Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles
Abstract
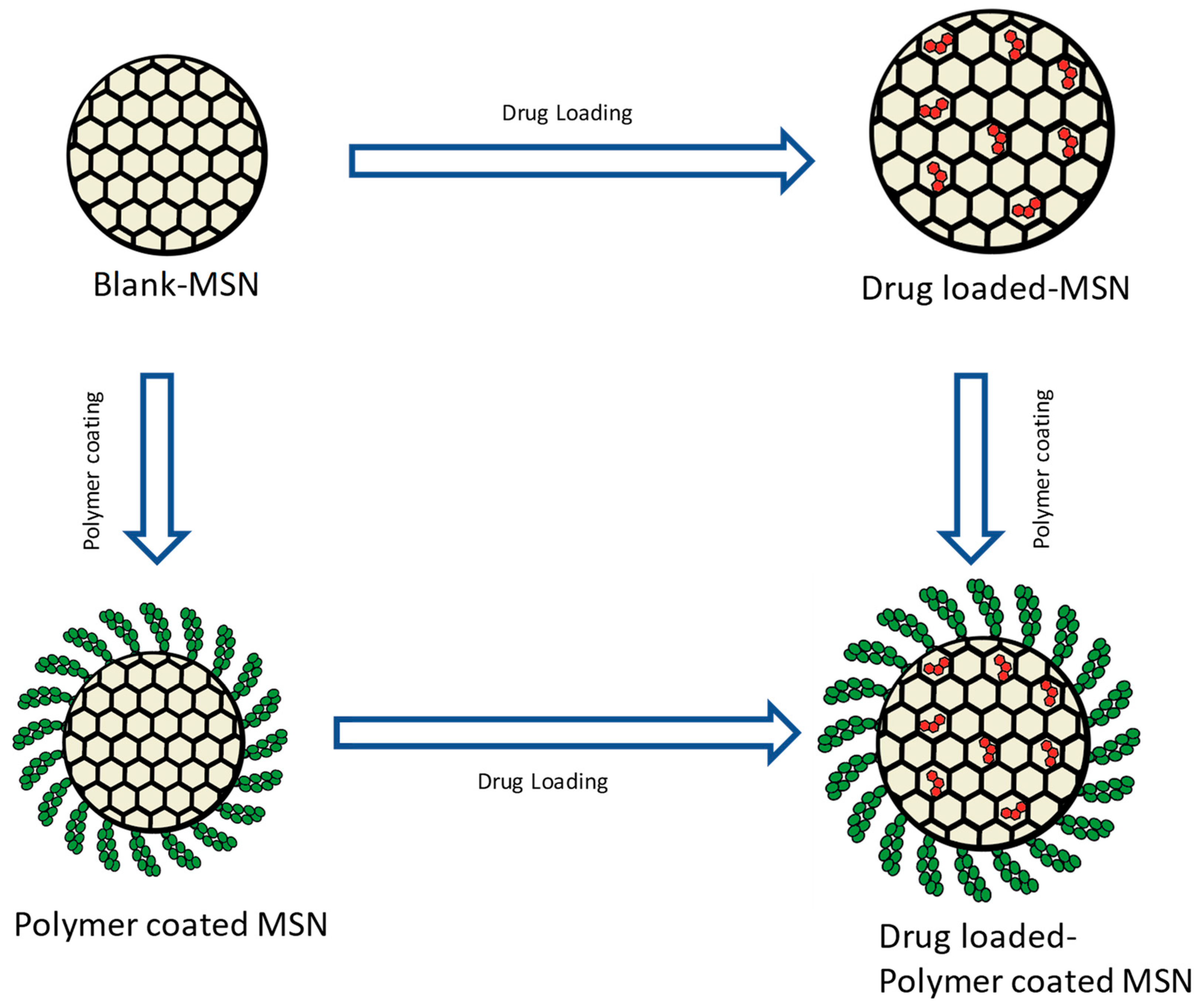
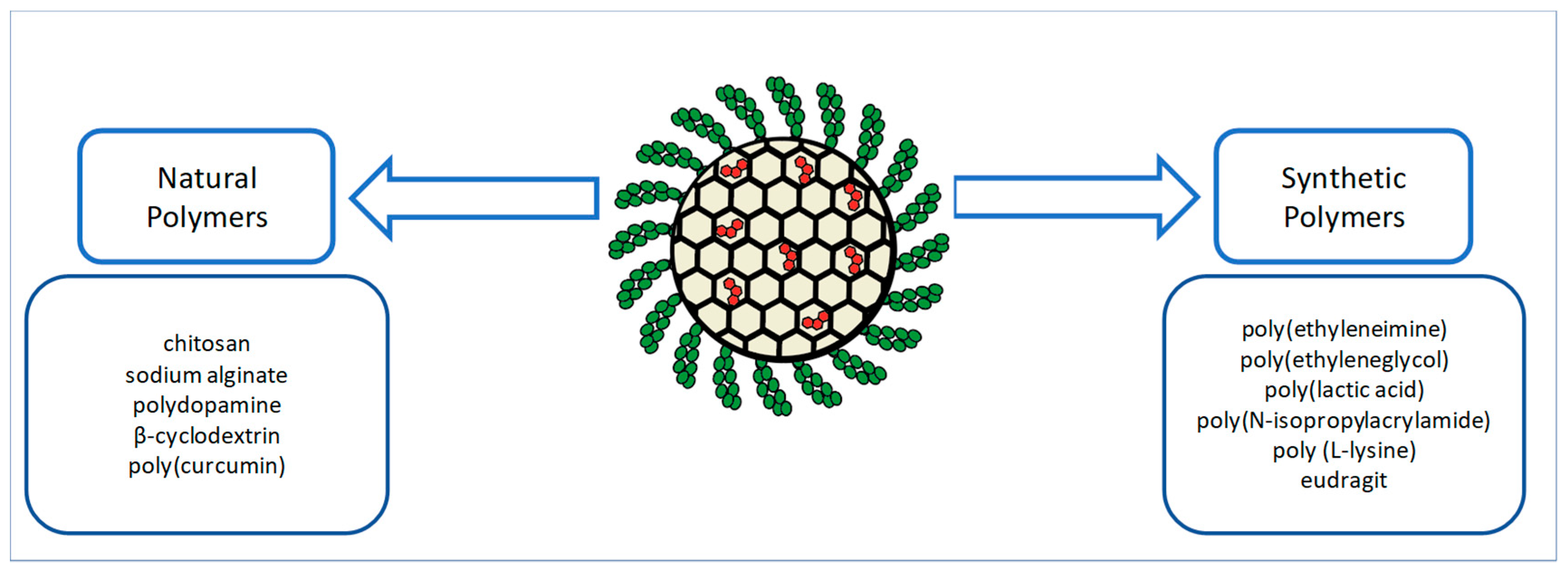
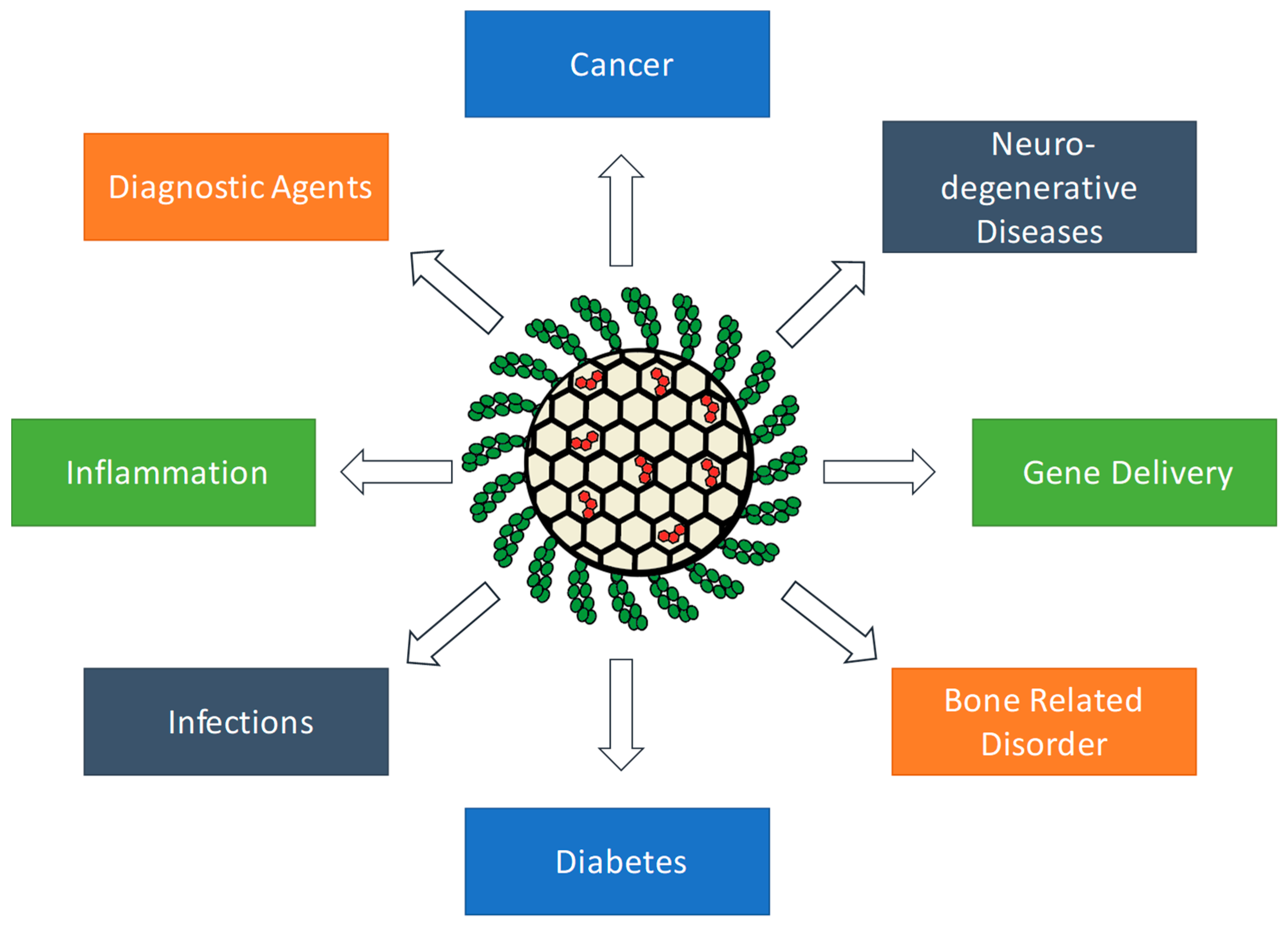
1. Introduction
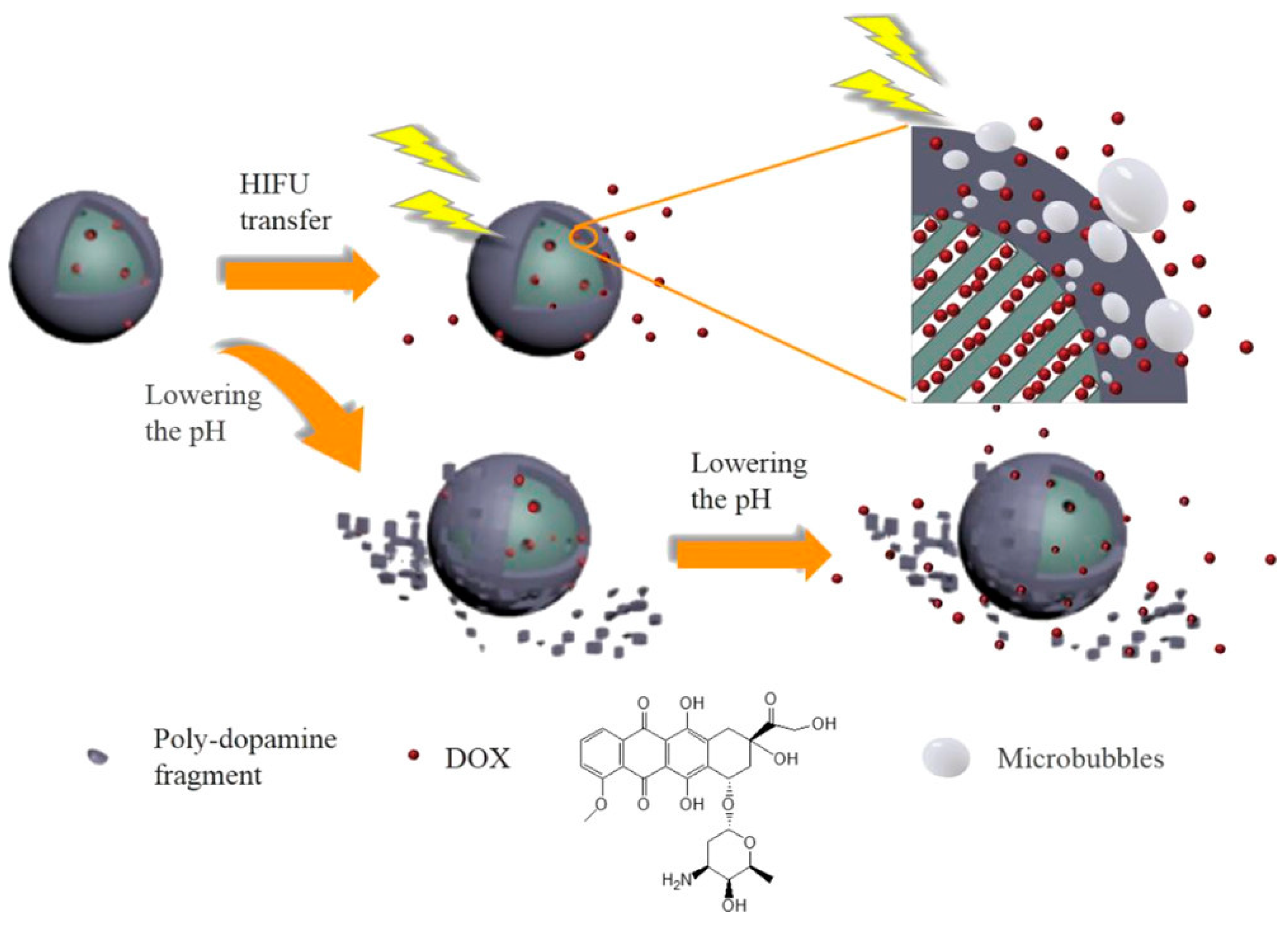
2. Polymer Coated MSNs for Cancer
3. Polymer Coated MSNs for Neurodegenerative Diseases
4. Polymer Coated MSNs for Inflammation
5. Polymer Coated MSNs for Infectious Diseases
6. Polymer Coated MSNs for Bone Related Disorders
7. Polymer Coated MSNs for Gene Delivery
8. Polymer Coated MSNs for Diabetes
9. Polymer Coated MSNs for Imaging
10. Toxicity Aspects of Polymers
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Zhao, Q.; Hu, Y.; Sun, L.; Bai, L.; Jiang, T.; Wang, S. Ordered nanoporous silica as carriers for improved delivery of water insoluble drugs: A comparative study between three dimensional and two dimensional macroporous silica. Int. J. Nanomed. 2013, 8, 4015. [Google Scholar] [CrossRef]
- Kilpeläinen, M.; Riikonen, J.; Vlasova, M.A.; Huotari, A.; Lehto, V.P.; Salonen, J.; Herzig, K.H.; Järvinen, K. In vivo delivery of a peptide, ghrelin antagonist, with mesoporous silicon microparticles. J. Control. Release 2009, 137, 166–170. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Yang, K.; Liu, F.; Li, H.; Xu, Y.; Sun, S. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem. Soc. Rev. 2017, 46, 6024–6045. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinšek, O.; Kros, A.; Gaberšček, M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef]
- Dogra, P.; Adolphi, N.L.; Wang, Z.; Lin, Y.S.; Butler, K.S.; Durfee, P.N.; Croissant, J.G.; Noureddine, A.; Coker, E.N.; Bearer, E.L.; et al. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics. Nat. Commun. 2018, 9, 4551. [Google Scholar] [CrossRef]
- Lindén, M. Biodistribution and Excretion of Intravenously Injected Mesoporous Silica Nanoparticles: Implications for Drug Delivery Efficiency and Safety. In Enzymes; Academic Press: Cambridge, MA, USA, 2018; Volume 43, pp. 155–180. ISBN 9780128151129. [Google Scholar]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef]
- Li, X.; Xie, C.; Xia, H.; Wang, Z. pH and Ultrasound Dual-Responsive Polydopamine-Coated Mesoporous Silica Nanoparticles for Controlled Drug Delivery. Langmuir 2018, 34, 9974–9981. [Google Scholar] [CrossRef]
- Rahoui, N.; Jiang, B.; Hegazy, M.; Taloub, N.; Wang, Y.; Yu, M.; Huang, Y.D. Gold modified polydopamine coated mesoporous silica nano-structures for synergetic chemo-photothermal effect. Colloids Surf. B Biointerfaces 2018, 171, 176–185. [Google Scholar] [CrossRef]
- Li, Y.; Duo, Y.; Bao, S.; He, L.; Ling, K.; Luo, J.; Zhang, Y.; Huang, H.; Zhang, H.; Yu, X. EpCAM aptamer-functionalized polydopamine-coated mesoporous silica nanoparticles loaded with DM1 for targeted therapy in colorectal cancer. Int. J. Nanomed. 2017, 12, 6239–6257. [Google Scholar] [CrossRef] [PubMed]
