Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study
Abstract
1. Introduction
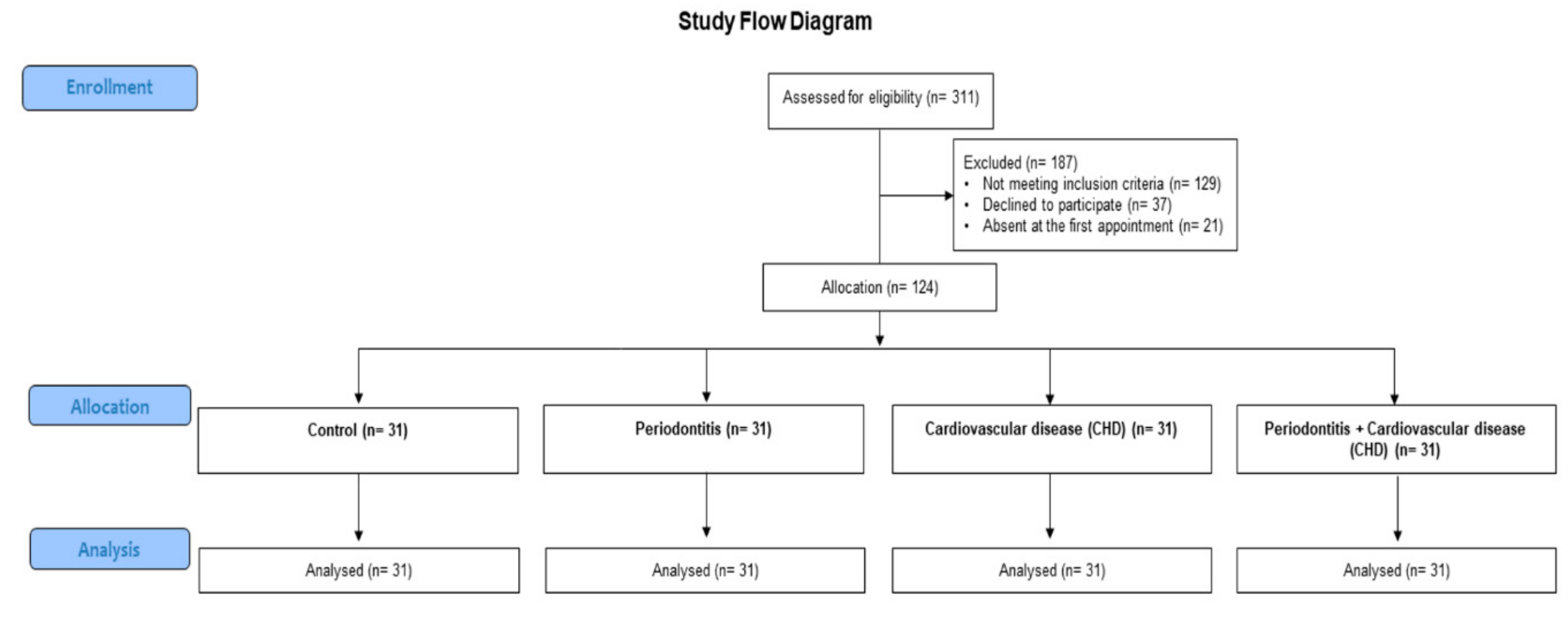
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of Salivary and Serum MPO
2.3. Statistical Analysis
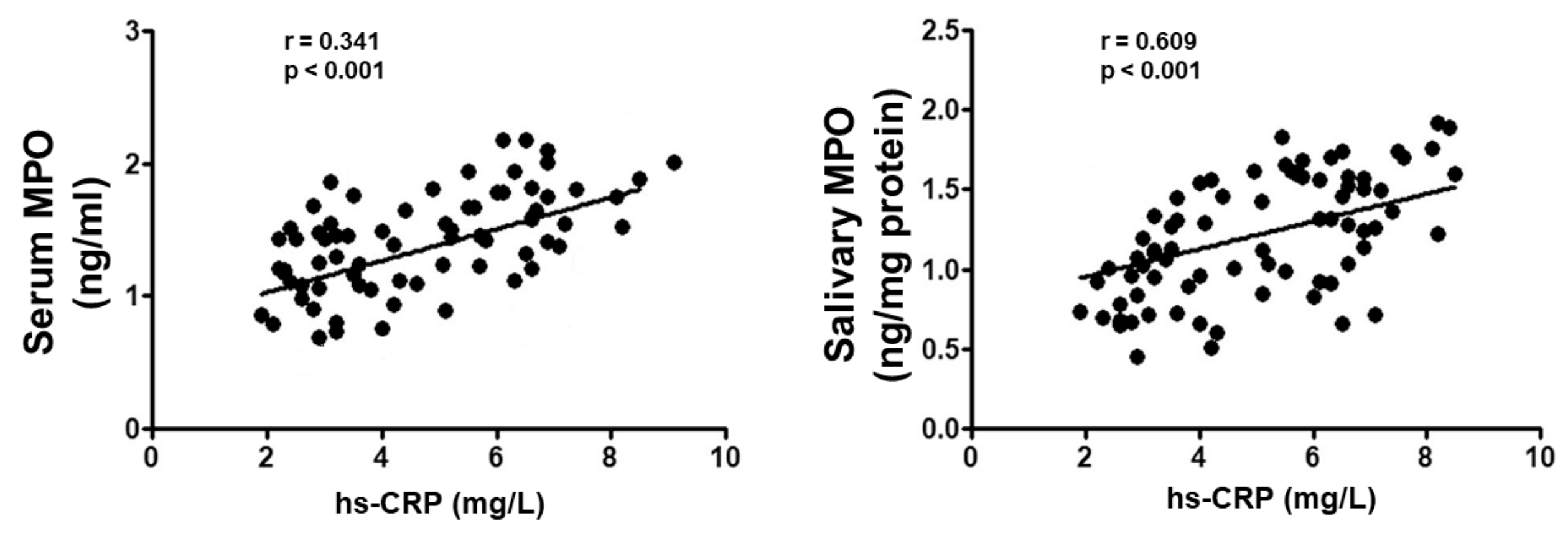
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar] [PubMed]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk Indicators for Periodontitis in US Adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef]
- Holmlund, A.; Holm, G.; Lind, L. Number of Teeth as a Predictor of Cardiovascular Mortality in a Cohort of 7,674 Subjects Followed for 12 Years. J. Periodontol. 2010, 81, 870–876. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Indelicato, F.; Ferlito, S. Analysis of Endothelin-1 concentrations in individuals with periodontitis. Sci. Rep. 2020. [Google Scholar] [CrossRef]
- Li, C.; Lv, Z.; Shi, Z.; Zhu, Y.; Wu, Y.; Li, L.; Iheozor-Ejiofor, Z. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst. Rev. 2017, 2017, CD009197. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Giudice, A.L. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef]
- Matarese, G.; Curro, M.; Isola, G.; Caccamo, D.; Vecchio, M.; Giunta, M.L.; Ramaglia, L.; Cordasco, G.; Williams, R.C.; Ientile, R. Transglutaminase 2 up-regulation is associated with RANKL/OPG pathway in cultured HPDL cells and THP-1-differentiated macrophages. Amino Acids 2015, 47, 2447–2455. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ramadori, G.; Moriconi, F. Modulation of Chemokine- and Adhesion-Molecule Gene Expression and Recruitment of Neutrophil Granulocytes in Rat and Mouse Liver after a Single Gadolinium Chloride or Zymosan Treatment. Int. J. Mol. Sci. 2018, 19, 3891. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.G.M.; Gomes, A.C.; Navarro, A.M.; Cunha, L.C.D.; Silva, M.A.C.; Junior, F.B.; Mota, J.F. Baru Almonds Increase the Activity of Glutathione Peroxidase in Overweight and Obese Women: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 11, 1750. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Giudice, G.L.; Briguglio, F.; Alibrandi, A.; Crupi, A.; Cordasco, G.; Ramaglia, L. A New Approach for the Treatment of Lateral Periodontal Cysts with an 810-nm Diode Laser. Int. J. Periodontics Restor. Dent. 2017, 37, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Anastasi, G.P.; Cutroneo, G.; Favaloro, A.; Vita, G.; Cordasco, G.; Milardi, D.; Zizzari, V.L.; Tetè, S.; et al. Transforming Growth Factor Beta 1 and Vascular Endothelial Growth Factor levels in the pathogenesis of periodontal disease. Eur. J. Inflamm. 2013, 11, 479–488. [Google Scholar] [CrossRef]
