Abstract
Triple-negative breast cancer (TNBC) is amongst the most challenging tumor subtypes because it presents itself without the estrogen, progesterone, and HER2 receptors. Hence, assessing new markers is an essential requirement for enhancing its targeted treatment. The survival of TNBC relies upon the advancement of hypoxia that contributes to treatment resistance, immune response resistance, and tumor stroma arrangement. Here, we explored bovine serum albumin (BSA) nanoparticle encapsulating the anti-cancer drug Paclitaxel (PTX) for cell-killing mediated by tumor hypoxia. For targeting hypoxia, we conjugated Acetazolamide (ATZ) with BSA nanoparticle that encapsulated PTX (referred hereon as BSA-PTX-ATZ) utilizing copper-free click chemistry, specifically the Strain-Promoted Alkyne Azide Cycloaddition (SPAAC). The in-vitro cell killing study uncovered that BSA-PTX-ATZ is more productive contrasted with free PTX. The evaluations of the physio-chemical properties of BSA-PTX-ATZ proves that the shelf-life is approximately two months when stored either at room or freezing temperatures or under refrigerated conditions. There is no leakage of PTX from the formulation during that period, while their nanoparticulate nature remained undisturbed. The BSA-PTX-ATZ nanoparticles indicated altogether higher cell killing in hypoxic conditions contrasted with normoxia proposing the hypoxia-mediated delivery mechanism of the activity of the formulation. Higher cell uptake found with fluorescent-marked BSA-PTX-ATZ shows CA-IX mediated cell uptake, substantiated by the prominent apoptotic cell death contrasted with free PTX.
1. Introduction
Cancer is endangering millions of lives and has been predicted to diagnose an estimated 1.5 million new cases [1]. Among many types of cancer, the therapeutic success of Triple-negative breast cancer (TNBC) is not satisfactory. The conventional therapeutic strategies for TNBC are DNA intercalating taxanes, platinum compounds, and tyrosine, and serine-threonine kinase inhibitors, such as Everolimus, Cetuximab. However, in the clinic, treatment with these drugs produces drug resistance to TNBC patients. Thus, it is essential to improve not only diagnostic tools for early detection of the tumor but also to develop selective ligands for therapy with very few adverse events in healthy tissues. Nanomedicine, especially the development of drug delivery systems that accumulate in the tumor actively as well as passively with the advantage of multi-targetability and show promise as conventional cancer therapy, is necessary. It is known that most of the current drug delivery systems are failing in clinical trials because of the lack of specific targetability, poor tumor penetration, tumor heterogeneity, and complex association of tumor-associated immune cells and stroma. Consequently, targeted drug delivery systems that utilize diverse ligands to understand unique biomarkers that are present on tumor cell components have turned out to be extremely critical in selective transport of medication and improving therapeutic efficacy. Targeted cancer remedy techniques are expected to differentiate between healthy and most cancer tissues. For this, the ligands might be decorated on the surface of the drug-carrier structures that use a reagent-free organic synthesis method for enhancing early prognosis and subsequent remedy in tumors. Our research presented in this study is linked to the concept of personalized medicine, that also has the convenience of designing the delivery system-drug combination on a need to need basis by utilizing targeting ligands-carrier combination using a synthesis approach that minimizes reagents like the copper-free cyclic Alkyne-Azide click reaction that doesn’t require any dangerous chemical substances, reagents, or any unique reaction conditions. The unique understanding accrued by this approach will provide a concept of development in drug delivery with the skills of rational designing of targeting ligand custom-designed drug carriers to address specific functions within the tumor microenvironment.
