CrossRef]
- Murray, B.W.; Cipher, D.J.; Pham, T.; Anthony, T. The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am. J. Surg. 2011, 202, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, W.J.; Gupta, K.; Itani, K.M.F. Association of Postoprative infection With Risk of Long-term Infection and Mortality. JAMA Surg. 2020, 155, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nespoli, A.; Gianotti, L.; Totis, M.; Bovo, G.; Nespoli, L.; Chiodini, P.; Brivio, F. Correlation between postoperative infections and long-term survival after colorectal resection for cancer. Tumori 2004, 90, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chuah, L.L.; Papamargaritis, D.; Pillai, D.; Krishnamoorthy, A.; le Roux, C.W. Morbidity and mortality of diabetes with surgery. Nutr. Hosp. 2013, 28, 47–52. [Google Scholar]
- Sørensen, L.T.; Hemmingsen, U.; Kallehave, F.; Wille-Jørgensen, P.; Kjærgaard, J.; Møller, L.N.; Jørgensen, T. Risk Factors for Tissue and Wound Complications in Gastrointestinal Surgery. Ann. Surg. 2005, 241, 654–658. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection - from underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Thelwall, S.; Harrington, P.; Sheridan, E.; Lamagni, T. Impact of obesity on the risk of wound infection following surgery: Results from a nationwide prospective multicentre cohort study in England. Clin. Microbiol. Infect. 2015, 21, 1008.e1–1008.e8. [Google Scholar] [CrossRef]
- Alexander, J.W.; Kaplan, J.Z.; Altemeier, W.A. Role of suture materials in the development of wound infection. Ann. Surg. 1967, 165, 192–199. [Google Scholar] [CrossRef]
- Kampf, G.; Kramer, A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin. Microbiol. Rev. 2004, 17, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Hubner, N.-O.; McBain, A.; Barbolt, T.; Rothenburger, S.; Wilson, P. Antimicrobial sutures and prevention of surgical site infection: Assessment of the safety of the antiseptic triclosan. Int. Wound J. 2011, 8, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Global Guidelines for the Prevention of Surgical Site Infection, second edition. World Health Organization: Geneva, 2018. Licence: CC BY-NC-SA 3.0 IGO. Available online: https://apps.who.int/iris/bitstream/handle/10665/277399/9789241550475-eng.pdf?ua=1 (accessed on 1 August 2019).
- Ban, K.A.; Minei, J.P.; Laronga, C.; Harbrecht, B.G.; Jensen, E.H.; Fry, D.E.; Itani, K.M.F.; Dellinger, E.P.; Ko, C.Y.; Duane, T.M. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J. Am. Coll. Surg. 2017, 224, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Surgical Site Infections: Prevention and Treatment NICE Guideline [NG125]. Available online: https://www.nice.org.uk/guidance/ng125 (accessed on 1 August 2019).