- Peniche-Palma, D.C.; Carrillo-Avila, B.A.; Sauri-Esquivel, E.A.; Acosta-Viana, K.; Esparza-Villalpando, V.; Pozos-Guillen, A.; Hernandez-Rios, M.; Martinez-Aguilar, V.M. Levels of Myeloperoxidase and Metalloproteinase-9 in Gingival Crevicular Fluid from Diabetic Subjects with and without Stage 2, Grade B Periodontitis. BioMed Res. Int. 2019, 2019, 5613514. [Google Scholar] [CrossRef]
- Currò, M.; Matarese, G.; Isola, G.; Caccamo, D.; Ventura, V.P.; Cornelius, C.; Lentini, M.; Cordasco, G.; Ientile, R. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Isola, G.; Williams, R.C.; Lo Gullo, A.; Ramaglia, L.; Matarese, M.; Iorio-Siciliano, V.; Cosio, C.; Matarese, G. Risk association between scleroderma disease characteristics, periodontitis, and tooth loss. Clin Rheumatol. 2017, 36, 2733–2741. [Google Scholar] [CrossRef]
- Andrukhov, O.; Haririan, H.; Bertl, K.; Rausch, W.-D.; Bantleon, H.-P.; Moritz, A.; Rausch-Fan, X. Nitric oxide production, systemic inflammation and lipid metabolism in periodontitis patients: Possible gender aspect. J. Clin. Periodontol. 2013, 40, 916–923. [Google Scholar] [CrossRef]
- Isola, G.; Alibrandi, A.; Rapisarda, E.; Matarese, G.; Williams, R.C.; Leonardi, R. Association of vitamin d in patients with periodontitis: A cross-sectional study. J. Periodontal Res. 2020, in press. [Google Scholar]
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Investig. 2014, 44, 1000–1009. [Google Scholar] [CrossRef]
- Tabeta, K.; Hosojima, M.; Nakajima, M.; Miyauchi, S.; Miyazawa, H.; Takahashi, N.; Matsuda, Y.; Sugita, N.; Komatsu, Y.; Sato, K.; et al. Increased serum PCSK9, a potential biomarker to screen for periodontitis, and decreased total bilirubin associated with probing depth in a Japanese community survey. J. Periodontal Res. 2018, 53, 446–456. [Google Scholar] [CrossRef]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar] [PubMed]
- Kendall, H.K.; Marshall, R.I.; Bartold, P.M. Nitric oxide, and tissue destruction. Oral Dis. 2001, 7, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Aurer, A.; Aleksic, J.; Ivic-Kardum, M.; Aurer, J.; Čulo, F. Nitric oxide synthesis is decreased in periodontitis. J. Clin. Periodontol. 2001, 28, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Ruest, L.B.; Ranjbaran, H.; Tong, E.J.; Svoboda, K.K.H.; Feng, J.Q. Activation of Receptor Activator of Nuclear Factor-κB Ligand and Matrix Metalloproteinase Production in Periodontal Fibroblasts by Endothelin Signaling. J. Periodontol. 