Further, nanoparticles are the drug delivery systems of choice for targeted delivery of anticancer drugs [2,3] in the pharmaceutical industry. Nanoparticles improve the delivery of hydrophobic drugs, lower their metabolic degradation in the eliminating organs, target their delivery to cancer cells directly by logical surface modifications on the delivery systems, and show controlled or extended or sustained drug delivery [4,5,6]. Besides having the capability to improve the solubility of hydrophobic drugs, nano-delivery systems can additionally target cancerous cells with the aid of two distinctive strategies: passive and active targeting. The functions of the passive targeting delivery systems depend on the ability of nano-delivery systems to exploit the Enhanced Permeability and Retention (EPR) effect [7,8]. As a result, the nano-molecules can extravasate and accumulate at the tumor site. However, not all tumors manifest the EPR effect, and even if they do, the angiogenesis is not uniform through the depths of the tumor resulting in unequal medication at different sites of cancer. Further, the EPR effect may not be as effective in some tumors as in the others [9,10]. Thus, developing targeted delivery systems taken up by active targeting via receptors on the tumor cells is the need of the hour.
This research uses albumin as carrier and acetazolamide as the ligand to target carbonic anhydrase (CA) receptor overexpressed in cancer cells in hypoxic conditions. Albumin is the most abundant plasma protein in the human body. Several research facilities and industry-related techniques are already known to use it for the delivery of different medicines [11,12,13,14]. The advantages of the bovine serum albumin (BSA) encompass the presence of multiple binding sites for the drugs, lower cost, non-immunogenicity, and naturally biodegradable property that has allowed its implementation as a perfect carrier for nanoparticles-based drug delivery systems [15,16]. These properties could result in developing promising delivery systems for biopharmaceutical challenging medications and improving their delivery and bioavailability in the body [17,18]. Carbonic Anhydrase (CA) is a family of zinc metalloenzyme involved in the reversible conversion of carbon dioxide to ionic bicarbonate form, producing a proton subsequently. Thus, it has a role in ion exchange and pH balance in the physiological system [19]. Of this family of receptors, CA IX is the most common and is overexpressed on the surface of most cancer cells [20,21] in hypoxic conditions developed when the rapidly growing tumor cells that overuse the oxygen supply.
For this reason, it has emerged as the primary target for most cancer remedies [22,23,24]. Sulfonamides, like Acetazolamide, are a subset of antibacterial agents that recently showed antitumor activity [25,26] via inhibition of carbonic anhydrase activity [27,28]. Our experiments use paclitaxel (PTX), a hydrophobic drug as the experimental drug due to its unique chemical structure and ability to polymerize tubulin to stable microtubules. It is also known to operate by inhibiting mitochondrial apoptosis inhibitor protein B-cell Leukemia 2 (Bcl-2) [27,28]. Hence, we believe that the combination of the bio-safe BSA, the targeting ligand ATZ specific for the hypoxia receptor CA IX, and PTX with its unique anti-cancer properties is a powerful system to target TNBC. The delivery system itself has the potential to be applied to a varied range of cancers apart from TNBC.
2. Materials
The details are provided in the Supplementary Materials.
3. Methods
Synthesis and characterization of the Targeted Drug Delivery System
The designing of the drug delivery system that is used to target hypoxia in this research paper follows the same procedure as that published in our previous work [29]. The process involves the use of a four-step reaction using a copper-free cyclic Alkyne-Azide click reaction. The steps include the introduction of complementary azide [30] and DBCO groups on BSA and acetazolamide respectively, followed by a simple click reaction between these two molecules. The drug is then loaded by the desolvation procedure described in our previous work [31,32,33,34]. The detailed information is given in the Supplementary Materials. FTIR and 1H NMR techniques were used to confirm the bond formation. Drug loading was calculated using HPLC and UV Spectrophotometer measured at 227 nm for PTX. Beckman Coulter Delsa Nano-C DLS Particle analyzer (Beckman Coulter, Inc., Fullerton, CA) and JEOL Transmission Electron Magnifying tool (JEM 2010, Tokyo, Japan) were used to analyze the particles size and morphology. Drug release was analyzed by HPLC at physiological condition at pH 7 and also at acidic conditions (pH 5) by extracting samples from the delivery system. Stability studies were performed similarly using the particle size analyzer and HPLC by extracting samples weekly for 12 weeks. In-vitro cytotoxicity of the formulation was assessed using MTT assay both in the normoxic and hypoxic conditions. Drug uptake studies were performed by treating the cells with rhodamine dye encapsulated in the targeted and non-targeted formulations to assess the efficiency of uptake. Apoptosis was assessed using Annexin V/7-AAD dual staining in Guava Easycyte flow Cytometer (EMD Millipore, USA) in a flow cytometer. To understand the significance of the difference between the responses to the free drug and the formulation in each cell line, statistical analysis using the t-test was done to calculate the p-value.