- Pada, A.-K.; Desai, D.; Sun, K.; Prakirth Govardhanam, N.; Törnquist, K.; Zhang, J.; Rosenholm, J.M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.; Desai, D.; Senthilkumar, R.; Johansson, E.M.; Råtts, N.; Odén, M.; Eriksson, J.E.; Sahlgren, C.; Toivola, D.M.; Rosenholm, J.M. Shape engineering vs organic modification of inorganic nanoparticles as a tool for enhancing cellular internalization. Nanoscale Res. Lett. 2012, 7, 358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, J.; Wang, J.; Qi, X.; Rosenholm, J.M.; Cai, K. Polydopamine Coatings in Confined Nanopore Space: Toward Improved Retention and Release of Hydrophilic Cargo. J. Phys. Chem. C 2015, 119, 24512–24521. [Google Scholar] [CrossRef]
- Chai, S.; Kan, S.; Sun, R.; Zhou, R.; Sun, Y.; Chen, W.; Yu, B. Fabricating polydopamine-coated MoSe2-wrapped hollow mesoporous silica nanoplatform for controlled drug release and chemo-photothermal therapy. Int. J. Nanomed. 2018, 13, 7607–7621. [Google Scholar] [CrossRef]
- Cheng, W.; Liang, C.; Xu, L.; Liu, G.; Gao, N.; Tao, W.; Luo, L.; Zuo, Y.; Wang, X.; Zhang, X.; et al. TPGS-Functionalized Polydopamine-Modified Mesoporous Silica as Drug Nanocarriers for Enhanced Lung Cancer Chemotherapy against Multidrug Resistance. Small 2017, 13, 1700623. [Google Scholar] [CrossRef]
- Szegedi, Á.; Shestakova, P.; Trendafilova, I.; Mihayi, J.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Momekova, D.; Konstantinov, S.; et al. Modified mesoporous silica nanoparticles coated by polymer complex as novel curcumin delivery carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 700–712. [Google Scholar] [CrossRef]
- Hu, Y.; Ke, L.; Chen, H.; Zhuo, M.; Yang, X.; Zhao, D.; Zeng, S.; Xiao, X. Natural material-decorated mesoporous silica nanoparticle container for multifunctional membrane-controlled targeted drug delivery. Int. J. Nanomed. 2017, 12, 8411–8426. [Google Scholar] [CrossRef]
- Iraji, S.; Ganji, F.; Rashidi, L. Surface modified mesoporous silica nanoparticles as sustained-release gallic acid nano-carriers. J. Drug Deliv. Sci. Technol. 2018, 47, 468–476. [Google Scholar] [CrossRef]
- Mu, S.; Liu, Y.; Wang, T.; Zhang, J.; Jiang, D.; Yu, X.; Zhang, N. Unsaturated nitrogen-rich polymer poly(l-histidine) gated reversibly switchable mesoporous silica nanoparticles using “graft to” strategy for drug controlled release. Acta Biomater. 2017, 63, 150–162. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Q.; Tang, Y.; Xu, Y.; Wang, Q.; Li, T.; Xu, H.; Wang, S.; Fan, K.; Liu, Z.; et al. PEGylated Lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: Design, characterization and its cytotoxic effect. Int. J. Pharm. 2018, 536, 272–282. [Google Scholar] [CrossRef]
