Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It
Abstract
1. Introduction
2. Theoretical Background and Consideration of Existing Literature
2.1. Principle of Water Repellent
2.2. Review Existing Literature
3. Materials and Specimens
3.1. Outline of Experiment
3.2. Materials
3.3. Specimens
3.4. Method of Experimental
3.4.1. Air Content, Flow, Compressive Strength
3.4.2. Contact Angle (Sessile Drop Method)
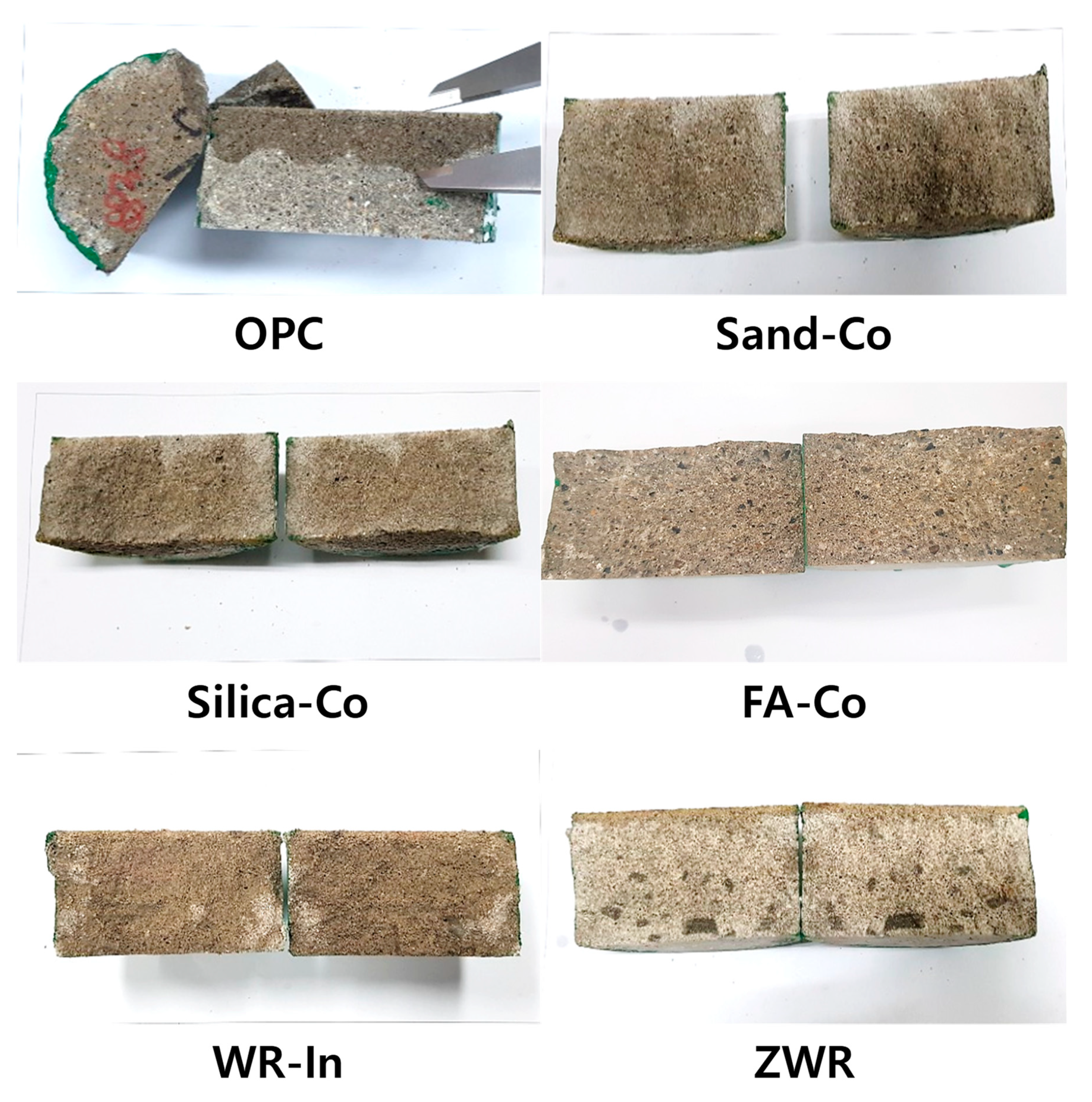
3.4.3. Water Penetration Test
3.4.4. Resist Chloride Ion Penetration Test (RCPT)
- Q: Total passing charge.