- Baracs, J.; Huszár, O.; Sajjadi, S.G.; Horváth, O.P. Surgical site infections after abdominal closure in colorectal surgery using triclosan-coated absorbable suture (PDS Plus) vs. uncoated sutures (PDS II): A randomized multicenter study. Surg. Infect. 2011, 12, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.K.; Knebel, P.; Kieser, M.; Schüler, P.; Schiergens, T.S.; Atanassov, V.; Neudecker, J.; Stein, E.; Thielemann, H.; Kunz, R.; et al. Effectiveness of triclosan-coated PDS Plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: The randomised controlled PROUD trial. Lancet 2014, 384, 142–152. [Google Scholar] [CrossRef]
- Justinger, C.; Slotta, J.E.; Ningel, S.; Gräber, S.; Kollmar, O.; Schilling, M.K. Surgical-site infection after abdominal wall closure with triclosan-impregnated polydioxanone sutures: Results of a randomized clinical pathway facilitated trial (NCT00998907). Surgery 2013, 154, 589–595. [Google Scholar] [CrossRef]
- Mattavelli, I.; Rebora, P.; Doglietto, G.; Dionigi, P.; Dominioni, L.; Luperto, M.; La Porta, A.; Garancini, M.; Nespoli, L.; Alfieri, S.; et al. Multi-Center Randomized Controlled Trial on the Effect of Triclosan-Coated Sutures on Surgical Site Infection after Colorectal Surgery. Surg. Infect. 2015, 16, 226–235. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Alonso, N.; Morales, V.; Llavero, C. Association between Triclosan-Coated Sutures for Abdominal Wall Closure and Incisional Surgical Site Infection after Open Surgery in Patients Presenting with Fecal Peritonitis: A Randomized Clinical Trial. Surg. Infect. 2015, 16, 588–594. [Google Scholar] [CrossRef]
- Mingmalairak, C.; Ungbhakorn, P.; Paocharoen, V. Efficacy of antimicrobial coating suture coated polyglactin 910 with tricosan (Vicryl plus) compared with polyglactin 910 (Vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report. J. Med. Assoc. Thai. 2009, 92, 770–775. [Google Scholar] [PubMed]
- Nakamura, T.; Kashimura, N.; Noji, T.; Suzuki, O.; Ambo, Y.; Nakamura, F.; Kishida, A. Triclosan-coated sutures reduce the incidence of wound infections and the costs after colorectal surgery: A randomized controlled trial. Surgery 2013, 153, 576–583. [Google Scholar] [CrossRef]
- Rasić, Z.; Schwarz, D.; Adam, V.N.; Sever, M.; Lojo, N.; Rasić, D.; Matejić, T. Efficacy of antimicrobial triclosan-coated polyglactin 910 (Vicryl* Plus) suture for closure of the abdominal wall after colorectal surgery. Coll. Antropol. 2011, 35, 439–443. [Google Scholar] [PubMed]