- Kienzle, A.; Kurch, S.; Schlöder, J.; Berges, C.; Ose, R.; Schupp, J.; Tuettenberg, A.; Weiss, H.; Schultze, J.; Winzen, S.; et al. Dendritic Mesoporous Silica Nanoparticles for pH-Stimuli-Responsive Drug Delivery of TNF-Alpha. Adv. Healthc. Mater. 2017, 6, 1700012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.M.; Kim, J.; Kim, W.J. Doxorubicin/Ce6-Loaded Nanoparticle Coated with Polymer via Singlet Oxygen-Sensitive Linker for Photodynamically Assisted Chemotherapy. Nanotheranostics 2017, 1, 196–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hegazy, M.; Zhou, P.; Wu, G.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, X.; Huang, Y. Construction of polymer coated core–shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Feng, Y.; Li, N.; Yin, H.; Chen, T.; Yang, Q.; Wu, M. Thermo- and pH-responsive, Lipid-coated, Mesoporous Silica Nanoparticle-based Dual Drug Delivery System To Improve the Antitumor Effect of Hydrophobic Drugs. Mol. Pharm. 2019, 16, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, T.; Ma, G.; Gu, X.; Wang, G.; Li, J. Glucose-responsive mesoporous silica nanoparticles to generation of hydrogen peroxide for synergistic cancer starvation and chemistry therapy. Int. J. Nanomed. 2019, 14, 2233–2251. [Google Scholar] [CrossRef]
- Yuan, N.; Li, S.; Li, G. Sodium alginate coated mesoporous silica for dual bio-responsive controlled drug delivery. J. Drug Deliv. Sci. Technol. 2018, 46, 348–353. [Google Scholar] [CrossRef]
- Avedian, N.; Zaaeri, F.; Daryasari, M.P.; Akbari Javar, H.; Khoobi, M. pH-sensitive biocompatible mesoporous magnetic nanoparticles labeled with folic acid as an efficient carrier for controlled anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 323–332. [Google Scholar] [CrossRef]
- Desai, D.; Karaman, D.S.; Prabhakar, N.; Tadayon, S.; Duchanoy, A.; Toivola, D.M.; Rajput, S.; Näreoja, T.; Rosenholm, J.M. Design considerations for mesoporous silica nanoparticulate systems in facilitating biomedical applications. Open Mater. Sci. 2014, 1, 16–43. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 2018, 157, 107–124. [Google Scholar] [CrossRef]
- Tao, J.; Fei, W.; Tang, H.; Li, C.; Mu, C.; Zheng, H.; Li, F.; Zhu, Z. Angiopep-2-Conjugated “Core–Shell” Hybrid Nanovehicles for Targeted and pH-Triggered Delivery of Arsenic Trioxide into Glioma. Mol. Pharm. 2019, 16, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Rodrigues, C.F.; Reis, C.A.; Costa, E.C.; Ferreira, P.; Correia, I.J. Development of poly-2-ethyl-2-oxazoline coated gold-core silica shell nanorods for cancer chemo-photothermal therapy. Nanomedicine 2018, 13, 2611–2627. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wu, H.; Tang, Y.; Xu, Y.; Qian, X.; Zhu, W. Temperature-sensitive copolymer-coated fluorescent mesoporous silica nanoparticles as a reactive oxygen species activated drug delivery system. Int. J. Pharm. 2018, 536, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Yuan, W.; Liu, Y.; Wang, Y. Lipid-coated mesoporous silica nanoparticles of hydroxycamptothecin for sustained release and cancer therapy. Pharmazie 2018, 73, 447–453. [Google Scholar]
- Ma, J.; Wu, H.; Li, Y.; Liu, Z.; Liu, G.; Guo, Y.; Hou, Z.; Zhao, Q.; Chen, D.; Zhu, X. Novel Core-Interlayer-Shell DOX/ZnPc Co-loaded MSNs@ pH-Sensitive CaP@PEGylated Liposome for Enhanced Synergetic Chemo-Photodynamic Therapy. Pharm. Res. 2018, 35, 57. [Google Scholar] [CrossRef] [PubMed]