- lo: Current immediately after the start of the test with voltage applied.
- l360: Current after 360 min have passed after applying voltage.
3.4.5. Mercury Intrusion Porosimetry (MIP)
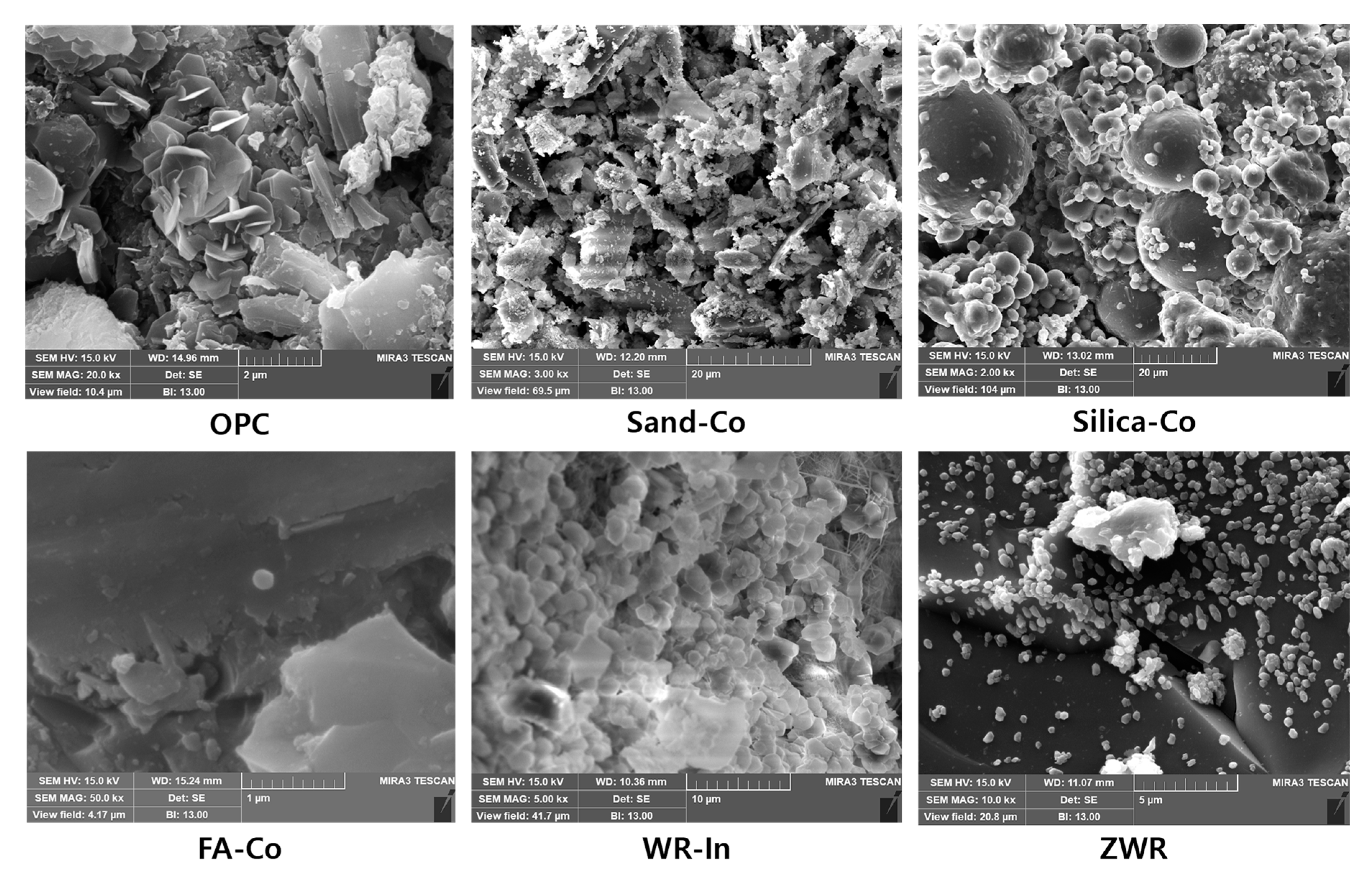
3.4.6. Scanning Electron Microscope (SEM)
4. Result and Discussion
4.1. Air Content, Flow, Compressive Strength
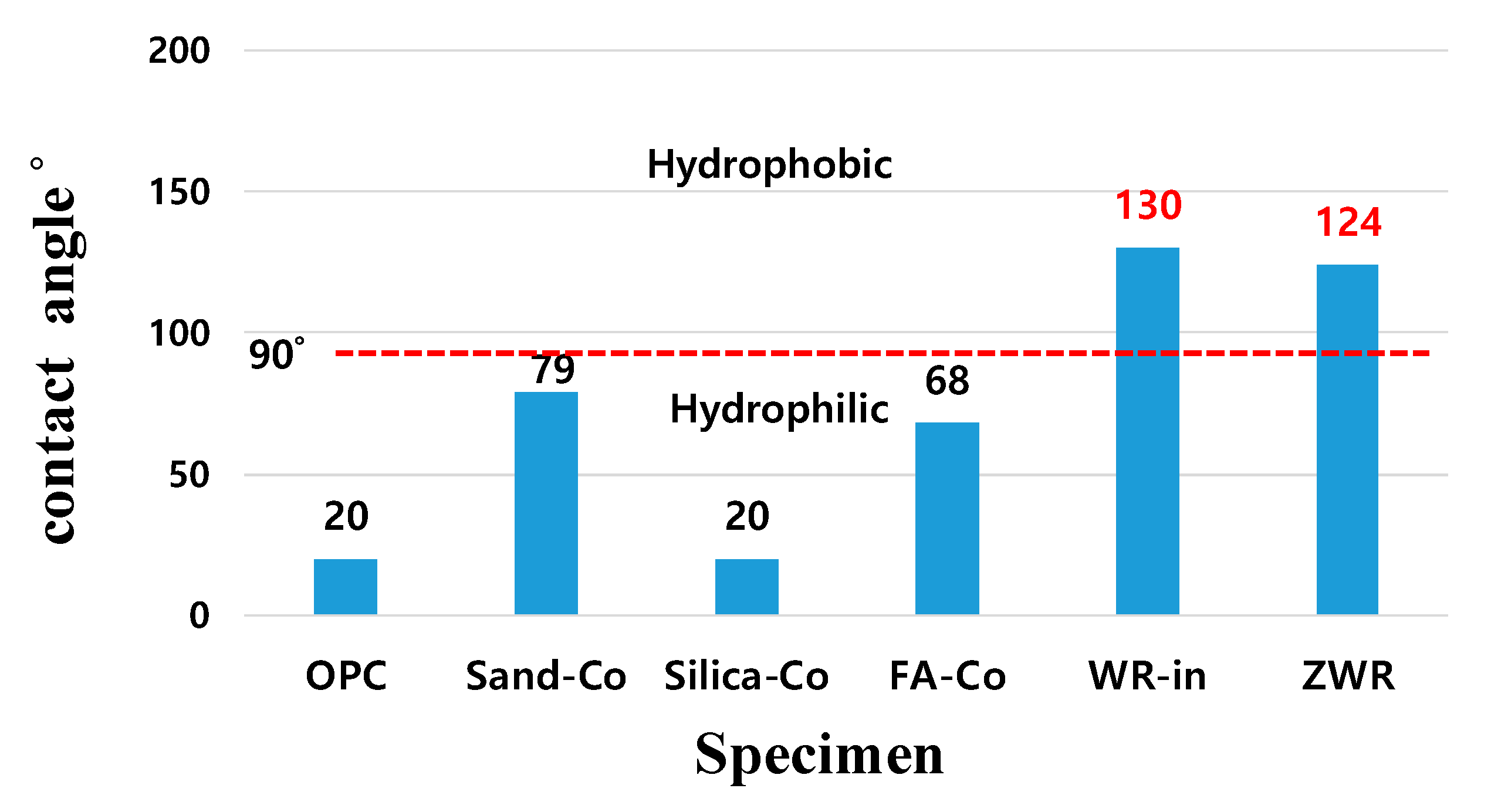
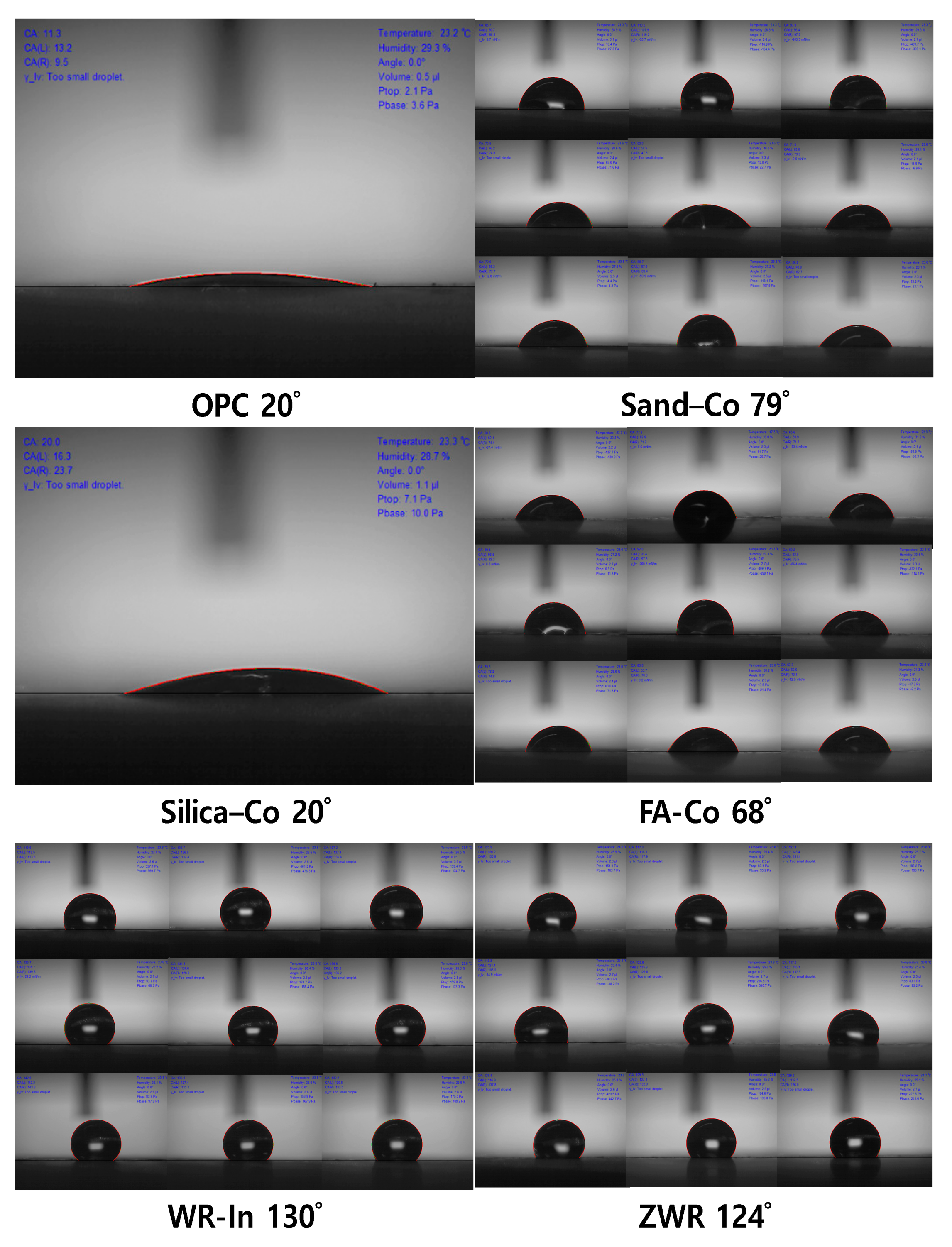
4.2. Contact Angle
4.3. Water Penetration
4.4. Result of Resistance of Concrete to Chloride Ion Penetration Test (RCPT)
4.5. Mercury Intrusion Porosimetry (MIP)
4.6. Scanning Electron Microscope (SEM)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoon, C.B.; Lee, H.S. Experimental Study on the Evaluation of Physical Performance and Durability of Cement Mortar Mixed with Water Repellent Impregnated Natural Zeolite. Materials 2020, 13, 3288. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.B.; Lee, M.S. An Experimental Study on Water Resistance of Penetrating Water Repellency of Emulsified Silicon Type Exposed to The Outdoor Environment. J. Korea Concr. Inst. 2004, 16, 477–484. [Google Scholar] [CrossRef]
- Kalaitzaki, P.M. Hydraulic lime mortars with siloxane for waterproofing historic masonry. Cem. Concr. Res. 2007, 37, 283–290. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Sudbrink, B.; Ley, M.T. Determining the effective service life of silane treatments in concrete bridge decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Müller, H.S.; Zhao, T. Influence