- Henriksen, N.A.; Deerenberg, E.B.; Venclauskas, L.; Fortelny, R.H.; Garcia-Alamino, J.M.; Miserez, M.; Muysoms, F.E. Triclosan-coated sutures and surgical site infection in abdominal surgery: The TRISTAN review, meta-analysis and trial sequential analysis. Hernia 2017, 21, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Uchino, M.; Mizuguchi, T.; Ohge, H.; Haji, S.; Shimizu, J.; Mohri, Y.; Yamashita, C.; Kitagawa, Y.; Suzuki, K.; Kobayashi, M.; et al. The Efficacy of Antimicrobial-Coated Sutures for Preventing Incisional Surgical Site Infections in Digestive Surgery: A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2018, 22, 1832–1841. [Google Scholar] [CrossRef] [PubMed]
- Mauskopf, J.A.; Sullivan, S.D.; Annemans, L.; Caro, J.; Mullins, C.D.; Nuijten, M.; Orlewska, E.; Watkins, J.; Trueman, P. Principles of good practice for budget impact analysis: Report of the ISPOR Task Force on good research practices--budget impact analysis. Value Health 2007, 10, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.D.; Mauskopf, J.A.; Augustovski, F.; Jaime Caro, J.; Lee, K.M.; Minchin, M.; Orlewska, E.; Penna, P.; Rodriguez Barrios, J.-M.; Shau, W.-Y. Budget impact analysis-principles of good practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014, 17, 5–14. [Google Scholar] [CrossRef]
- Gianotti, L.; Braga, M.; Frei, A.; Greiner, R.; Di Carlo, V. Health care resources consumed to treat postoperative infections: Cost saving by perioperative immunonutrition. Shock 2000, 14, 325–330. [Google Scholar] [CrossRef]
- Wang, G.J.; Hungerford, D.S.; Savory, C.G.; Rosenberg, A.G.; Mont, M.A.; Burks, S.G.; Mayers, S.L.; Spotnitz, W.D. Use of fibrin sealant to reduce bloody drainage and hemoglobin loss after total knee arthroplasty: A brief note on a randomized prospective trial. J. Bone Jt. Surg. Am. 2001, 83, 1503–1505. [Google Scholar] [CrossRef]
- Daoud, F.C.; Edmiston, C.E.; Leaper, D. Meta-analysis of prevention of surgical site infections following incision closure with triclosan-coated sutures: Robustness to new evidence. Surg. Infect. 2014, 15, 165–181. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Singh, N.; Bandong, A.N.; Madriaga, G. Triclosan-coated sutures reduce the risk of surgical site infections: A systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 2015, 36, 169–179. [Google Scholar] [CrossRef]
- Guo, J.; Pan, L.-H.; Li, Y.-X.; Yang, X.-D.; Li, L.-Q.; Zhang, C.-Y.; Zhong, J.-H. Efficacy of triclosan-coated sutures for reducing risk of surgical site infection in adults: A meta-analysis of randomized clinical trials. J. Surg. Res. 2016, 201, 105–117. [Google Scholar] [CrossRef]
- Sandini, M.; Mattavelli, I.; Nespoli, L.; Uggeri, F.; Gianotti, L. Systematic review and meta-analysis of sutures coated with triclosan for the prevention of surgical site infection after elective colorectal surgery according to the PRISMA statement. Medicine 2016, 95, e4057. [Google Scholar] [CrossRef] [PubMed]