2016, 87, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Periodontol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Lindhe, J.; Ranney, R.; Lamster, I.; Charles, A.; Chung, C.-P.; Flemmig, T.; Kinane, D.; Listgarten, M.; Löe, H.; Schoor, R.; et al. Consensus Report: Chronic Periodontitis. Ann. Periodontol. 1999, 4, 38. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef]
- Bassand, J.P.; Hamm, C.W.; Ardissino, D.; Boersma, E.; Budaj, A.; Fernández-Avilés, F.; Fox, K.A.; Hasdai, D.; Ohman, E.M.; Wallentin, L.; et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur. Heart J. 2007, 28, 1598–1660. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lo Giudice, G.; Lo Giudice, R.; Matarese, G.; Isola, G.; Cicciù, M.; Terranova, A.; Palaia, G.; Romeo, U. Evaluation of magnification systems in restorative dentistry. An in-vitro study. Dental Cadmos 2015, 83, 296–305. [Google Scholar] [CrossRef]
- Mazzoli, A.; Crescenzo, R.; Cigliano, L.; Spagnuolo, M.S.; Cancelliere, R.; Gatto, C.; Iossa, S. Early Hepatic Oxidative Stress and Mitochondrial Changes Following Western Diet in Middle Aged Rats. Nutrients 2019, 11, 2670. [Google Scholar] [CrossRef]
- Lahdentausta, L.; Paju, S.; Mäntylä, P.; Buhlin, K.; Pietiäinen, M.; Tervahartiala, T.; Nieminen, M.S.; Sinisalo, J.; Sorsa, T.; Pussinen, P.J. Smoking confounds the periodontal diagnostics using saliva biomarkers. J. Periodontol. 2019, 90, 475–483. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Ramaglia, L.; Pedullà, E.; Rapisarda, E.; Iorio-Siciliano, V. Association between periodontitis and glycosylated haemoglobin before diabetes onset: A cross-sectional study. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Valenzuela, M.; Draibe, J.; Quero, M.; Fulladosa, X.; Cruzado, J.M.; Bestard, O.; Torras, J.; Valenzuela, L.M. Exploring Frequencies of Circulating Specific Th17 Cells against Myeloperoxidase and Proteinase 3 in ANCA Associated Vasculitis. Int. J. Mol. Sci. 2019, 20, 5820. [Google Scholar] [CrossRef]
- Przepiera-Będzak, H.; Fischer, K.; Brzosko, M. Serum Interleukin-18, Fetuin-A, Soluble Intercellular Adhesion Molecule-1, and Endothelin-1 in Ankylosing Spondylitis, Psoriatic Arthritis, and SAPHO Syndrome. Int. J. Mol. Sci. 2016, 17, 1255. [Google Scholar] [CrossRef]
- Magán-Fernández, A.; O’Valle, F.; Abadía-Molina, F.; Muñoz, R.; Puga-Guil, P.; Mesa, F. Characterization and comparison of neutrophil extracellular traps in gingival samples of periodontitis and gingivitis: A pilot study. J. Periodontal Res. 2019, 54, 218–224. [Google Scholar] [CrossRef]