- Tzankov, B.; Tzankova, V.; Aluani, D.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Avramova, K.; Valoti, M.; Yoncheva, K. Development of MCM-41 mesoporous silica nanoparticles as a platform for pramipexole delivery. J. Drug Deliv. Sci. Technol. 2019, 51, 26–35. [Google Scholar] [CrossRef]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood–brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef]
- Cheng, C.S.; Liu, T.P.; Chien, F.C.; Mou, C.Y.; Wu, S.H.; Chen, Y.P. Codelivery of Plasmid and Curcumin with Mesoporous Silica Nanoparticles for Promoting Neurite Outgrowth. ACS Appl. Mater. Interfaces 2019, 11, 15322–15331. [Google Scholar] [CrossRef]
- Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Domazet Jurašin, D.; Sikirić, M.D.; Šegota, S. Enhanced Protection of Biological Membranes during Lipid Peroxidation: Study of the Interactions between Flavonoid Loaded Mesoporous Silica Nanoparticles and Model Cell Membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef]
- Gulin-Sarfraz, T.; Jonasson, S.; Wigenstam, E.; von Haartman, E.; Bucht, A.; Rosenholm, J. Feasibility Study of Mesoporous Silica Particles for Pulmonary Drug Delivery: Therapeutic Treatment with Dexamethasone in a Mouse Model of Airway Inflammation. Pharmaceutics 2019, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Trendafilova, I.; Zgureva, D.; Kalvachev, Y.; Boycheva, S.; Novak Tušar, N.; Szegedi, A. Polymer-coated mesoporous silica nanoparticles for controlled release of the prodrug sulfasalazine. J. Drug Deliv. Sci. Technol. 2018, 44, 415–420. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Chen, Q.; Guan, G.; Hu, W.; Zhao, X.; Qiao, M.; Hu, H.; Liang, Y.; Zhu, H.; et al. Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int. J. Nanomed. 2015, 10, 4747. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.-V.; Shim, K.; Vo Thi, T.-T.; Kook, J.-K.; An, S.S.A.; Lee, S.-W. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lü, S.; Wu, C.; Wang, Z.; Feng, C.; Wen, N.; Liu, M.; Zhang, X.; Liu, Z.; Liu, Y.; et al. Curcumin polymer coated, self-fluorescent and stimuli-responsive multifunctional mesoporous silica nanoparticles for drug delivery. Microporous Mesoporous Mater. 2018, 271, 234–242. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, H.; Wang, B.; Kong, Y.; Chen, J. Silver-Incorporated Mussel-Inspired Polydopamine Coatings on Mesoporous Silica as an Efficient Nanocatalyst and Antimicrobial Agent. ACS Appl. Mater. Interfaces 2018, 10, 1792–1801. [Google Scholar] [CrossRef]
- Yunessnia lehi, A.; Shagholani, H.; Nikpay, A.; Ghorbani, M.; Soleimani lashkenari, M.; Soltani, M. Synthesis and modification of crystalline SBA-15 nanowhiskers as a pH-sensitive metronidazole nanocarrier system. Int. J. Pharm. 2019, 555, 28–35. [Google Scholar] [CrossRef]