4. Results
4.1. Characterization of Targeting Drug Delivery System
Peaks in the region of 1500–2000 cm−1 corresponding to the (azide) stretching in FTIR (Supplementary Figure S1) were observed. Further, –N-H, -C=O, and –C-H bond stretching at 3300–3500 cm−1, 1670–1820 cm−1, and 1050–1150 cm−1, respectively, were seen. The 1H NMR spectrum (Supplementary Figure S2) showed readings between 6.8–7.4 ppm corresponding to the DBCO hydrogens and between 2.4–3.8 ppm of the azide hydrogens. These results have helped us confirm the bond formation in the final molecule that is shown in Figure 1.
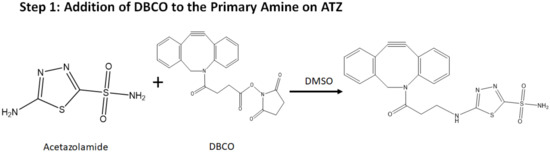
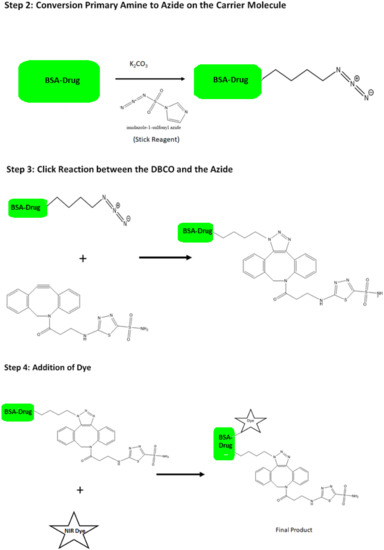
Figure 1.
Scheme for the synthesis of the targeted drug delivery (‘Dye’ represents NIR dye).
4.2. Drug Loading
Drug-loading was calculated from the standard graph measured for absorbance at 227 nm to be around 10% w/w for PTX.
4.3. Particle Size Analysis
The particle size was found to be (Figure 2a) 284.7 nm and the PDI was around 0.146 which lies in the desired range of 200–300 nm suitable for both active and passive uptake into cells. Further, the TEM analysis showed a particle size (Figure 2b) between 12.5 nm and 18.0 nm, affirming that the nanoparticle is in the preferred nanoscale.
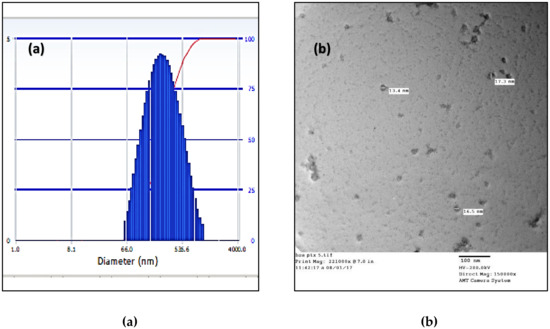
Figure 2.
(a) DLS of BSA-PTX-ATZ; (b) TEM analysis of BSA-PTX-ATZ.
4.4. Drug Release Studies
A steady percentage release of the drug for 72 h was seen with a release of about 26.6% at 24 h and 48.14% at 72 h (Figure 3). Hence, the product can be classified as a sustained/extended release formulation. This was performed both in the physiologic pH as well as the acidic pH which is found in the tumor microenvironment. Our formulation has shown a much more rapid release in the acidic pH characteristic of the tumor microenvironment.
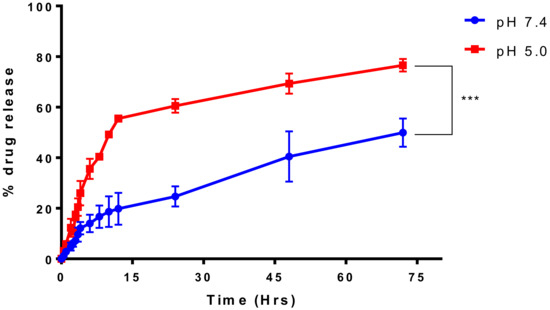
Figure 3.