- Cavuoti, S.; Matarese, G.; Isola, G.; Abdolreza, J.; Femiano, F.; Perillo, L. Combined orthodontic-surgical management of a transmigrated mandibular canine: A case report. Angle Orthod. 2016, 86, 681–691. [Google Scholar] [CrossRef]
- Matejka, M.; Partyka, L.; Ulm, C.; Solar, P.; Sinzinger, H. Nitric oxide synthesis is increased in periodontal disease. J. Periodontal Res. 1998, 33, 517–518. [Google Scholar] [CrossRef]
- Perillo, L.; Isola, G.; Esercizio, D.; Iovane, M.; Triolo, G.; Matarese, G. Differences in craniofacial characteristics in Southern Italian children from Naples: A retrospective study by cephalometric analysis. Eur. J. Paediatr. Dent. 2013, 14, 195–198. [Google Scholar] [PubMed]
- Ozer, L.; Elgün, S.; Özdemir, B.; Pervane, B.; Özmeriç, N. Arginine–Nitric Oxide–Polyamine Metabolism in Periodontal Disease. J. Periodontol. 2011, 82, 320–328. [Google Scholar] [CrossRef]
- Bodis, S.; Haregewoin, A. Significantly reduced salivary nitric oxide levels in smokers. Ann. Oncol. 1994, 5, 371–372. [Google Scholar] [CrossRef]
- Vasconcelos, D.F.P.; Da Silva, F.R.P.; Pinto, M.E.S.C.; Santana, L.D.A.B.; Souza, I.G.; De Souza, L.K.M.; Oliveira, N.C.M.; Ventura, C.A.; Novaes, P.D.; Barbosa, A.L.D.R.; et al. Decrease of Pericytes is Associated with Liver Disease Caused by Ligature-Induced Periodontitis in Rats. J. Periodontol. 2017, 88, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: A randomized, controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Perillo, L.; Migliorati, M.; Matarese, M.; Dalessandri, D.; Grassia, V.; Alibrandi, A.; Matarese, G. The impact of temporomandibular joint arthritis on functional disability and global health in patients with juvenile idiopathic arthritis. Eur. J. Orthod. 2019, 41, 117–124. [Google Scholar] [CrossRef]
- Isola, G.; Anastasi, G.P.; Matarese, G.; Williams, R.C.; Cutroneo, G.; Bracco, P.; Piancino, M.G. Functional and molecular outcomes of the human masticatory muscles. Oral Dis. 2018, 24, 1428–1441. [Google Scholar] [CrossRef]
- Isola, G.; Alibrandi, A.; Pedullà, E.; Grassia, V.; Ferlito, S.; Perillo, L.; Rapisarda, E. Analysis of the Effectiveness of Lornoxicam and Flurbiprofen on Management of Pain and Sequelae Following Third Molar Surgery: A Randomized, Controlled, Clinical Trial. J. Clin. Med. 2019, 8, 325. [Google Scholar] [CrossRef]