- Tamanna, T.; Bulitta, J.B.; Landersdorfer, C.B.; Cashin, V.; Yu, A. Stability and controlled antibiotic release from thin films embedded with antibiotic loaded mesoporous silica nanoparticles. RSC Adv. 2015, 5, 107839–107846. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, L.; Wang, T.; Tang, S.; Li, Q.; Tang, T.; Wei, S.; Qian, J.; Wei, J.; Su, J. Lithium doped silica nanospheres/poly(dopamine) composite coating on polyetheretherketone to stimulate cell responses, improve bone formation and osseointegration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 965–976. [Google Scholar] [CrossRef]
- Sun, T.; Sun, Y.; Zhang, H. Phospholipid-Coated Mesoporous Silica Nanoparticles Acting as Lubricating Drug Nanocarriers. Polymers 2018, 10, 513. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Morry, J.; Gu, S.; Castro, D.J.; Goodyear, S.M.; Sangvanich, T.; Reda, M.M.; Lee, R.; Mihelic, S.A.; Beckman, B.L.; et al. Cationic Polymer Modified Mesoporous Silica Nanoparticles for Targeted siRNA Delivery to HER2 + Breast Cancer. Adv. Funct. Mater. 2015, 25, 2646–2659. [Google Scholar] [CrossRef] [PubMed]
- Zarei, H.; Kazemi Oskuee, R.; Hanafi-Bojd, M.Y.; Gholami, L.; Ansari, L.; Malaekeh-Nikouei, B. Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles. Pharm. Dev. Technol. 2019, 24, 127–132. [Google Scholar] [CrossRef] [PubMed]
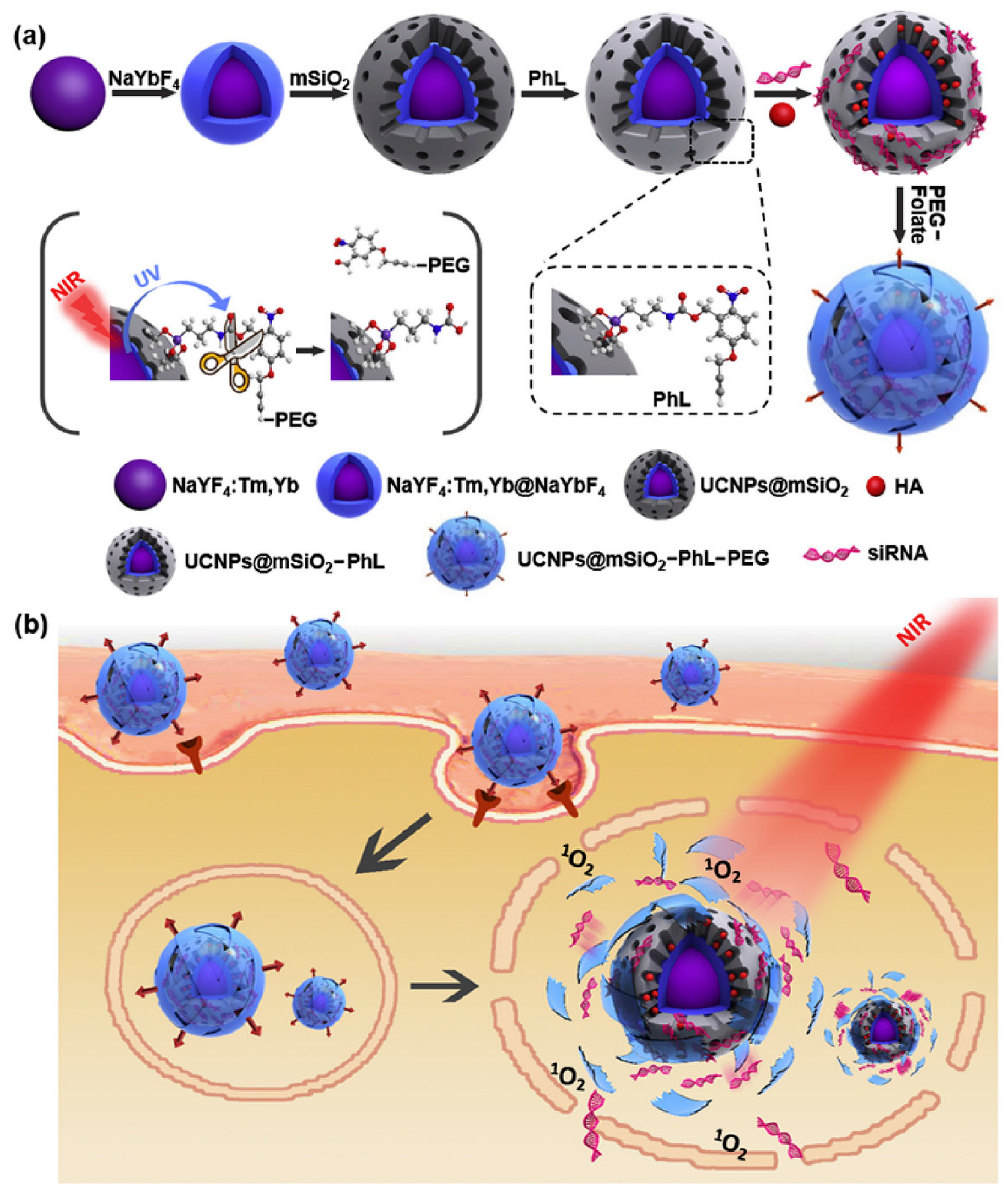
- Zhang, Y.; Ren, K.; Zhang, X.; Chao, Z.; Yang, Y.; Ye, D.; Dai, Z.; Liu, Y.; Ju, H. Photo-tearable tape close-wrapped upconversion nanocapsules for near-infrared modulated efficient siRNA delivery and therapy. Biomaterials 2018, 163, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Mousavi, S.N. Synthesis of a novel structure for the oral delivery of insulin and the study of its effect on diabetic rats. Life Sci. 2017, 186, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.; Hussein, M.; Nasser, H.A.; Ali, A.A.A. Multidisciplinary Role of Mesoporous Silica Nanoparticles in Brain Regeneration and Cancers: From Crossing the Blood-Brain Barrier to Treatment. Part. Part. Syst. Charact. 2019, 36, 1900195. [Google Scholar] [CrossRef]
- Le Joncour, V.; Karaman, S.; Laakkonen, P.M. Predicting in vivo payloads delivery using a blood-brain tumor-barrier in a dish. J. Vis. Exp. 2019, 146, e59384. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Yordanov, Y.; Aluani, D.; Spassova, I.; Kovacheva, D.; Lambov, N.; Tzankova, V. Development and in vitro safety evaluation of pramipexole-loaded hollow mesoporous silica (HMS) particles. Biotechnol. Biotechnol. Equip. 2019, 33, 1204–1215. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Manner, S.; Rosenholm, J.M. Mesoporous silica nanoparticles as diagnostic and therapeutic tools: How can they combat bacterial infection? Ther. Deliv. 2018, 9, 241–244. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Sarwar, S.; Desai, D.; Björk, E.M.; Odén, M.; Chakrabarti, P.; Rosenholm, J.M.; Chakraborti, S. Shape engineering boosts antibacterial activity of chitosan coated mesoporous silica nanoparticle doped with silver: A mechanistic investigation. J. Mater. Chem. B 2016, 4, 3292–3304. [Google Scholar] [CrossRef]