Drug release studies for BSA-PTX-ATZ at normal blood pH and acidic pH in the tumor conditions.
4.5. Stability Studies
The parameters indicating stability over three months have been shown in Figure 4. which indicate very slight fluctuations showing great stability of the formulation over a long time. The particle size seemed to be around 287.5 nm at room temperature but was more stable when in the frozen state. The PDI (polydispersity index) was around 0.168 at the end of 12 weeks and has stayed stable throughout. The drug loss was 1.1% at room temperature and was of the order of 0.8% in the frozen conditions showing minimal drug loss during the storage. Hence, it can be inferred that the formulation has better stability in frozen state than at room temperature indicating an increase in shelf life when stored frozen. The p-value > 0.05 indicates that there is not much difference in the variance in terms of particle size, PDI and drug retained within the formulation over a period, showing the stability of the formulation.
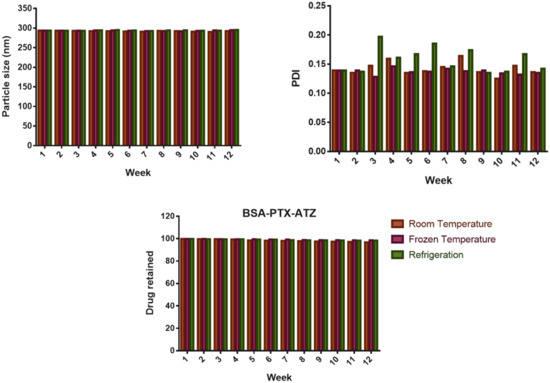
Figure 4.
Stability studies for 12 weeks or 3 months.
4.6. In-Vitro Cytotoxicity Studies
MDA-MB-231 showed an IC50 of the drug at approximately 1 μM in the targeted formulation as compared to the 0.5 μM in MDA-MB-468. Dose-dependent killing and lower cell viability were observed when treated with targeted formulation when compared to those treated with the non-targeted formulation and free-drug in both the cell lines (Figure 5a). The cell viability of the order of 80%–90% (high) for the control carrier-ligand treatment shows that the delivery system itself is safe for use. Also, it is seen that the cytotoxicity of targeted formulation in the normoxic conditions (healthy cells) is lesser than in the hypoxia in cancer cells (Figure 5b). There is a significant difference in response to free drug and formulation in the cell line MDA-MB-468 with a p-value of 0.004123 and in the cell line MDA-MB-231 with a p-value of 0.004295.
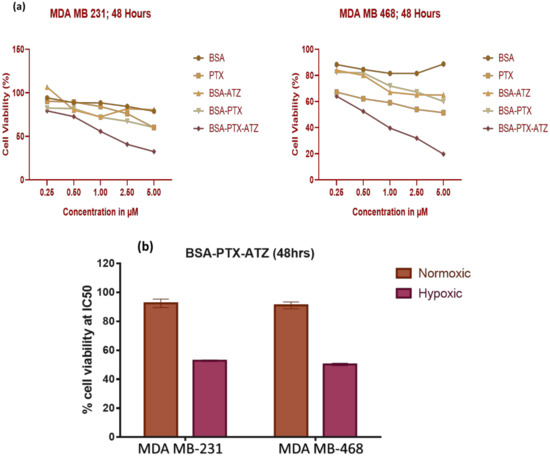
Figure 5.
(a) Dose dependent cytotoxic assay for MDA-MB-231 and MDA-MB-468; (b) Cytotoxicity studies in MDA-MB-231 and MDA-MB-468 cell lines.
4.7. Drug Uptake Studies
The fluorescence spectroscopic assay showed that drug is taken up in a time and concentration-dependent. It can be inferred that the amount of formulation entering into the two cell lines increases in a time-dependent manner (Figure 6). Also, the targeted formulation is taken up more than the non-targeted formulation in the two cell lines. A p-value < 0.05 indicates that there is a significant difference in variance between the non-targeted and targeted formulations.
