Isothermal Crystallization Kinetics of Poly(ethylene terephthalate) Copolymerized with Various Amounts of Isosorbide
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
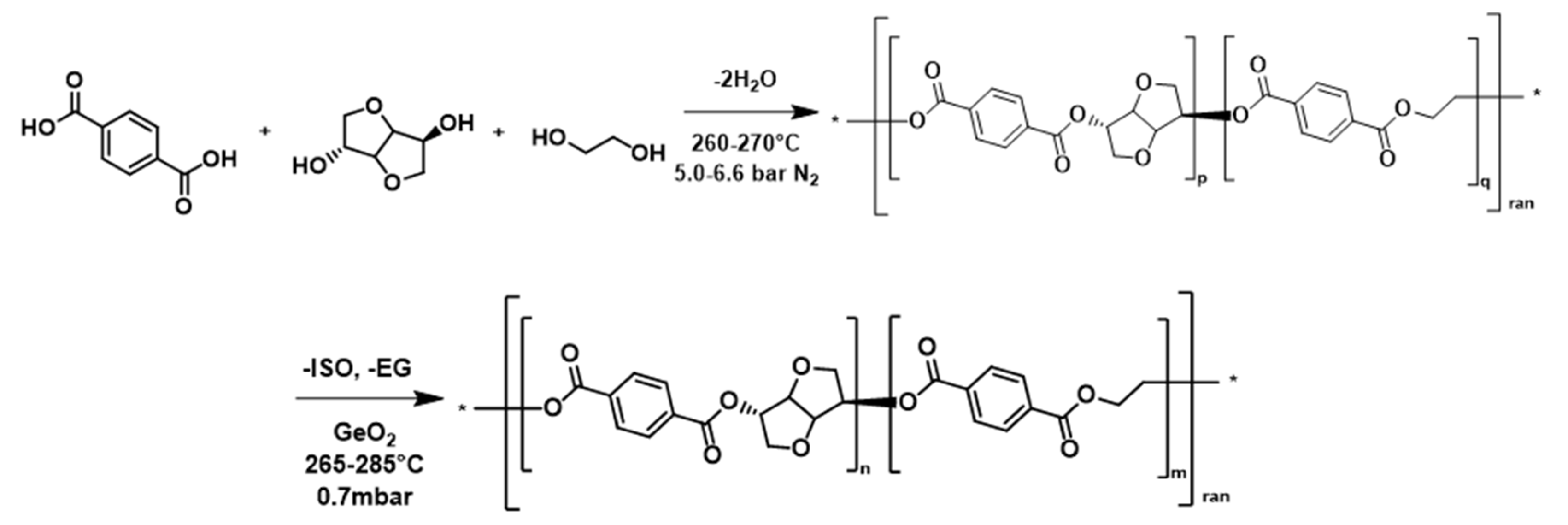
2.2. General Procedure for PEIT (and PET) Synthesis
3. Polymer Characterization
3.1. Reduced Viscosity
3.2. 1H-NMR Analysis
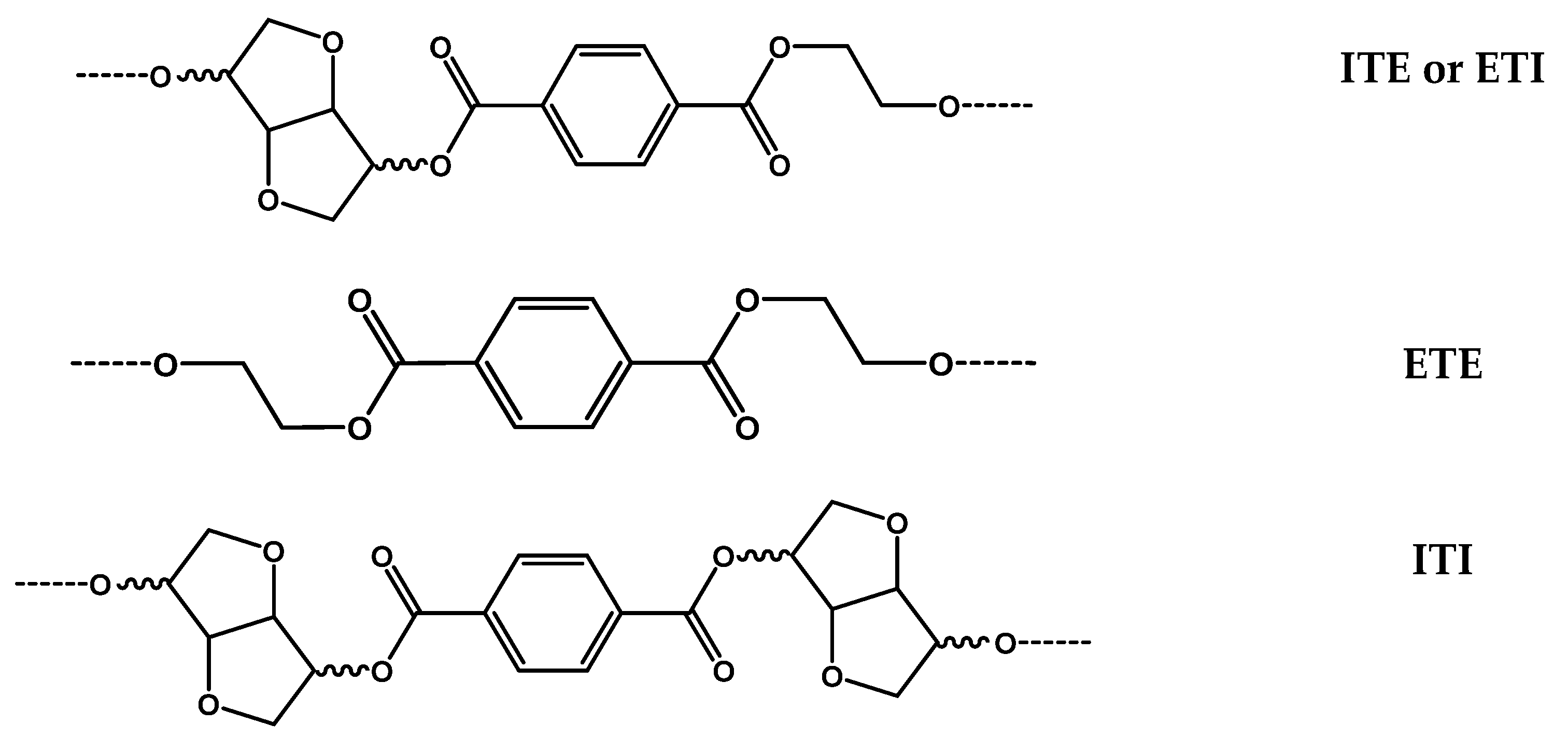
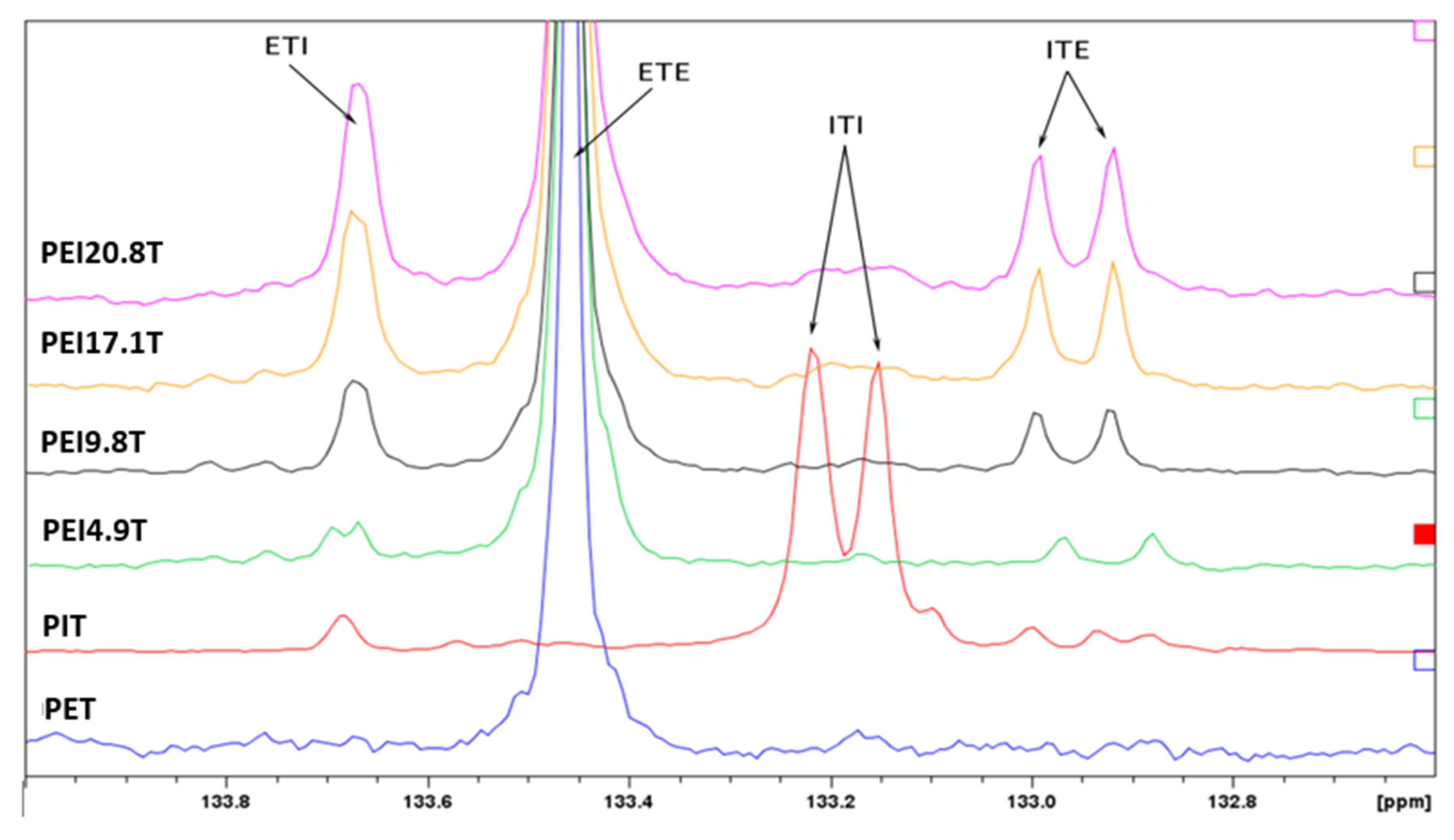
3.3. 13C-NMR Analysis
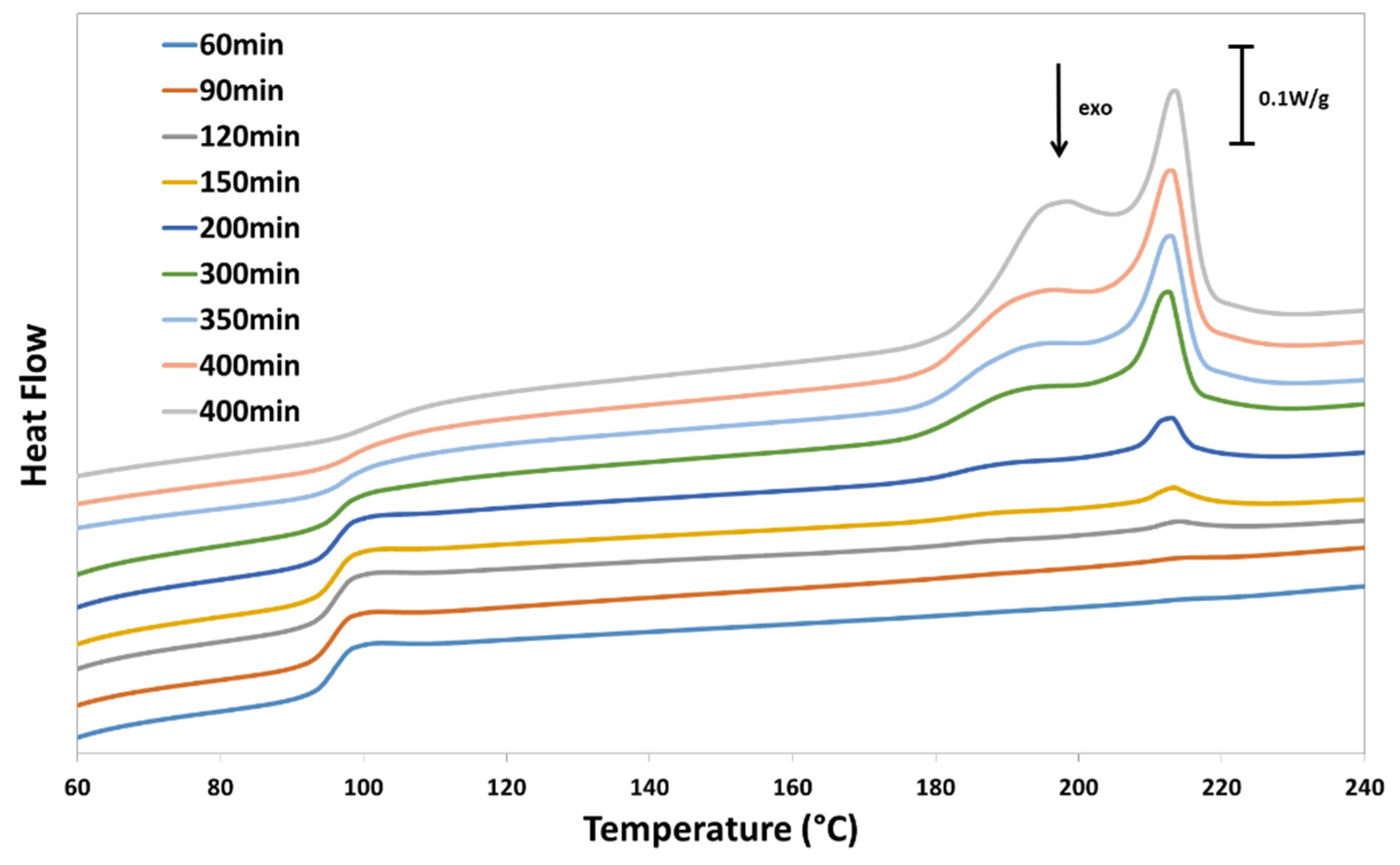
3.4. Differential Scanning Calorimetry (DSC)
3.5. Powder X-ray diffraction (PXRD)
4. Results
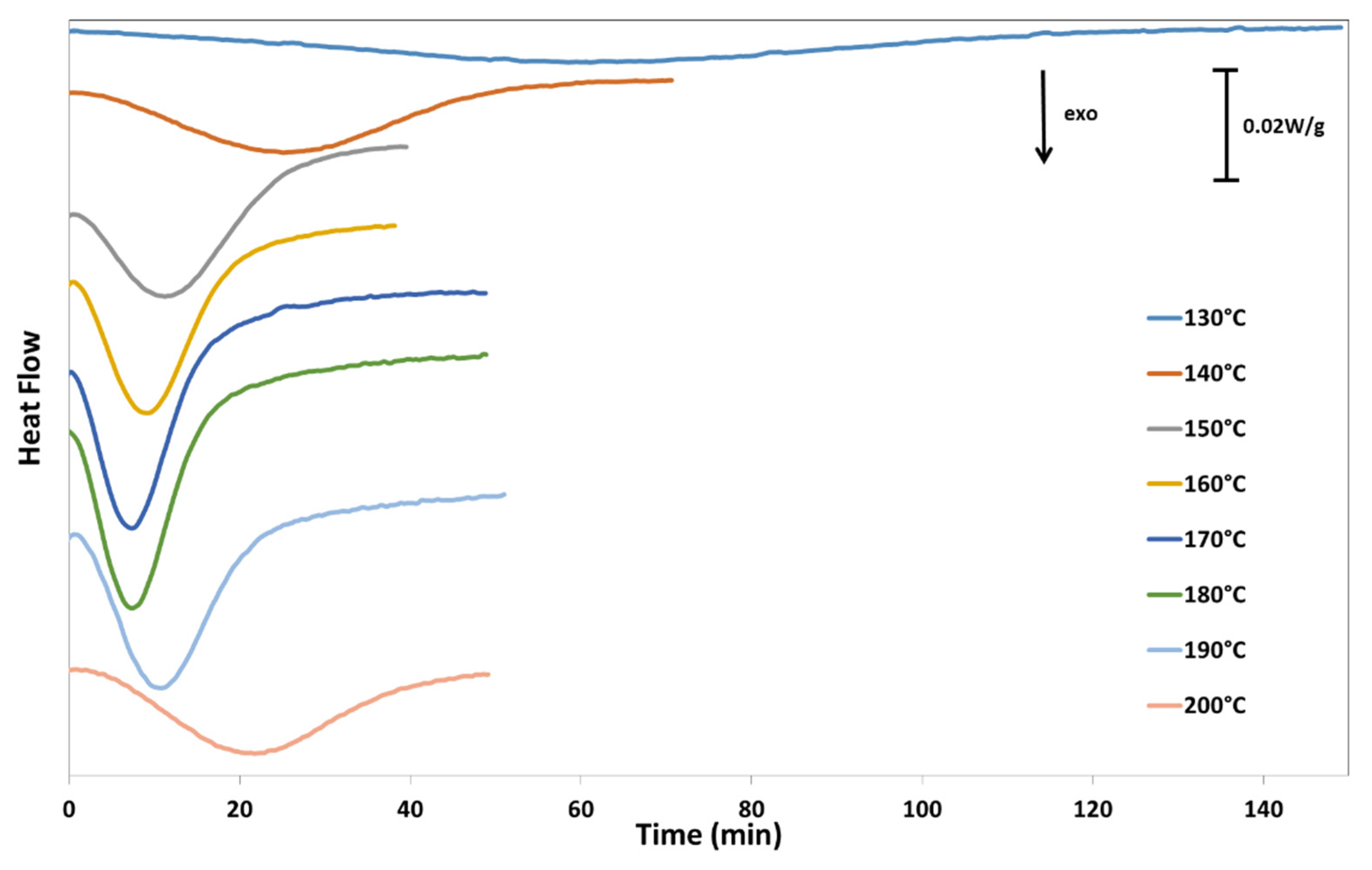
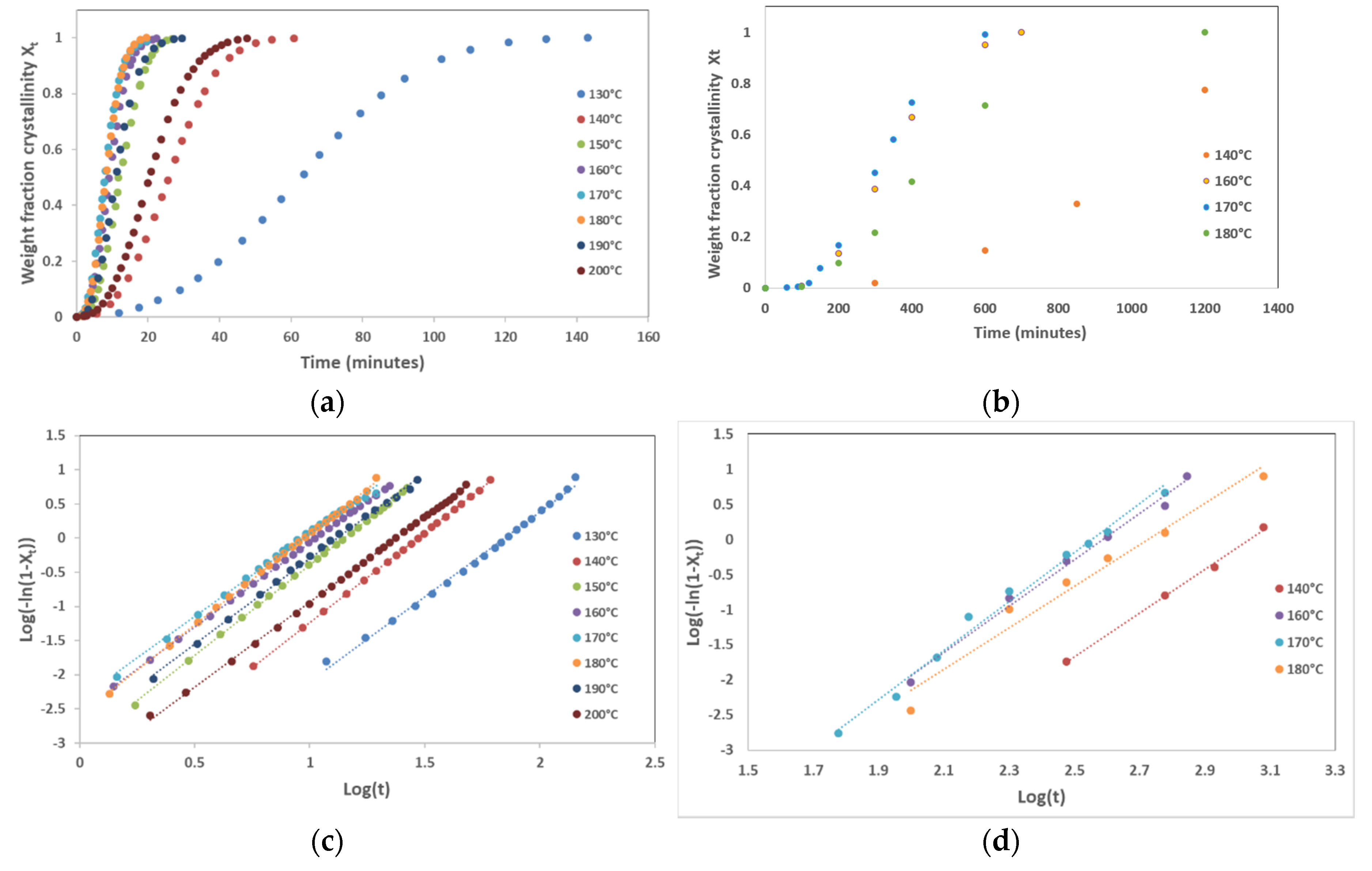
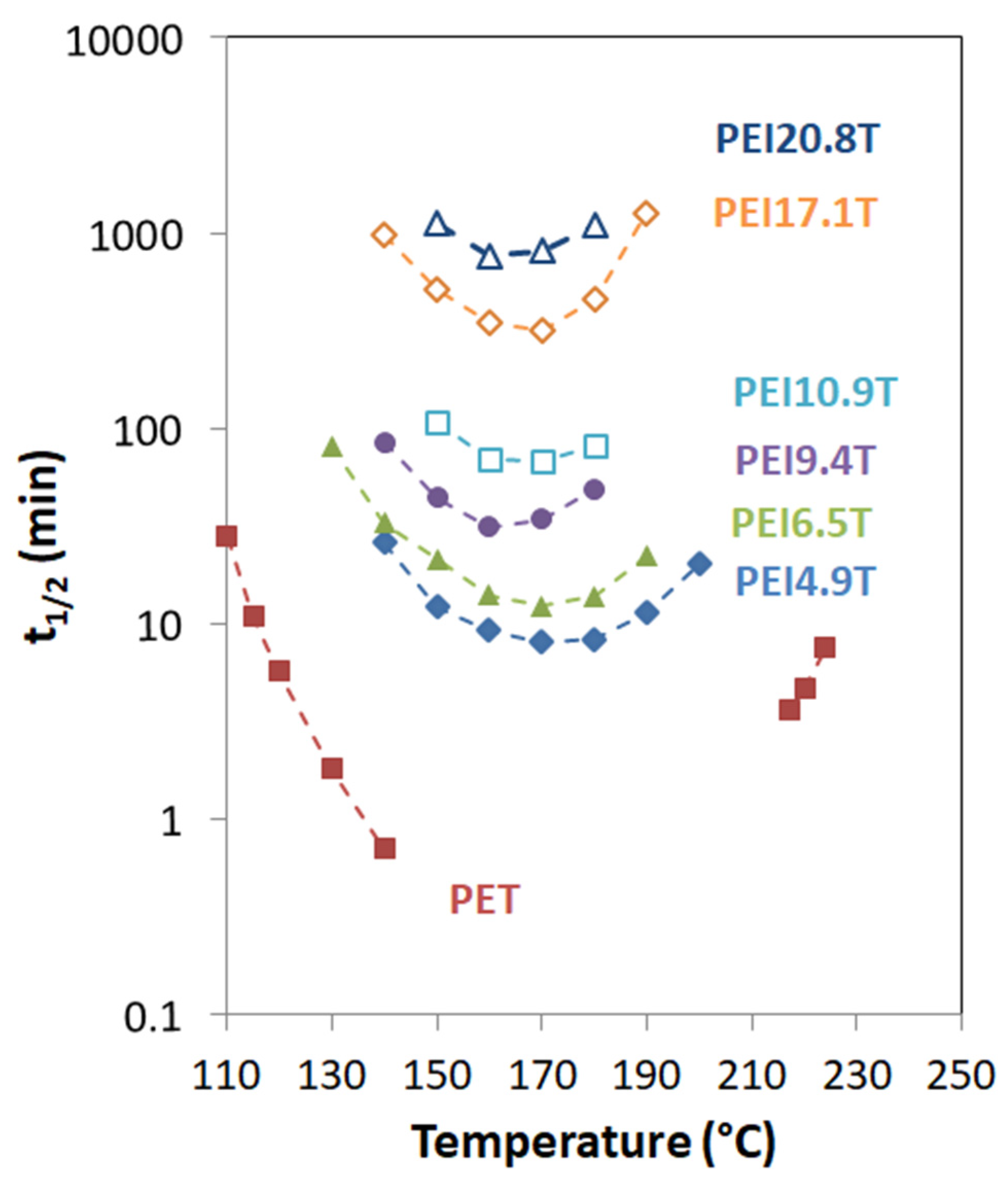
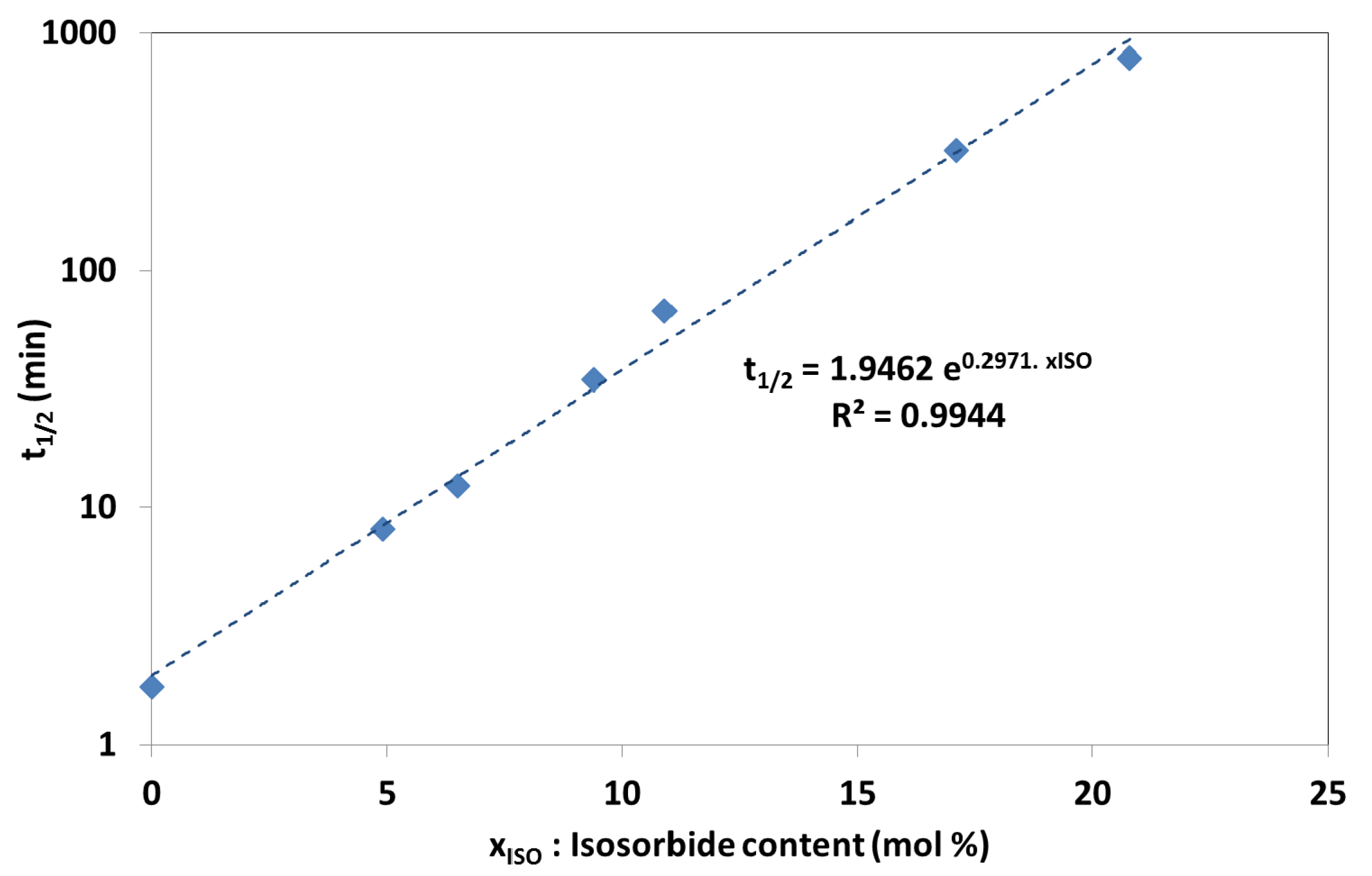
Isothermal crystallization kinetics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1, 4: 3, 6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Kricheldorf, H.R. Sugar Diols as Building Blocks of Polycondensates. J. Macromol. Sci. Part C Polym. Rev. 1997, 37, 599–631. [Google Scholar] [CrossRef]