- Camacho-Alonso, F.; Davia-Peña, R.S.; Vilaplana-Vivo, C.; Tudela-Mulero, M.R.; Merino, J.J.; Martínez-Beneyto, Y. Synergistic effect of photodynamic therapy and alendronate on alveolar bone loss in rats with ligature-induced periodontitis. J. Periodontal Res. 2017, 53, 306–314. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Alibrandi, A.; Dalessandri, D.; Migliorati, M.; Pedullà, E.; Rapisarda, E. Comparison of Effectiveness of Etoricoxib and Diclofenac on Pain and Perioperative Sequelae After Surgical Avulsion of Mandibular Third Molars: A Randomized, Controlled, Clinical Trial. Clin. J. Pain 2019, 35, 908–915. [Google Scholar] [CrossRef]
- Mohammed, H.; Varoni, E.M.; Cochis, A.; Cordaro, M.; Gallenzi, P.; Patini, R.; Staderini, E.; Lajolo, C.; Rimondini, L.; Rocchetti, V. Oral Dysbiosis in Pancreatic Cancer and Liver Cirrhosis: A Review of the Literature. Biomedicines 2018, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Gallenzi, P.; Spagnuolo, G.; Cordaro, M.; Cantiani, M.; Amalfitano, A.; Arcovito, A.; Callà, C.; Mingrone, G.; Nocca, G. Correlation Between Metabolic Syndrome, Periodontitis and Reactive Oxygen Species Production. A Pilot Study. Open Dent. J. 2017, 11, 621–627. [Google Scholar] [CrossRef]
- Caccianiga, G.; Paiusco, A.; Perillo, L.; Nucera, R.; Pinsino, A.; Maddalone, M.; Cordasco, G.; Giudice, A.L. Does Low-Level Laser Therapy Enhance the Efficiency of Orthodontic Dental Alignment? Results from a Randomized Pilot Study. Photomed. Laser Surg. 2017, 35, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Nucera, R.; Leonardi, R.; Paiusco, A.; Baldoni, M.; Caccianiga, G. A Comparative Assessment of the Efficiency of Orthodontic Treatment with and Without Photobiomodulation during Mandibular Decrowding in Young Subjects: A Single-Center, Single-Blind Randomized Controlled Trial. Photobiomodul. Photomed. Laser Surg. 2020. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Currò, M.; Zinellu, A.; Caccamo, D.; Isola, G.; Ventura, V.; Carru, C.; Matarese, G.; Ientile, R. Influence of MTHFR genetic background on P16 and MGMT methylation in oral squamous cell cancer. Int. J. Mol. Sci. 2017, 18, 724. [Google Scholar] [CrossRef]
- Cutroneo, G.; Piancino, M.G.; Ramieri, G.; Bracco, P.; Vita, G.; Isola, G.; Vermiglio, G.; Favaloro, A.; Anastasi, G.P.; Trimarchi, F. Expression of muscle-specific integrins in masseter muscle fibers during malocclusion disease. Int. J. Mol. Med. 2012, 30, 235–242. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Briguglio, F.; Grassia, V.; Picciolo, G.; Fiorillo, L.; Matarese, G. Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials 2019, 12, 2187. [Google Scholar] [CrossRef]