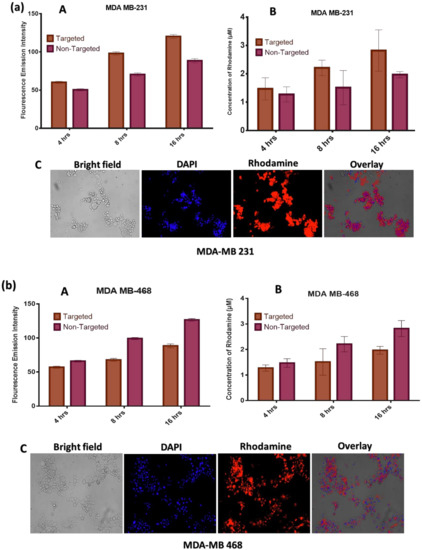
Figure 6.
Cell uptake studies in (a) MDA-MB-231: (A) Uptake based on Fluorescence Intensity; (B)Uptake based on Concentration of Rhodamine; (C) Uptake from Fluorescence Microscopy and (b) MDA-MB-468 cell lines: (A) Uptake based on Fluorescence Intensity; (B)Uptake based on Concentration of Rhodamine; (C) Uptake from Fluorescence Microscopy.
4.8. Apoptosis Assay by Flow Cytometry
The extent of apoptosis was greater in the two cell lines treated with the targeted formulation in comparison with the free-drug or non-targeted formulation. The comparative early and late apoptotic cell percentages in the MDA-MB-231 cell line was found to be 56.1% and 15%, respectively, in cells treated with targeted formulation; and 28.5% and 8.6%, respectively in cells treated with non-targeted formulation (Figure 7a). Similarly, in the MDA-MB-468 cell line early and late apoptotic cells were around 57.6% and 13.9%, respectively, for targeted formulation and 41.8% and 2.5% for non-targeted formulation (Figure 7c). Thus, the inference is that the targeted formulation is better than the non-targeted formulation. Furthermore, the control formulation showed generally low apoptosis, demonstrating the safety of the carrier system.
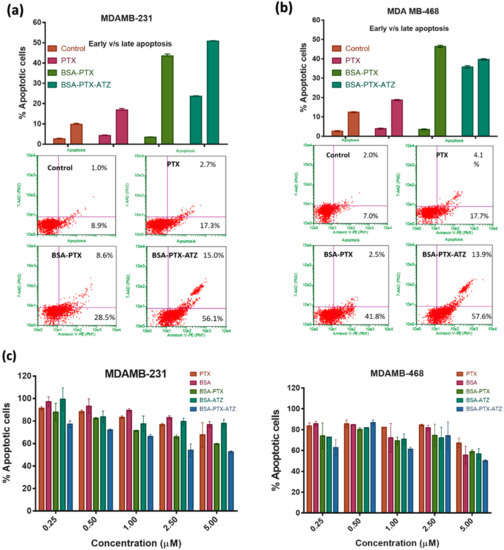
Figure 7.
(a) Apoptosis assay studies in MDA-MB-231 for the hypoxia targeting drug delivery system BSA-PTX-ATZ; (b) Apoptosis assay studies in MDA-MB-468 for the hypoxia targeting drug delivery system BSA-PTX-ATZ; (c) Apoptosis assay studies in MDA-MB-231 and MDA-MB-468 based on the concentration of BSA-PTX-ATZ.