- Blache, H.; Mechin, F.; Rousseau, A.; Fleury, E.; Pascault, J.P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New bio-based thermoplastic polyurethane elastomers from isosorbide and rapeseed oil derivatives. Ind. Crops Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Bersot, J.C.; Jacquel, N.; Saint-Loup, R.; Fuertes, P.; Rousseau, A.; Pascault, J.P.; Spitz, R.; Fenouillot, F.; Monteil, V. Efficiency Increase of Poly (ethylene terephthalate-co-isosorbide terephthalate) Synthesis using Bimetallic Catalytic Systems. Macromol. Chem. Phys. 2011, 212, 2114–2120. [Google Scholar] [CrossRef]
- Adelman, D.J.; Charbonneau, L.F.; Ung, S. Process for Making Poly(ethylene-co-isosorbide) Terephthalate Polymer. U.S. Patent 20030232959, 14 June 2002. [Google Scholar]
- Stanley, N.; Chenal, T.; Delaunay, T.; Saint-Loup, R.; Jacquel, N.; Zinck, P. Bimetallic catalytic systems based on Sb, Ge and Ti for the synthesis of poly(ethylene terephthalate-co-isosorbide terephthalate). Polymers 2017, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Adelman, D.J.; Greene, R.N.; Putzig, D.E. Poly (1, 3-propylene-co-1, 4: 3, 6-dianhydro-D-sorbitol terephthalate) and Manufacturing Process. U.S. Patent 20030232960, 14 June 2002. [Google Scholar]
- Kricheldorf, H.R.; Behnken, G.; Sell, M. Influence of Isosorbide on Glass-Transition Temperature and Crystallinity of Poly (butylene terephthalate). J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 679–684. [Google Scholar] [CrossRef]
- Sablong, R.; Duchateau, R.; Koning, C.E.; Wit, G.; Es, D.; Koelewijn, R.; Haveren, J. Incorporation of isosorbide into poly (butylene terephthalate) via solid-state polymerization. Biomacromolecules 2008, 9, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Legrand, S.; Jacquel, N.; Amedro, H.; Saint-Loup, R.; Pascault, J.P.; Rousseau, A.; Fenouillot, F. Synthesis and properties of poly(1,4-cyclohexanedimethylene-co-isosorbide terephthalate), a biobased copolyester with high performances. Eur. Polym. J. 2019, 115, 22–29. [Google Scholar] [CrossRef]