- Piancino, M.G.; Isola, G.; Cannavale, R.; Cutroneo, G.; Vermiglio, G.; Bracco, P.; Anastasi, G.P.; Grazia, P.M.; Gaetano, I.; Rosangela, C.; et al. From periodontal mechanoreceptors to chewing motor control: A systematic review. Arch. Oral Boil. 2017, 78, 109–121. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Nucera, R.; Perillo, L.; Paiusco, A.; Caccianiga, G. Is low-level laser therapy an effective method to alleviate pain induced by active orthodontic alignment archwire? A randomized clinical trial. J. Evid. Based Dent. Pract. 2019, 19, 71–78. [Google Scholar] [CrossRef]
- Leonardi, R.; Lo Giudice, A.; Rugeri, M.; Muraglie, S.; Cordasco, G.; Barbato, E. Three-dimensional evaluation on digital casts of maxillary palatal size and morphology in patients with functional posterior crossbite. Eur. J. Orthod. 2018, 40, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.L.; Caccianiga, G.; Crimi, S.; Cavallini, C.; Leonardi, R. Frequency and type of ponticulus posticus in a longitudinal sample of nonorthodontically treated patients: Relationship with gender, age, skeletal maturity, and skeletal malocclusion. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Cannavale, R.; Matarese, G.; Isola, G.; Grassia, V.; Perillo, L. Early treatment of an ectopic premolar to prevent molar-premolar transposition. Am. J. Orthod. Dentofacial Orthop. 2013, 143, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Isola, G.; Alibrandi, A.; Lo Gullo, A.; Bagnato, G.; Cordasco, G.; Perillo, L. Occlusal and MRI characterizations in systemic sclerosis patients: A prospective study from Southern Italian cohort. Joint. Bone. Spine. 2016, 83, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Cordasco, G.; Rotondo, F.; Crupi, A.; Ramaglia, L. Anticoagulant therapy in patients undergoing dental interventions: a critical review of the literature and current perspectives. Minerva Stomatol. 2015, 64, 21–46. [Google Scholar] [PubMed]
- Matarese, G.; Isola, G.; Ramaglia, L.; Dalessandri, D.; Lucchese, A.; Alibrandi, A.; Fabiano, F.; Cordasco, G. Periodontal biotype: characteristic, prevalence and dimensions related to dental malocclusion. Minerva Stomatol. 2016, 65, 231–238. [Google Scholar]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease-Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]

| Controls (N = 31) | Periodontitis (N = 31) | CVD (N = 31) | Periodontitis + CVD (N = 31) | |
|---|---|---|---|---|