- Morry, J.; Ngamcherdtrakul, W.; Gu, S.; Reda, M.; Castro, D.J.; Sangvanich, T.; Gray, J.W.; Yantasee, W. Targeted Treatment of Metastatic Breast Cancer by PLK1 siRNA Delivered by an Antioxidant Nanoparticle Platform. Mol. Cancer Ther. 2017, 16, 763–772. [Google Scholar] [CrossRef]
- Prabhakar, N.; Zhang, J.; Desai, D.; Casals, E.; Gulin-Sarfraz, T.; Näreoja, T.; Westermarck, J.; Rosenholm, J.M. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int. J. Nanomed. 2016, 11, 6591–6608. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Gulin-Sarfraz, T.; Mamaeva, V.; Niemi, R.; Özliseli, E.; Desai, D.; Antfolk, D.; von Haartman, E.; Lindberg, D.; Prabhakar, N.; et al. Prolonged Dye Release from Mesoporous Silica-Based Imaging Probes Facilitates Long-Term Optical Tracking of Cell Populations In vivo. Small 2016, 12, 1578–1592. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Von Haartman, E.; Jiang, H.; Khomich, A.A.; Zhang, J.; Burikov, S.A.; Dolenko, T.A.; Ruokolainen, J.; Gu, H.; Shenderova, O.A.; Vlasov, I.I.; et al. Core-shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery I: Fabrication. J. Mater. Chem. B 2013, 1, 2358–2366. [Google Scholar] [CrossRef]
- Hong, S.; Kim, K.Y.; Wook, H.J.; Park, S.Y.; Lee, K.D.; Lee, D.Y.; Lee, H. Attenuation of the in vivo toxicity of biomaterials by polydopamine surface modification. Nanomedicine 2011, 6, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Svirshchevskaya, E.V.; Zubareva, A.A.; Boyko, A.A.; Shustova, O.A.; Grechikhina, M.V.; Shagdarova, B.T.; Varlamov, V.P. Analysis of toxicity and biocompatibility of chitosan derivatives with different physico-chemical properties. Appl. Biochem. Microbiol. 2016, 52, 483–490. [Google Scholar] [CrossRef]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Cosmetic Ingredient Review Expert Panel; Andersen, F.A. Final report of the safety assessment of hyaluronic acid, potassium hyaluronate, and sodium hyaluronate. Int. J. Toxicol. 2009, 28, 5–67. [Google Scholar] [CrossRef]
- Johnson, R.P.; John, J.V.; Kim, I. Poly(l-histidine)-containing polymer bioconjugate hybrid materials as stimuli-responsive theranostic systems. J. Appl. Polym. Sci. 2014, 131, 40796. [Google Scholar] [CrossRef]
- Isaksson, K.; Åkerberg, D.; Posaric-Bauden, M.; Andersson, R.; Tingstedt, B. In vivo toxicity and biodistribution of intraperitoneal and intravenous poly-l-lysine and poly-l-lysine/poly-l-glutamate in rats. J. Mater. Sci. Mater. Med. 2014, 25, 1293–1299. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A two-stage poly(ethylenimine)-mediated cytotoxicity: Implications for gene transfer/therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef]
- Kazemi Oskuee, R.; Dabbaghi, M.; Gholami, L.; Taheri-Bojd, S.; Balali-Mood, M.; Mousavi, S.H.; Malaekeh-Nikouei, B. Investigating the influence of polyplex size on toxicity properties of polyethylenimine mediated gene delivery. Life Sci. 2018, 197, 101–108. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 1–12. [Google Scholar] [CrossRef]