5. Discussion
Our research is a probable solution to deal with the problems currently prevalent with the nano-delivery formulations because we demonstrate the use of a reagent free click chemistry technique to make a formulation that gives the flexibility to design a multi-targeted ligand library for nanoparticles with a wide choice of biologically safe carriers on a need-to-need basis. Studies reported, and techniques developed to successfully perform click chemical reactions proved them to be anything but difficult, and that they can be accomplished briskly with high yield to produce non-toxic products. Some scientists now say that click chemistry is a calculatedly logical approach for effective improvement of the new molecular delivery systems. This specific but easy method relies essentially on carbon-heteroatom bonds using spring-loaded reactants. The growing extent of application of these reactions in designing delivery systems is observed in most of the specializations of cutting-edge pharmaceutical sciences discipline from drug delivery strategies to materials science [35,36]. Due to the simplicity of click chemistry, it has been gaining importance in drug discovery, drug delivery, and bioconjugation reactions [37]. The system synthesized in this research is this sort of simple chemical procedure. This gives us the flexibility to create drug delivery systems for a choice of ligands and carriers for numerous hydrophobic drugs from different classes no longer confined to the cancer treatment. The biosafety of the carrier systems accordingly evolved is the key achievement of these formulation systems [38,39]. The nanoparticles formed are in the nanoscale with a low PDI indicating nanoparticles to be of identical size as the average size of the particle. This characteristic feature of the particles promotes the uptake of the formulation into the cancerous cells via a weak vasculature system by way of the EPR effect [40,41]. The formulation is noted to be robust with a minimum drug loss over a period of time establishing a viably long shelf-life by way of this formulation approach. Further, this approach creates a novel drug delivery system that shows sustained release properties too. Mechanistically speaking, the molecule is intended for use to deliver the drug to the core of tumor. To this end, it has been equipped with a targeting ligand, acetazolamide that will target a receptor that is highly expressed in the core of the hypoxic solid tumor. Carbonic anhydrase (CA IX) receptor is expressed in most of the solid tumors. Essentially, it is seen to be overexpressed in the core of the solid tumors where there is a shortage of oxygen. Hypoxia is a physiological condition of lack of oxygen that leads to acidosis which induces CA IX on the tumor cell surface that can be easily targeted using a suitable inhibitor like acetazolamide [19,29,33,34]. Moreover, the BSA used for designing this nanoparticle serves as a biomimetic protein that easily circulates in the blood without producing any immunogenic effects. The incorporation of the NIR dye adds a diagnostic advantage to our drug delivery system. Putting all of these elements together with the advantages mentioned earlier of the synthesis technique and the nanoparticle design, the drug will be delivered with high specificity to the core of the tumor to show its cytotoxicity.To confirm the efficacy of our approach, we can show from the results of the cytotoxic studies, the formulation is observed to have more effect in the MDA-MB-468 that extensively overexpresses CA IX receptor in hypoxic conditions compared to MDA-MB-231 which is known to be a more difficult-to-treat strain [42]. However, the fact that the system has a consistent performance on MDA-MB-231 also shows that it is a promising means treatment for this more aggressive variant also [43]. The hypoxic condition enhances the effect of our formulation because the acidic pH of rapidly growing cancer cells that causes enhanced expression of this receptor. Hence, such conditions provide a greater opportunity for the formulation to enter the cells more efficiently. The difference in the extent of cell death between the non-targeted and targeted formulations indicates that the formulation is entering not only via the EPR effect but also by specific uptake by CA IX receptor; i.e., receptor-mediated cell uptake [41]. The more viability of cells in the blank formulation treatments is evidence for the safety of the delivery system by itself. The amount of the formulation taken up by the cells of each of the non-targeted by EPR effect and targeted formulations by active targeting, respectively, indicates that the hypoxia-targeted formulation has a preference for entering cells in the hypoxia in a time-dependent manner. It can also be said, considering the cytotoxicity studies, that the formulation shows the receptor-mediated uptake [5,23,44,45,46,47] alongside the EPR effect. In addition, the higher percentage of the apoptotic cells in each cell lines shows a better performance of the targeted formulation over the non-targeted formulation suggesting the advantage of the hypoxia-targeting ligand. The low percentage of total apoptosis in the cell treated with the delivery system only indicates that the formulation in itself is safe for healthy cells.