| Age (years) | 52 (48; 56) | 53 (47; 57) | 52 (46; 58) | 53 (47; 56) |
| Gender (male/female) | 15/16 | 16/15 | 14/17 | 16/15 |
| Education level | ||||
| Primary school, n (%) | 11 (35.4) | 12 (38.7) | 11 (35.4) | 12 (38.7) |
| High school, n (%) | 14 (45.1) | 13 (41.9) | 15 (48.3) | 14 (45.1) |
| College/university, n (%) | 6 (19.3) | 6 (19.3) | 5 (16.1) | 5 (16.1) |
| Body mass index (kg/m2) | 24.4 (21.8; 27.8) | 23.9 (22.4; 26.4) | 24.8 (21.6; 27.1) | 24.5 (22.3; 26.1) |
| Fasting glucose (mg/dl) | 87.9 (82.1; 93.2) | 88.7 (82.3; 105.3) | 88.1 (80.6; 114.6) | 91.1 (85.1; 109.2) |
| Smokers, n (%) | 2 (6.4) | 3 (9.6) | 2 (6.4) | 2 (9.6) |
| Never smokers, n (%) | 27 (88.2) | 27 (85.2) | 28 (88.2) | 26 (85.2) |
| Past smokers, n (%) | 2 (6.4) | 1 (3.2) | 1 (3.2) | 3 (9.6) |
| Current smokers, n (%) | 2 (6.4) | 3 (6.4) | 2 (6.4) | 2 (6.4) |
| Comorbidities | ||||
| Diabetes, n (%) | - | 3 (9.6) ** | 2 (6.4) ** | 2 (9.6) ** |
| Previous CVD | ||||
| Atrial fibrillation, n (%) | - | - | 6 (19.3) **,§§ | 5 (16.1) **,§§ |
| Angina pectoris, n (%) | - | - | 12 (38.7) **,§§ | 13 (41.9) **,§§ |
| Stroke, n (%) | - | - | 5 (16.1) **,§§ | 7 (22.6) **,§§ |
| Heart failure, n (%) | - | - | 6 (19.3) **,§§ | 5 (16.1) **,§§ |
| Antihypertensive, n (%) | - | - | 10 (32.2) **,§§ | 10 (32.2) **,§§ |
| Statins, n (%) | - | - | 10 (32.2) **,§§ | 9 (29) **,§§ |
| Low-dose aspirin, n (%) | - | - | 7 (22.6) **,§§ | 7 (22.6) **,§§ |
| Beta blockers, n (%) | - | - | 6 (19.3) **,§§ | 8 (25.8) **,§§ |
| hs-CRP (mg/L) | 2.5 (2.1; 2.9) | 3.1 (2.5; 3.9) * | 5.6 (4.8; 6.2) ** | 6.7 (5.8; 7.1) **,§§,# |
| Total cholesterol (mg/dl) | 162 (139; 181) | 164 (133; 181) | 173 (139; 197) | 176 (178; 201) |
| Triglycerids (mg/dl) | 121 (91; 145) | 102 (66; 128) | 141 (122; 166) | 139 (103; 158) |
| Controls (N = 31) | Periodontitis (N = 31) | CVD (N = 31) | Periodontitis + CVD (N = 31) | |
|---|---|---|---|---|
| N° of teeth | 26 (21; 27) | 20 (15; 21)** | 23 (19; 26) **,§§ | 18 (12; 20) **,## |
| CAL (mm) | 1 (0.9; 1.3) | 3.6 (3.1; 4.2) ** | 2 (1.8; 2.4) **,§§ | 3.4 (3; 4.1) **,## |
| CAL 4–5 mm (% sites) | - | 38.7 (35.4; 42.9) ** | - | 40.6 (35.5; 46.1) **,## |
| CAL ≥6 mm (% sites) | - | 18.7 (19.6; 22.3) ** | - | 17.4 (15.8; 22.7) **,## |
| PD (mm) | 1.5 (1.3; 1.9) | 4.4 (3.7; 4.6) ** | 2.1 (1.8; 2.4) **,§§ | 3.9 (3.7; 4.4) **,## |
| PD 4–5 mm (% sites) | - | 40.9 (38.6; 45.7) ** | - | 44.1 (42.7; 55.3) **,## |
| PD ≥6 mm (% sites) | - | 23.1 (19.8; 25.2) ** | - | 22.7 (20.3; 26.5) **,§§,## |