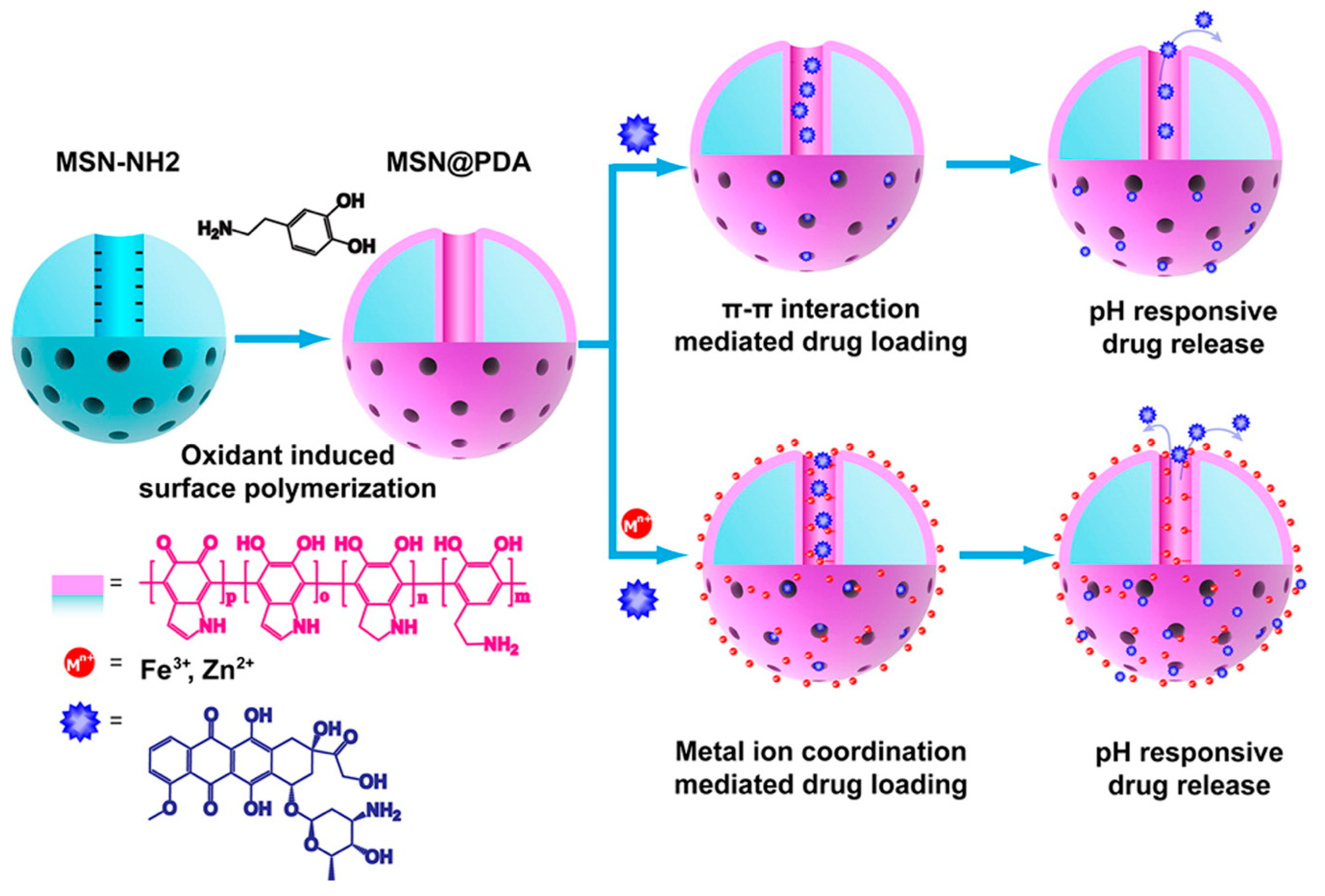

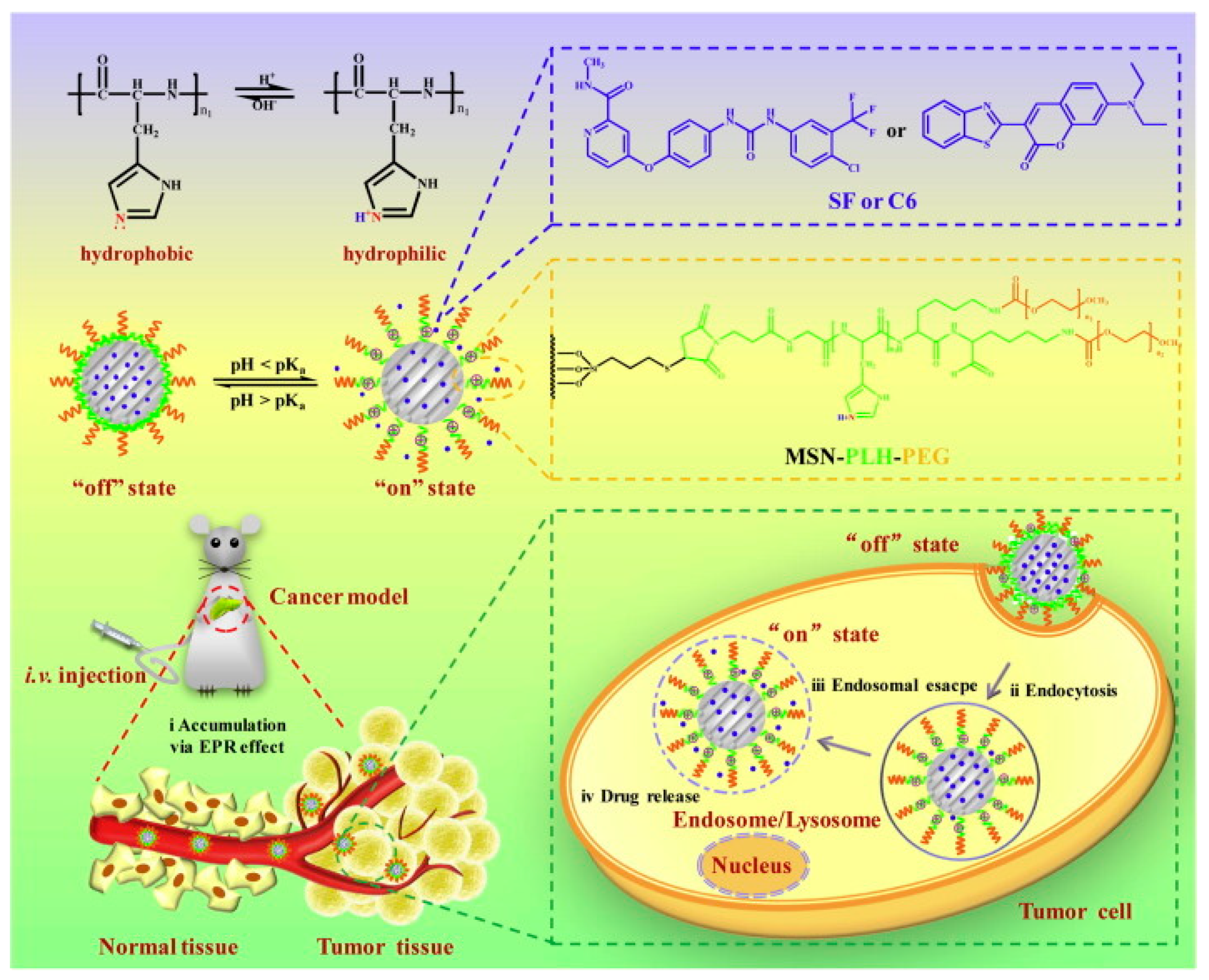


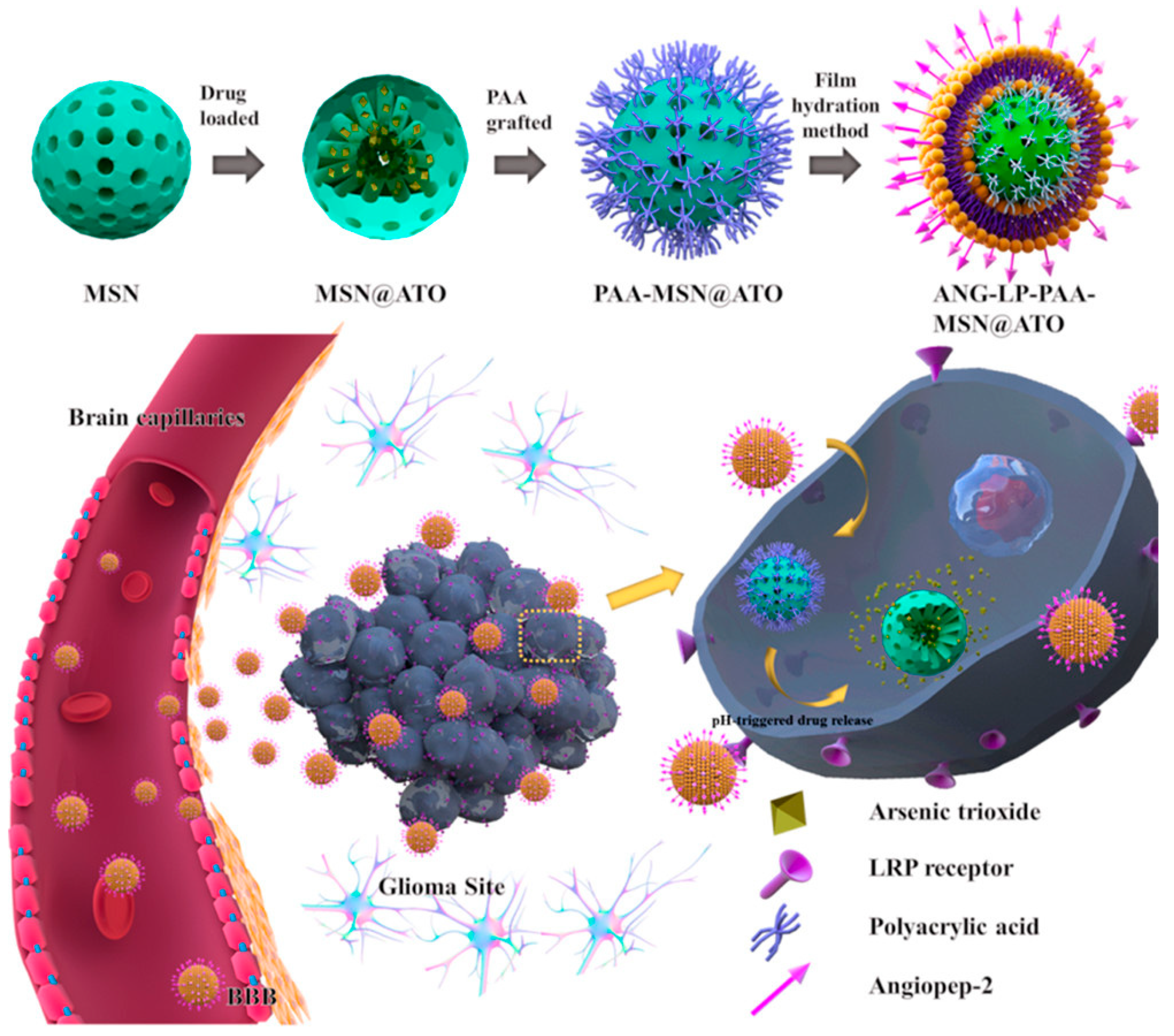

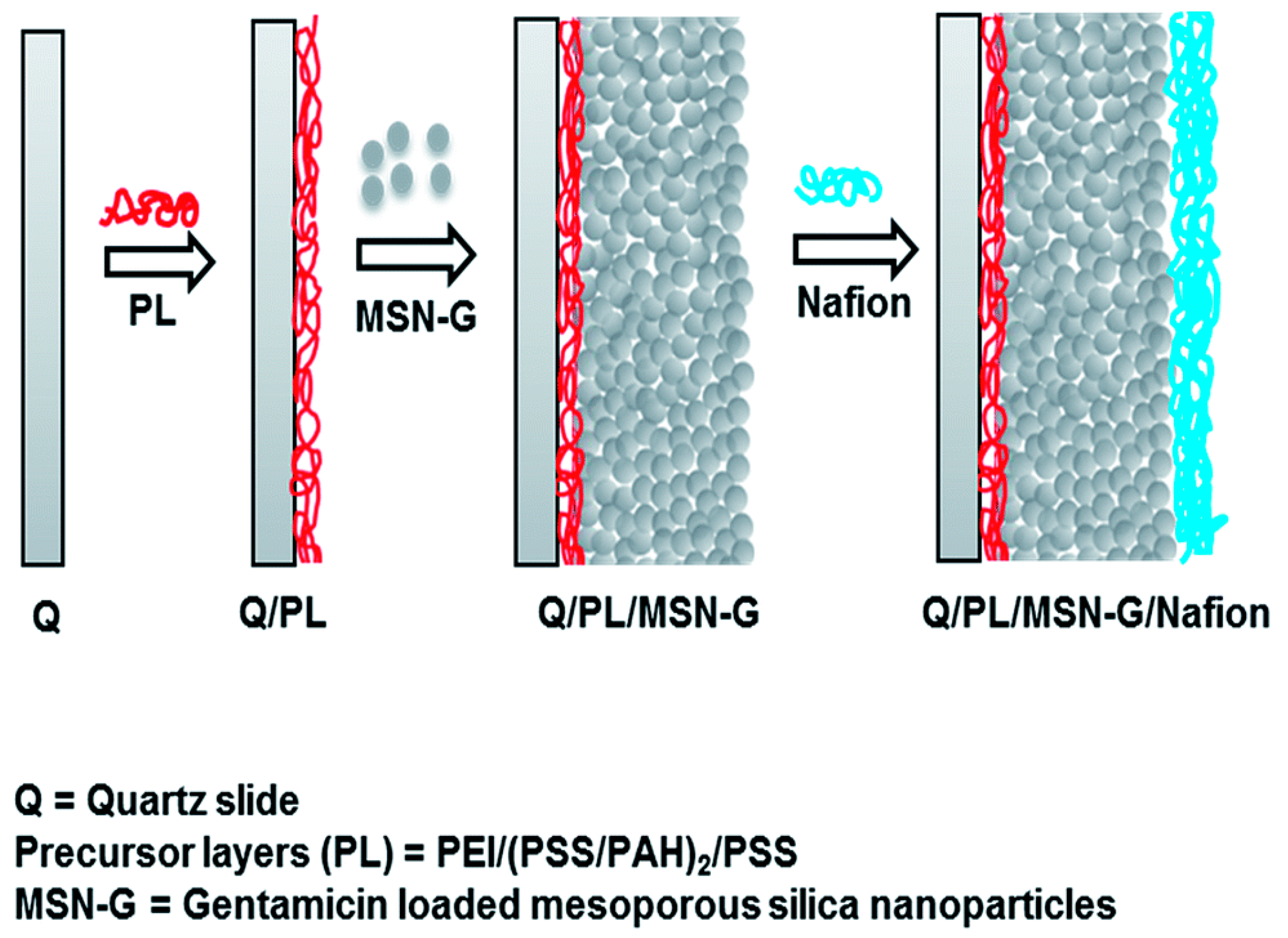

| S. No | Title | Submission Date | Identifier | Recruitment Status |
|---|---|---|---|---|
| 1 | Plasmonic Photothermal Therapy of Flow-Limiting Atherosclerotic Lesions with Silica-Gold Nanoparticles: a First-in-Man Study | 30 December 2010 | NCT01270139 | Completed |
| 2 | Targeted Silica Nanoparticles for Real-Time Image-Guided Intraoperative Mapping of Nodal Metastases | 3 April 2014 | NCT02106598 | Recruiting |
| 3 | Molecular Phenotyping and Image-Guided Surgical Treatment of Prostate Cancer Using Ultra small Silica Nanoparticles | 18 November 2019 | NCT04167969 | Recruiting |
| Sr No | Investigators | Silica Core | Polymer Used | Drug | Application |
|---|---|---|---|---|---|
| 1 | Li et al. [20] | MSNs | Polydopamine | DOX | Cancer |
| 2 | Rahoui et al. [21] | Gold modified MSNs | Polydopamine | DOX | Cancer |
| 3 | Yang et al. [22] | MSNs | Polydopamine, PEG and EpCAM aptamer | DM-1 | Colorectal cancer |
| 4 | Chai et al. [26] | MoSe2 wrapped MSNs | Polydopamine | DOX | Cancer |
| 5 | Cheng et al. [27] | MSNs | Alpha-tocopheryl polyethylene glycol 1000 succinate functionalized polydopamine | DOX | Cancer |
| 6 | Szegedi et al. [28] | KIL-2 and KIT-6 | k-Carrageenan and Chitosan | Curcumin | Cancer |
| 7 | Hu et al. [29] | MSNs | Folic acid modified Chitosan and Hyaluronic acid | DOX | Cancer |
| 8 | Iraji et al. [30] | MSNs | Chitosan | Gallic acid | Cancer |
| 9 | Mu et al. [31] | MSNs | Poly-(L-Histidine) and PEG | Sorafenib | Cancer |
| 10 | Hegazy et al. [35] | Core-shell magnetic MSNs | Poly(N-isopropylacrylamide) (PNIPAAm) | DOX | Cancer |
| 11 | Feng et al. [36] | MSNs | Poly(N-Isopropylacrylamide-co-methacrylic acid) and DSPE-PEG2000 | Evodiamine and Berberine | Cancer |
| 12 | Du et al. [37] | MSNs | Poly(L-lysine) and Hyaluronic acid | Glucose Oxidase and Paclitaxel | Cancer |
| 13 | Yuan et al. [38] | MSNs | Sodium Alginate | DOX | Cancer |
| 14 | Avedian et al. [39] | Fe3O4 core MSNs | Polyethyleneimine and Folic acid | Erlotinib | Cancer |
| 15 | Moreira et al. [43] | Gold core MSNs | Poly-2-ethyl-2-oxazoline | DOX | Cancer |
| 16 | Yu et al. [44] | MSNs | 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl acrylate polymer | DOX | Cancer |
| 17 | Liu et al. [45] | MSNs | Lipid Galactosyl-Ceramide | Hydroxy-campothecine | Cancer |
| 18 | Ma et al. [46] | MSNs encapsulated in liposomes | Calcium Phosphate | DOX and Zinc Phthalocyanine | Cancer |
| 19 | Liu et al. [41] | Gold Nanorods coated with Mesoporous silica | β-cyclodextrin and (2,3-dimethylmaleic anhydride modified chitosan oligosaccharide-b-poly(ethylene glycol)) | Indocyanine Green dye | Cancer |
| 20 | Lin et al. [32] | Highly ordered MSNs | PEGylated Lipid bilayer | Paclitaxel and Curcumin | Cancer |
| 21 | Kienzle et al. [33] | Dendritic MSNs | Polyethylene imine and Poly ethylene glycol | TNF-α | Cancer |