of freeze-thaw cycles on capillary absorption and chloride penetration into concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, B.J.; Kim, J.S.; Kim, W.Y. Effect of Concrete Strength on Chloride Ion Penetration Resistance and Chemical Resistance of Concrete Coated by Siloxane-based Water Repellent. J. Korea Concr. Inst. 2018, 30, 583–590. [Google Scholar] [CrossRef]
- Namoulniara, K.; Mahieux, P.Y.; Lux, J.; Mokhtar, A.A.; Turcry, P. Efficiency of water repellent surface treatment: Experiments on low performance concrete and numerical investigation with pore network model. Constr. Build. Mater. 2019, 227, 116638. [Google Scholar] [CrossRef]
- Oh, B.H. Durability Design for Carbonation in Concrete Structures. J. Korea Concr. Inst. 2005, 5, 30–60. [Google Scholar]
- Lee, J.S. Comparative Study on Repellent Ability of Silane Repellent According to Type and Treatment Method. Master’s Thesis, University of Ulsan, Ulsan, South Korea, 2004. [Google Scholar]
- Lee, S.Y.; Nam, G.Y.; Kim, J.H. Effects of Water-Repellent on the Physical Properties of Water Paint. J. Korea Inst. Build. Constr. 2004, 14, 259–265. [Google Scholar] [CrossRef][Green Version]
- Folker, H. Effective Chloride Barrier for Reinforced Concrete Structures in Order to Extend the Service-Life. In Advances in Construction Materials; Springer: Berlin/ Heidelberg, Germany, 2007; pp. 427–437. [Google Scholar]
- Zhu, Y.G.; Kou, S.C.; Poon, C.S.; Dai, J.G.; Li, Q.Y. Influence of silane-based water repellent on the durability properties of recycled aggregate concrete. Cem. Concr. Compos. 2013, 35, 32–38. [Google Scholar] [CrossRef]