- Storbeck, R.; Ballauff, M. Synthesis and thermal analysis of copolyesters deriving from 1, 4: 3, 6-dianhydrosorbitol, ethylene glycol, and terephthalic acid. J. Appl. Polym. Sci. 1996, 59, 1199–1202. [Google Scholar] [CrossRef]
- Göltner, W. Relationship between Polyester Quality and Processability: Hands-On Experience. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolymers; John Wiley & Sons Inc.: New York, NY, USA, 2004; pp. 435–493. ISBN 9780470090688. [Google Scholar]
- Jacquel, N.; Saint-Loup, R.; Pascault, J.P.; Rousseau, A.; Fenouillot, F. Bio-based alternatives in the synthesis of aliphatic-aromatic polyesters dedicated to biodegradable film applications. Polymer 2015, 59, 234–242. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal crystallization kinetics and melting behaviour of poly(ethylene terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Aoki, Y.; Li, L.; Amari, T.; Nishimura, K.; Arashiro, Y. Dynamic Mechanical Properties of Poly(ethylene terephthalate)/Poly(ethylene 2,6-naphthalate) Blends. Macromolecules 1999, 32, 1923–1929. [Google Scholar] [CrossRef]
- Storbeck, R.; Rehahn, M.; Ballauff, M. Synthesis and properties of high-molecular-weight polyesters based on 1, 4: 3, 6-dianhydrohexitols and terephthalic acid. Die Makromol. Chem. 1993, 194, 53–64. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Q.; Wang, L.; Bai, Y. Implementing plant-derived isosorbide and isomannide as comonomers for polyester synthesis: Effects of crystallization properties on optical properties. J. Appl. Polym. Sci. 2017, 134, 45444. [Google Scholar] [CrossRef]
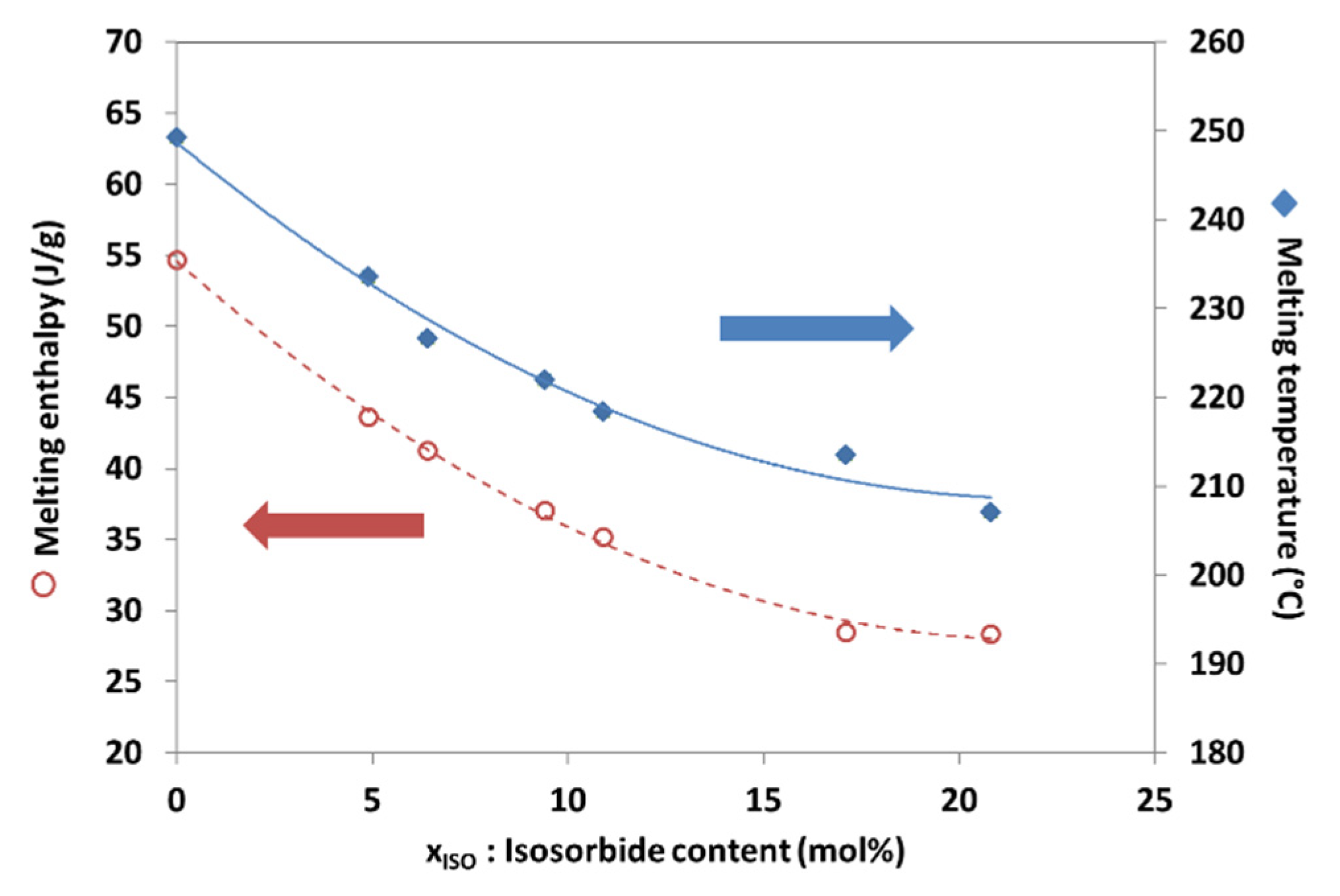
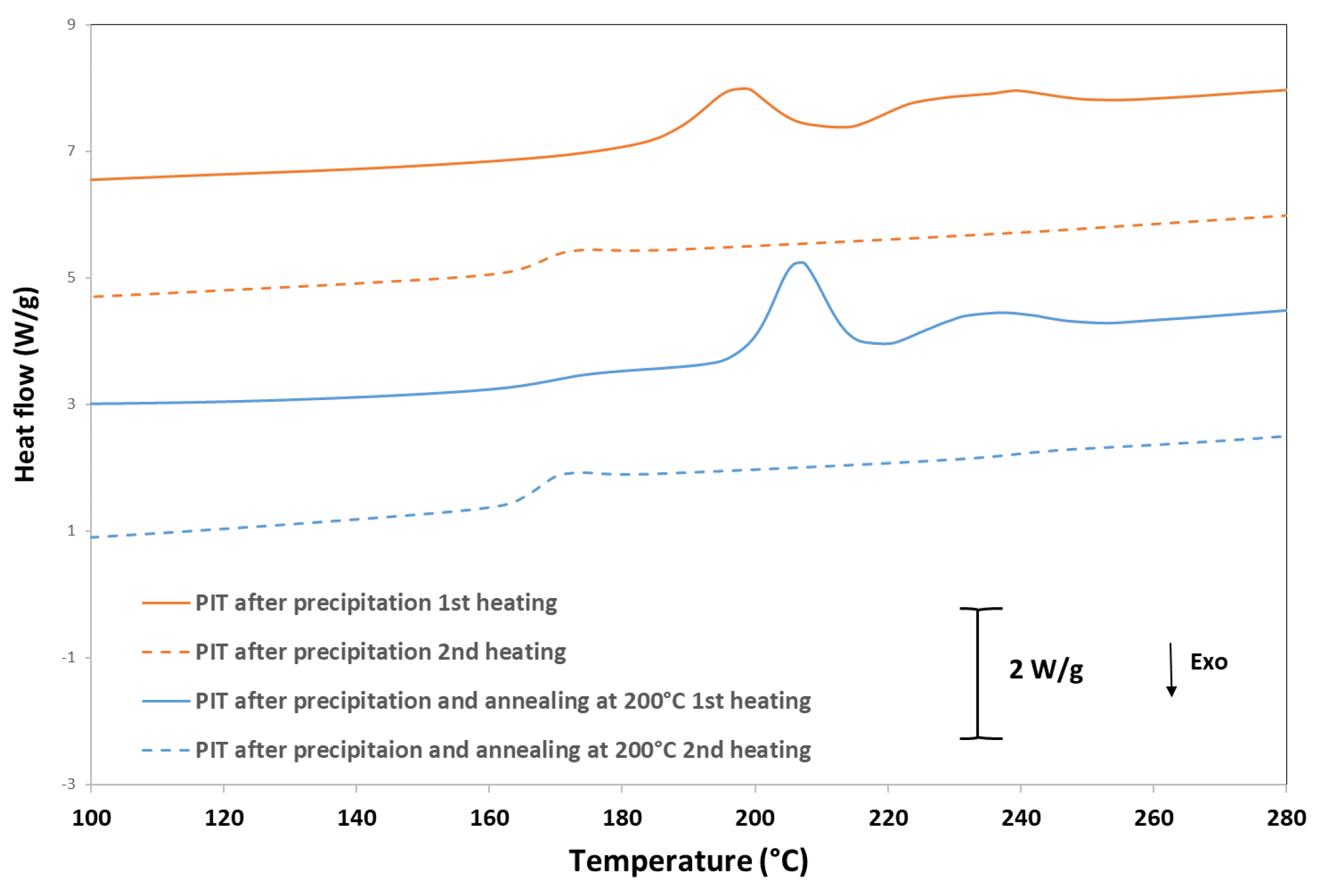
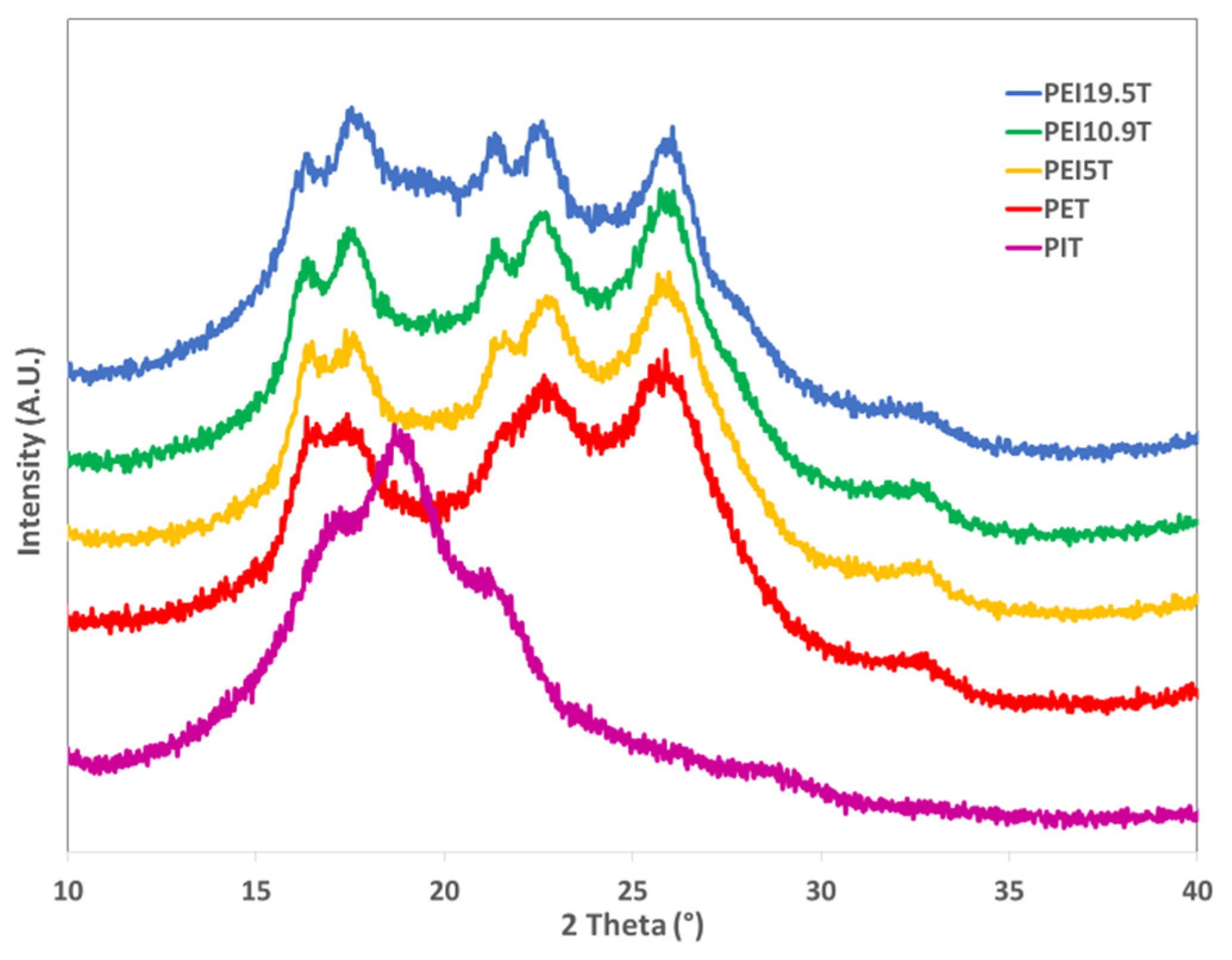
| Samples Code | Isosorbide in Polymer | Reduced Viscosity (dL/g) | Triad Molar Fraction | Average Sequence Lengths | Degree of Randomness (R) | Coloration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITI | ITE + ETI | ETE | n ET | n IT | L* | a* | b* | ||||
| PET | 0 | 0.71 | - | - | - | - | - | - | - | - | - |