- Elsolh, B.; Zhang, L.; Patel, S.V. The Effect of Antibiotic-Coated Sutures on the Incidence of Surgical Site Infections in Abdominal Closures: A Meta-Analysis. J. Gastrointest. Surg. 2017, 21, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Konstantelias, A.A.; Andriakopoulou, C.S.I.; Mourgela, S. Triclosan-coated sutures for the prevention of surgical-site infections: A meta-analysis. Acta Chir. Belg. 2017, 117, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Olmez, T.; Berkesoglu, M.; Turkmenoglu, O.; Colak, T. Effect of Triclosan-Coated Suture on Surgical Site Infection of Abdominal Fascial Closures. Surg. Infect. 2019, 20, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Noda, H.; Kikugawa, R.; Hasegawa, F.; Obitsu, T.; Ishioka, D.; Fukuda, R.; Yoshizawa, A.; Tsujinaka, S.; Rikiyama, T. Effect of triclosan-coated sutures on the incidence of surgical site infection after abdominal wall closure in gastroenterological surgery: A double-blind, randomized controlled trial in a single center. Surgery 2018. [Google Scholar] [CrossRef]
- Wang, Z.X.; Jiang, C.P.; Cao, Y.; Ding, Y.T. Systematic review and meta-analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br. J. Surg. 2013, 100, 465–473. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Leaper, D.J.; Edmiston, C.E.; Holy, C.E. Meta-analysis of the potential economic impact following introduction of absorbable antimicrobial sutures. Br. J. Surg. 2017, 104, e134–e144. [Google Scholar] [CrossRef]
- Galal, I.; El-Hindawy, K. Impact of using triclosan-antibacterial sutures on incidence of surgical site infection. Am. J. Surg. 2011, 202, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Fleck, T.; Moidl, R.; Blacky, A.; Fleck, M.; Wolner, E.; Grabenwoger, M.; Wisser, W. Triclosan-coated sutures for the reduction of sternal wound infections: Economic considerations. Ann. Thorac. Surg. 2007, 84, 232–236. [Google Scholar] [CrossRef] [PubMed]
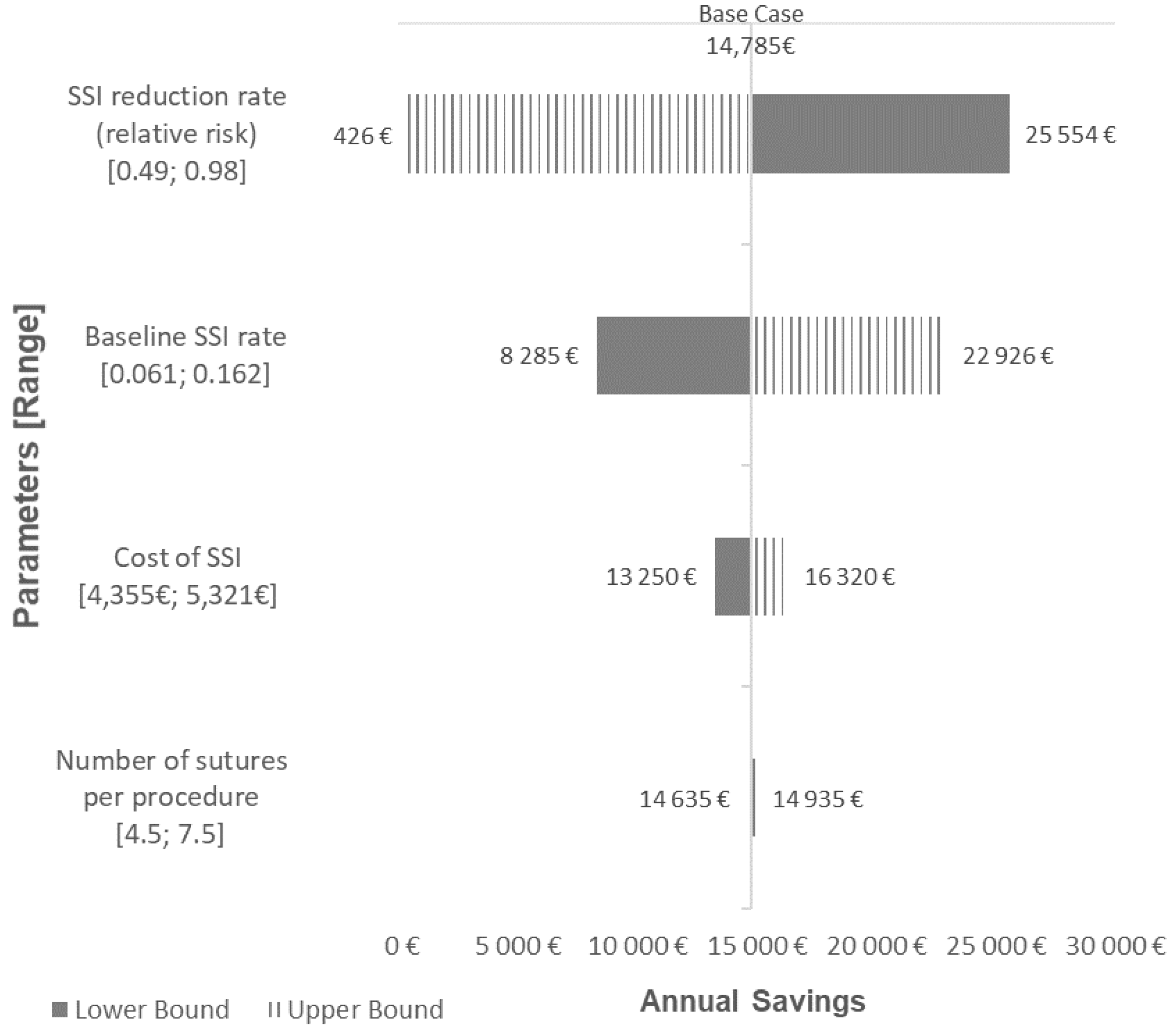
| PSA Parameters | Base Case Value | Lower Range | Upper Range | Distribution |
|---|---|---|---|---|
| Baseline SSI rate | 10.6% | 6.1% | 16.2% | Beta |
| Cost of SSI | €4838 | €4355 | €5321 | Gamma |
| Number of sutures per procedure | 6 | 4.5 | 7.5 | Gamma |
| SSI reduction rate (relative risk) | 0.70 | 0.49 | 0.98 | Log-normal |
| Study | N. studies | N. pts | Surgeries | Population | Publication Bias | Results for Abdominal Surgery and RCTs Only |
|---|---|---|---|---|---|---|
| Wang et al. [42] | 17 RCTs | 3720 TCS:1726; CS:1994 | Abdominal Breast Cardiovascular | Adult (15 studies) Pediatric (2 studies) | None | RR = 0.69 95%CI: 0.50–0.97 p = 0.03; I2 = 34% |
| Daoud et al. [34] | 15 RCTs | 4800 TCS: 2323; CS = 2477 | Neurosurgery Abdominal/CR Breast Cardiovascular Various | Adult (14) Pediatric (3) | None | RR = 0.67 95%CI: 0.54–0.84 p = 0.00053; I2 = N/A |