- Wittmann, F.H.; Xian, Y.Z.; Zhao, T.J.; Beltzung, F.; Giessler, S. Drying and shrinkage of integral water repellent concrete. Restor. Build. Monum. 2006, 12, 229–242. [Google Scholar] [CrossRef]
- Feng, H.; Le, H.T.N.; Wang, S.; Zhang, M.H. Effects of silanes and silane derivatives on cement hydration and mechanical properties of mortars. Constr. Build. Mater. 2016, 129, 48–60. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, F.; Zhao, T. Effects of surface modification of silane coupling agent on the properties of concrete with freeze-thaw damage. KSCE J. Civ. Eng. 2018, 22, 657–669. [Google Scholar] [CrossRef]
- Dai, J.G.; Akira, Y.; Wittmann, F.H.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- Oh, S.K.; Ahn, S.D.; Shim, S.M. A Study on Effect of Silane Solution of Capillary Coating Type as Protection Agent of Absorption for the Durability Improvement in Concrete Surface Layer. J. Archit. Inst. Korea Struct. Constr. 2001, 17, 149–158. [Google Scholar]
- Xian, Y.Z.; Wittmann, F.H.; Zhao, T.J.; Giessler, S. Chloride penetration into integral water repellent concrete. Restor. Build. Monum. 2007, 13, 17–24. [Google Scholar] [CrossRef]
- Wittmann, F.H.; Wittmann, A.D.A.; Wang, P.G. Capillary absorption of integral water repellent and surface impregnated concrete. Restor. Build. Monum. 2014, 20, 281–290. [Google Scholar]