| 22 | Lee et al. [34] | Chlorin-e6 loaded MSNs | PEG | DOX | Cancer |
| 23 | Tao et al. [42] | MSNs encapsulated in liposomes | Poly(acrylic acid) | Arsenic trioxide | Glioma |
| 24 | Tzankov et al. [47] | MCM-41 | Chitosan and Sodium alginate | Pramipexole | Neuroblastoma |
| 25 | Shen et al. [48] | MSNs | Poly(lactic acid) | Resveratrol | Parkinson’s disease |
| 26 | Cheng et al. [49] | MSNs | plasmid RhoG-DsRed | Curcumin | Oxidative stress |
| 27 | Mandic et al. [50] | MSNs | PEG | Quercetin, myricetin and myricitrin | Oxidative stress |
| 28 | Peralta et al. [51] | MSNs | Poly(N-isopropylacrylamide-co-3-(methacryloxypropyl) trimethoxysilane) | Ibuprofen | Inflammation |
| 29 | Gulin-Sarfaz et al. [52] | MSNs | PEI and PEG | Dexamethasone | Pulmonary Inflammation |
| 30 | Popova et al. [53] | SBA-15 and MCM- 41 | Eudragit S and Eudragit RL | Sulfasalazine | inflammatory bowel disease |
| 31 | Liu et al. [54] | Gold nanorods coated mesoporous silica | PEG | Indocyanine green | Light-induced imaging-guided cancer therapy |
| 32 | Tran et al. [55] | FITC-MSNs | Polydopamine and Graphene oxide | Cisplatin | Theranostic Cancer therapy |
| 33 | Xu et al. [56] | MSNs | Poly Curcumin | DOX | Theranostic Cancer therapy |
| 34 | Song et al. [57] | SBA-15 | Polydopamine | Silver | Antibacterial therapy |
| 35 | Lehi et al. [58] | SBA-15 nanowishkers | Tannic acid | Metronidazole | Trichomonasis |
| 36 | Tamanna et al. [59] | MSNs | Nafion Polymer | Gentamicin | Immunoassay |
| 37 | Zhang et al. [60] | Lithium Doped Silica Nanospheres | Polydopamine | Polyetherether ketone implant | Bone regeneration |
| 38 | Sun et al. [61] | MSNs | Distearoyl phosphatidylcholine | Rhodamine- B | Osteoarthritis |
| 39 | Ngamcherdtrakul et al. [62] | MSNs | PEI and PEG | siRNA | Gene Delivery |
| 40 | Zarei et al. [63] | Phosphonate modified MSNs | PEI | Plasmid DNA and chlooquine | Gene delivery |
| 41 | Zhang et al. [64] | Lanthanide doped upconversion Silica nanoparticles | PEG | siRNA and hypocrellin A | Gene delivery |
| 42 | Esmaeli et al. [65] | MCM-41 | PAMAM dendrimer and chitosan-gelatine scaffold | Insulin and cinnamaldeyde | Diabetes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bansal, K.K.; Mishra, D.K.; Rosling, A.; Rosenholm, J.M. Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles. Appl. Sci. 2020, 10, 289. https://doi.org/10.3390/app10010289
Bansal KK, Mishra DK, Rosling A, Rosenholm JM. Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles. Applied Sciences. 2020; 10(1):289. https://doi.org/10.3390/app10010289
Chicago/Turabian StyleBansal, Kuldeep K., Deepak K. Mishra, Ari Rosling, and Jessica M. Rosenholm. 2020. "Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles" Applied Sciences 10, no. 1: 289. https://doi.org/10.3390/app10010289
APA StyleBansal, K. K., Mishra, D. K., Rosling, A., & Rosenholm, J. M. (2020). Therapeutic Potential of Polymer-Coated Mesoporous Silica Nanoparticles. Applied Sciences, 10(1), 289. https://doi.org/10.3390/app10010289