| Apisarnthanarak et al. [35] | 29 22 RCTs 7 non-RCTs | 11,942 TCS: 5,802; CS: 6,140 | Neurosurgery Abdominal/CR Breast Cardiovascular Head/neck Pelvic surgery Various | NR | None | RR: 0.56 95% CI: 0.41–0.77 p < 0.05; I2 = 64.6% |
| Guo et al. [36] | 13 RCTs | 5256 TCS: 2,592; CS: ,2592 | Abdominal Breast Cardiovascular Vascular Various | Adult | Low | RR = 0.70 95% CI: 0.50–0.99 p = 0.04; I2 = 52% |
| Sandini et al. [37] | 6 RCTs | 2168 TCS: 1,102; CS: 1,066 | Colorectal | Adult | None | OR = 0.81 95% CI: 0.58–1.13 p = 0.220; I2 = 44.9% |
| Elsolh et al. [38] | 5 RCTs | 3117 | Abdominal | Adult | None | OR = 0.79 95%CI: 0.57–1.09 p = 0.15; I2 = 44% |
| Konstantelias et al. [39] | 30 RCTs: 19 non-RCTs: 11 | 15,385 | Neurosurgery Abdominal/CR Breast Cardiovascular Head and neck Spine Various | NR | None | RCTs: RR = 0.74 95%CI: 0.44–1.27 p = NS; I2 = 54% |
| Henriksen et al. [28] | 8 RCTs | 3502 TCS: 1,797; CS: 1,705 | Abdominal/CR | Adult | NR | OR = 0.67 95%CI: 0.46–0.98 p = 0.04; I2 = 56% |
| Uchino et al. [29] | 15 RCTs: 10 non-RCTs: 5 | 5703 TCS: 2,889; CS: 2,814 | Abdominal/CR | Adult | Minimal | RCTs: RR = 0.67 95% CI: 0.48–0.94 p = 0.02; I2 = 55% |
| First Author | N° of pts (TCS/CS) | Type of Surgery | Additional Preventive Strategies for SSI | Follow-Up | SSI Rates | ||
|---|---|---|---|---|---|---|---|
| TCS | CS | p-Value | |||||
| Baracs et al. [20] | 385 (188/197) | Elective open colorectal surgery | antibiotic prophylaxis | Clinical examination during hospital stay, telephonic follow-up at 30 d | 12.2% | 12.2% | 0.98 |
| Diener et al. [21] | 1185 (587/598) | Elective midline laparotomies | antibiotic prophylaxis | Clinical examination at 10 and 30 d from discharge | 14.8% | 16.1% | 0.64 |
| routine scrub | |||||||
| site preparation | |||||||
| Justinger et al. [22] | 856 (485/371) | Emergency or elective laparotomies | bowel preparation | Clinical examination during hospital stay and at 14 d | 6.4% | 11.3% | <0.05 |
| iodine shower | |||||||
| site preparation | |||||||
| antibiotic prophylaxis | |||||||
| Mattavelli et al. [23] | 281 (140/141) | Elective open colorectal surgery | Hair removal, skin disinfection, antibiotic prophylaxis, prevention of hypothermia | Weekly examination until 30 d from discharge | 12.9% | 10.6% | 0.564 |
| Mingmalairak et al. [25] | 100 (50/50) | Open appendectomy | Antibiotic prophylaxis | Clinical examination at 1,3,7,14, 30 d | 10% | 8% | 0.727 |
| Nakamura et al. [26] | 410 (206/204) | Elective colorectal surgery | Antibiotic prophylaxis and wound protector | Daily during hospital stay, weekly until 30 d after discharge | 4.3% | 9.3% | 0.047 |
| Rasic et al. [27] | 184 (91/93) | Elective colorectal surgery | Antibiotic prophylaxis | Clinical evaluation during hospital stay | 4.3% | 13.2% | 0.039 |