| PEI4.9T | 4.9 | 0.65 | 0 | 9.4 | 90.6 | 20.3 | 1.0 | 1.05 | 51.8 | 0.2 | −1.9 |
| PEI6.5T | 6.5 | 0.59 | - | - | - | - | - | - | 55.8 | 0.1 | 0.6 |
| PEI9.8T | 9.8 | 0.66 | 3.2 | 17.7 | 79.1 | 9.9 | 1.4 | 0.84 | 52.1 | −0.1 | 1.4 |
| PEI10.9T | 10.9 | 0.59 | - | - | - | - | - | - | 57.5 | −0.2 | 1.3 |
| PEI17.1T | 17.1 | 0.61 | 4.2 | 26.7 | 69.0 | 6.2 | 1.3 | 0.92 | 52.8 | −0.2 | −0.6 |
| PEI19.5T | 19.5 | 0.60 | - | - | - | - | - | - | 56.1 | −0.6 | 2.7 |
| PEI20.8T | 20.8 | 0.56 | 6.3 | 30.2 | 63.6 | 5.2 | 1.4 | 0.90 | 50.0 | −0.6 | 1.4 |
| PIT | 100 | 0.21 | - | - | - | - | - | - | - | - | - |
| Samples Code | T (°C) | K (min−1) | n | t1/2 (min) |
|---|---|---|---|---|
| PET | 110 | 0.03 | 2.08 | 27.7 |
| 115 | 0.07 | 2.07 | 10.8 | |
| 120 | 0.15 | 2.12 | 5.7 | |
| 130 | 0.47 | 2.01 | 1.8 | |
| 140 | 1.11 | 1.93 | 0.7 | |
| 217 | 0.24 | 2.43 | 3.6 | |
| 220 | 0.19 | 2.74 | 4.7 | |
| 224 | 0.12 | 2.62 | 7.5 | |
| PEI4.9T | 150 | 0.071 | 2.65 | 12.2 |
| 160 | 0.09 | 2.46 | 9.2 | |