- Korean Industrial Standards. KS L. 5105, Testing Method for Compressive Strength of Hydraulic Cement Mortar; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017. [Google Scholar]
- ASTM International. ASTM A C150, Standard Specification for Portland Cement; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- Korean Industrial Standards. KS L. 5405, Fly Ash; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2018. [Google Scholar]
- Korean Industrial Standards. KS F. 2567, Standard Specification on Silica Fume for Concrete; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2019. [Google Scholar]
- Korean Industrial Standards. KS E. 3076, Methods for X-ray Fluorescence Spectrometric Analysis of Silica Stone and Silica Sand; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017. [Google Scholar]
- Korean Industrial Standards. KS F. 2421, Standard Test Method for Air of Fresh Concrete by the Pressure Method (Air Receiver Method); Korean Industrial Standards: Chungcheongbuk-do, Korea, 2016. [Google Scholar]
- Korean Industrial Standards. KS F. 2405, Standard Test Method for Compressive Strength of Concrete; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017. [Google Scholar]
- Jung, Y.S. A Numerical Method for Determining Surface Tension of Sessile Drop. J. Korean Ceram. Soc. 1996, 33, 1325–1330. [Google Scholar]
- Vivian, I.F.; Hejazi, V.; Kozhukhova, M.I.; Nosonovsky, M.; Sobolev, K. Self-assembling particle-siloxane coatings for superhydrophobic concrete. ACS Appl. Mater. Interfaces 2013, 5, 13284–13294. [Google Scholar] [CrossRef]