6. Conclusions
The challenge to target hypoxia, which is a common feature of all solid tumors, is because of its existence in the core. The rapidly-proliferating tumor cells create a dearth of oxygen and a decrease in pH within the cancerous cells, which results in the enhanced expression of the receptor CA IX that will accommodate the uptake of the targeted formulation to the tumor core and, thus, efficiently deliver the drug at the targeted site. The research presented here is hoped to show the proof of the concept that delivery could be accomplished through CA IX receptor using ligands like Acetazolamide and a biologically safe carrier such as bovine serum albumin, and therefore is non-immunogenic. We can say that our reports here prove the effectiveness of the formulation designed and characterized herein. The delivery system guides the delivery of medicine to specific sites, therefore, removing the adverse outcomes in the healthy cells. The flexibility of the molecule to accommodate ligands and dyes with equal ease may be stated as the strength of the formulation encouraging further studied in-vivo.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/3/1075/s1, Figure S1: FTIR for the hypoxia targeting drug delivery system BSA-PTX-ATZ; Figure S2: NMR spectrum for the hypoxia targeting drug delivery system BSA-PTX-ATZ.
Author Contributions
The research was performed by the efforts of K.T., S.S., A.K. I. and M.A.R. have intellectual contributions and guidance in data analysis, respectively. A.K.I. has supervised the research entirely as the Principal Investigator. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wayne State University Start Up funding to A.K.I. S.S. acknowledges the support of Burroughs’s welcome fund collaborative research travel grant (BWFCRTG). A.K.I. acknowledges US Department of Defense CDMRP KCRP Idea Development Award # W81XWH1810471, US National Institutes of Health, National Cancer Institute (NIH/NCI) grant R21CA179652 and Wayne State University start-up funding for funding Iyer lab.
Acknowledgments
K.T. would like to acknowledge AGRADE scholarship from the Wayne State University Graduate School to pursue Ph.D. Studies in Iyer Lab, Department of Pharmaceutical Sciences, Wayne State University (WSU). The authors also wish to acknowledge the support extended by their colleagues Kaustub A. Gawde, Ketki S. Bhise, Rami Alzhrani, and Hashem O. Alsaab.
Conflicts of Interest
The authors have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- CDC. Expected New Cancer Cases and Deaths in 2020; Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/cancer/dcpc/research/articles/cancer_2020.htm (accessed on 19 November 2019).
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surf. B Biointerfaces 2015, 132, 138–145. [Google Scholar] [CrossRef]
- Amjad, M.W.; Amin, M.C.I.M.; Katas, H.; Butt, A.M.; Kesharwani, P.; Iyer, A.K. In Vivo Antitumor Activity of Folate-Conjugated Cholic Acid-Polyethylenimine Micelles for the Codelivery of Doxorubicin and siRNA to Colorectal Adenocarcinomas. Mol. Pharm. 2015, 12, 4247–4258. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic Acid Engineered Nanomicelles Loaded with 3,4-Difluorobenzylidene Curcumin for Targeted Killing of CD44+ Stem-Like Pancreatic Cancer Cells. Biomacromolecules 2015, 16, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012, 9, e161–e166. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Agarwalla, P.; Mukherjee, S.; Bag, I.; Sreedhar, B.; Pal-Bhadra, M.; Patra, C.R.; Banerjee, R. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale 2014, 6, 6745–6754. [Google Scholar] [CrossRef]
- Stockett, M.H.; Kjær, C.; Linder, M.K.; Detty, M.R.; Nielsen, S.B. Luminescence spectroscopy of chalcogen substituted rhodamine cations in vacuo. Photochem. Photobiol. Sci. 2017, 16, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.P.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Deliv. Sci. Technol. 2017, 39, 147–155. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef]
- Švastová, E.; Hulıíková, A.; Rafajová, M.; Zat’ovičová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004, 577, 439–445. [Google Scholar] [CrossRef]