| 170 | 0.11 | 2.43 | 8.1 | |
| 180 | 0.11 | 2.66 | 8.3 | |
| 190 | 0.076 | 2.51 | 11.3 | |
| 200 | 0.043 | 2.5 | 20.2 | |
| PEI6.5T | 150 | 0.041 | 2.76 | 21.4 |
| 160 | 0.062 | 2.58 | 13.9 | |
| 170 | 0.07 | 2.52 | 12.3 | |
| 180 | 0.063 | 2.62 | 13.9 | |
| 190 | 0.039 | 2.61 | 22.1 | |
| PEI9.8T | 140 | 0.0103 | 2.86 | 85.1 |
| 150 | 0.019 | 2.51 | 44.3 | |
| 160 | 0.029 | 2.63 | 31.1 | |
| 170 | 0.025 | 2.44 | 34.4 | |
| 180 | 0.018 | 2.87 | 49.1 | |
| PEI10.9T | 150 | 0.0081 | 2.72 | 107.8 |
| 160 | 0.012 | 2.26 | 69.6 | |
| 170 | 0.013 | 2.66 | 67.3 | |
| 180 | 0.01 | 2.26 | 82.1 | |
| PEI17.1T | 140 | 0.00091 | 3.11 | 976.5 |
| 160 | 0.0025 | 2.76 | 348.7 | |
| 170 | 0.0028 | 2.91 | 318.9 | |
| 180 | 0.0019 | 2.31 | 455.5 | |
| 190 | 0.00067 | 2.03 | 1247.5 | |
| PEI20.8T | 150 | 0.00081 | 3.73 | 1122 |
| 160 | 0.00116 | 3.03 | 765 | |
| 170 | 0.00109 | 2.99 | 813 | |