| BOP (%) | 7.9 (6.5; 8.3) | 41.2 (34.3; 46.5) ** | 8.1 (6.4; 8.9) **,§§ | 42.3 (41.5; 50.1) **,§§,## |
| Rx alveolar bone loss (mm) | 0.2 (0.1; 0.5) | 2.9 (2.5; 3.4) ** | 0.3 (0.2; 0.9) **,§§ | 3.3 (2.1; 4.6) **,## |
| PI (%) | 6.5 (4.7; 9.3) | 35.4 (31.7; 37.9) ** | 11.7 (9.1; 12.8) **,§§ | 32.4 (27.2; 36.1) **,## |
| Serum MPO Levels | UNIVARIATE | MULTIVARIATE | |||||
|---|---|---|---|---|---|---|---|
| Variable | B | 95% CI | p | B | 95% CI | p | |
| CVD | 0.442 | 0.314; 0.558 | <0.001 | 0.122 | −0.221; 0.559 | 0.578 | |
| Periodontitis | 0.289 | 0.18; 0.274 | 0.024 | 0.231 | −0.066; 0.319 | 0.226 | |
| hs-CRP | 0.278 | 0.028; 0.136 | <0.001 | 0.314 | 0.065; 0.189 | <0.001 | |
| Age (years) | −0.047 | −0.287; 0.056 | 0.064 | −0.039 | −0.111; 0.315 | 0.287 | |
| Female gender | 0.212 | −0.78; 0.549 | 0.227 | 0.219 | −0.88; 0.428 | 0.112 | |
| Education SES | −0.114 | −0.178; 0.123 | 0.287 | −0.066 | −0.312; 0.398 | 0.312 | |
| Salivary MPO Levels | |||||||
| CVD | 0.319 | 0.112; 0.552 | <0.001 | −0.065 | −0.412; 0.289 | 0.451 | |
| Periodontitis | 0.078 | −0.069; 0.328 | 0.411 | 0.007 | −0.178; 0.213 | 0.665 | |
| hs-CRP | 0.082 | 0.027; 0.287 | <0.001 | 0.066 | 0.021; 0.133 | <0.001 | |
| Age (years) | −0.051 | −0.110; 0.036 | 0.399 | 0.047 | −0.028; 0.087 | 0.741 | |
| Female gender | 0.074 | −0.112; 0.211 | 0.398 | 0.071 | −0.065; 0.412 | 0.321 | |
| Total Cholesterol | −0.057 | −0.151; −0.062 | 0.037 | −0.65 | −0.041; 0.314 | 0.035 | |
| Serum MPO | 0.122 | −0.021; 0.557 | 0.057 | −0.036 | −0.369; 0.166 | 0.331 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polizzi, A.; Torrisi, S.; Santonocito, S.; Di Stefano, M.; Indelicato, F.; Lo Giudice, A. Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study. Appl. Sci. 2020, 10, 1037. https://doi.org/10.3390/app10031037
Polizzi A, Torrisi S, Santonocito S, Di Stefano M, Indelicato F, Lo Giudice A. Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study. Applied Sciences. 2020; 10(3):1037. https://doi.org/10.3390/app10031037
Chicago/Turabian StylePolizzi, Alessandro, Salvatore Torrisi, Simona Santonocito, Mattia Di Stefano, Francesco Indelicato, and Antonino Lo Giudice. 2020. "Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study" Applied Sciences 10, no. 3: 1037. https://doi.org/10.3390/app10031037
APA StylePolizzi, A., Torrisi, S., Santonocito, S., Di Stefano, M., Indelicato, F., & Lo Giudice, A. (2020). Influence of Myeloperoxidase Levels on Periodontal Disease: An Applied Clinical Study. Applied Sciences, 10(3), 1037. https://doi.org/10.3390/app10031037