| Ruiz-Tovar et al. [24] | 101 (50/51) | Emergency laparotomies for fecal peritonitis | Antibiotic prophylaxis wound irrigation; sterile-drape | Clinical examination at 5, 30 and 60 d | 10% | 35.3% | 0.004 |
| Preoperative Measures |
| Shower before surgery with either plain or antimicrobial soap |
| Mupirocin 2% decolonization in S. aureus nasal carrier in cardiac and orthopedic surgery |
| Not remove patient hair, or if necessary, prefer clipper to shaver |
| Antibiotic prophylaxis in 120 min preceding surgical incision |
| Adequate surgical hand scrubbing |
| Oral administration of multiple nutrient-enhanced formula before surgery |
| Not interrupt immunosuppressive treatment |
| Intraoperative Measures |
| Skin preparation with alcohol-based chlorhexidine gluconate solution |
| Use wound protector devices |
| Consider prophylactic negative pressure therapy in high-risk wound |
| Not use plastic adhesive incise drapes |
| Not use antimicrobial sealants after surgical site skin preparation |
| Administration of oxygen with 80% FiO2 |
| Use warming device |
| Use protocol for intensive blood glucose control |
| Wound irrigation with aqueous povidone iodine solution before closure |
| Not perform antibiotic wound irrigation |
| Use of triclosan-coated sutures for the purpose of reducing the risk of SSI, independent of the type of surgery |
| Postoperative Measures |
| Not prolong antibiotic prophylaxis in postoperative period |
| Not continue antibiotic prophylaxis due to presence of drain |
| Administration of oxygen with 80% FiO2 for 2–6 h post-op |
| Appropriate wound evaluation and management |
| Not use advanced dressing of any sort, prefer standard dressing |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceresoli, M.; Carissimi, F.; Piemontese, A.; Paragò, V.; Galvain, T.; Tommaselli, G.A.; Gianotti, L. The Clinical and Economic Value of Triclosan-Coated Surgical Sutures in Abdominal Surgery. Appl. Sci. 2020, 10, 1090. https://doi.org/10.3390/app10031090
Ceresoli M, Carissimi F, Piemontese A, Paragò V, Galvain T, Tommaselli GA, Gianotti L. The Clinical and Economic Value of Triclosan-Coated Surgical Sutures in Abdominal Surgery. Applied Sciences. 2020; 10(3):1090. https://doi.org/10.3390/app10031090
Chicago/Turabian StyleCeresoli, Marco, Francesca Carissimi, Alessandra Piemontese, Vito Paragò, Thibaut Galvain, Giovanni A. Tommaselli, and Luca Gianotti. 2020. "The Clinical and Economic Value of Triclosan-Coated Surgical Sutures in Abdominal Surgery" Applied Sciences 10, no. 3: 1090. https://doi.org/10.3390/app10031090
APA StyleCeresoli, M., Carissimi, F., Piemontese, A., Paragò, V., Galvain, T., Tommaselli, G. A., & Gianotti, L. (2020). The Clinical and Economic Value of Triclosan-Coated Surgical Sutures in Abdominal Surgery. Applied Sciences, 10(3), 1090. https://doi.org/10.3390/app10031090