| 180 | 0.00082 | 3.41 | 1094 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Descamps, N.; Fernandez, F.; Heijboer, P.; Saint-Loup, R.; Jacquel, N. Isothermal Crystallization Kinetics of Poly(ethylene terephthalate) Copolymerized with Various Amounts of Isosorbide. Appl. Sci. 2020, 10, 1046. https://doi.org/10.3390/app10031046
Descamps N, Fernandez F, Heijboer P, Saint-Loup R, Jacquel N. Isothermal Crystallization Kinetics of Poly(ethylene terephthalate) Copolymerized with Various Amounts of Isosorbide. Applied Sciences. 2020; 10(3):1046. https://doi.org/10.3390/app10031046
Chicago/Turabian StyleDescamps, Nicolas, Florian Fernandez, Pierre Heijboer, René Saint-Loup, and Nicolas Jacquel. 2020. "Isothermal Crystallization Kinetics of Poly(ethylene terephthalate) Copolymerized with Various Amounts of Isosorbide" Applied Sciences 10, no. 3: 1046. https://doi.org/10.3390/app10031046
APA StyleDescamps, N., Fernandez, F., Heijboer, P., Saint-Loup, R., & Jacquel, N. (2020). Isothermal Crystallization Kinetics of Poly(ethylene terephthalate) Copolymerized with Various Amounts of Isosorbide. Applied Sciences, 10(3), 1046. https://doi.org/10.3390/app10031046