- Pastorekova, S.; Pastorek, J. Carbonic Anhydrase, Its Inhibitors and Activators, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Lloyd, M.C.; Bui, M.M.; Gillies, R.J.; Morse, D.L. Chapter 13: Carbonic Anhydrase IX as an Imaging and Therapeutic Target for Tumors and Metastases. In Subcell Biochem; Frost, S., McKenna, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 75, pp. 221–254. [Google Scholar]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef]
- Sneddon, D.; Poulsen, S.A. Agents described in the Molecular Imaging and Contrast Agent Database for imaging carbonic anhydrase IX expression. J. Enzyme Inhib. Med. Chem. 2014, 29, 753–763. [Google Scholar] [CrossRef]
- Mohan, R.; Banerjee, M.; Ray, A.; Manna, T.; Wilson, L.; Owa, T.; Bhattacharyya, B.; Panda, D. Antimitotic sulfonamides inhibit microtubule assembly dynamics and cancer cell proliferation. Biochemistry 2006, 45, 5440–5449. [Google Scholar] [CrossRef]
- Owa, T.; Yoshino, H.; Okauchi, T.; Yoshimatsu, K.; Ozawa, Y.; Sugi, N.H.; Nagasu, T.; Koyanagi, N.; Kitoh, K. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J. Med. Chem. 1999, 42, 3789–3799. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W. Renal and Electrolyte Disorders; Little Brown and Company: Boston, MA, USA, 1976. [Google Scholar]
- Chegwidden, W.R.; Spencer, I.M. Sulfonamide inhibitors of carbonic anhydrase inhibit the growth of human lymphoma cells in culture. Inflammopharmacology 1995, 3, 231–239. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Gawde, K.A.; Iyer, A.K. Copper-Free ‘Click’ Chemistry-Based Synthesis and Characterization of Carbonic Anhydrase-IX Anchored Albumin-Paclitaxel Nanoparticles for Targeting Tumor Hypoxia. Int. J. Mol. Sci. 2018, 19, 838. [Google Scholar] [CrossRef] [PubMed]
- Goddard-Borger, E.D.; Stick, R.V. An efficient, inexpensive, and shelf-stable diazotransfer reagent: Imidazole-1-sulfonyl azide hydrochloride. Org. Lett. 2007, 9, 3797–3800. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Alsaab, H.O.; Sau, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surf. B Biointerfaces 2017, 157, 490–502. [Google Scholar] [CrossRef]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent Folate-Dendrimer-Coated Iron Oxide Theranostic Nanoparticles for Simultaneous Magnetic Resonance Imaging and Precise Cancer Cell Targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Lock, F.E.; McDonald, P.C.; Lou, Y.; Serrano, I.; Chafe, S.C.; Ostlund, C.; Aparicio, S.; Winum, J.Y.; Supuran, C.T.; Dedhar, S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013, 32, 5210–5219. [Google Scholar] [CrossRef]
- Ivanova, L.; Zandberga, E.; Siliņa, K.; Kalniņa, Z.; Ābols, A.; Endzeliņš, E.; Vendina, I.; Romanchikova, N.; Hegmane, A.; Trapencieris, P.; et al. Prognostic relevance of carbonic anhydrase IX expression is distinct in various subtypes of breast cancer and its silencing suppresses self-renewal capacity of breast cancer cells. Cancer Chemother. Pharmacol. 2015, 75, 235–246. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne ‘click’ chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Nandivada, H.; Jiang, X.; Lahann, J. Click chemistry: Versatility and control in the hands of materials scientists. Adv. Mater. 2007, 19, 2197–2208. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Iyer, A.K.; Greish, K.; Seki, T.; Okazaki, S.; Fang, J.; Takeshita, K.; Maeda, H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007, 15, 496–506. [Google Scholar] [CrossRef]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Tafreshi, N.K.; Lloyd, M.C.; Proemsey, J.B.; Bui, M.M.; Kim, J.; Gillies, R.J.; Morse, D.L. Evaluation of CAIX and CAXII expression in breast cancer at varied O2Levels: CAIX is the superior surrogate imaging biomarker of tumor hypoxia. Mol. Imaging Biol. 2016, 18, 219–231. [Google Scholar] [CrossRef]
- Alsaab, H.; Alzhrani, R.M.; Kesharwani, P.; Sau, S.; Boddu, S.H.; Iyer, A.K. Folate decorated nanomicelles loaded with a potent curcumin analogue for targeting retinoblastoma. Pharmaceutics 2017, 9, 15. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the design of ligand-targeted cancer therapeutics and imaging agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.T.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Control. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).