- She, W.; Wang, X.; Miao, C.; Zhang, Q.; Zhang, Y.; Yang, J.; Hong, J. Biomimetic superhydrophobic surface of concrete: Topographic and chemical modification assembly by direct spray. Constr. Build. Mater. 2018, 181, 347–357. [Google Scholar] [CrossRef]
- Li, G.; Yue, J.; Guo, C.; Ji, Y. Influences of modified nanoparticles on hydrophobicity of concrete with organic film coating. Constr. Build. Mater. 2018, 169, 1–7. [Google Scholar] [CrossRef]
- Lange, A.; Hirata, T.; Plank, J. Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem. Concr. Res. 2014, 60, 45–50. [Google Scholar] [CrossRef]
- Korean Industrial Standards. KS F. 4919, Cement-Polymer Modified Waterproof Coationgs; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017. [Google Scholar]
- ASTM International. ASTM C 1202 Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. In Annual Book of American Society for Testing Materials Standards; ASTM International: West Conshohocken, PA, USA, 1993; Volume C04.02. [Google Scholar]
- Korean Industrial Standards. KS F. 2711, Standard Test Method for Resistance of Concrete to Chloride Ion Penetration by Electrical Conductance; Korean Industrial Standards: Chungcheongbuk-do, Korea, 2017. [Google Scholar]
- Cong, X.; Lu, S.; Gao, Y.; Yao, Y.; Elchalakani, M.; Shi, X. Effects of microwave, thermomechanical and chemical treatments of sewage sludge ash on its early-age behavior as supplementary cementitious material. J. Clean. Prod. 2020, 258, 120647. [Google Scholar] [CrossRef]


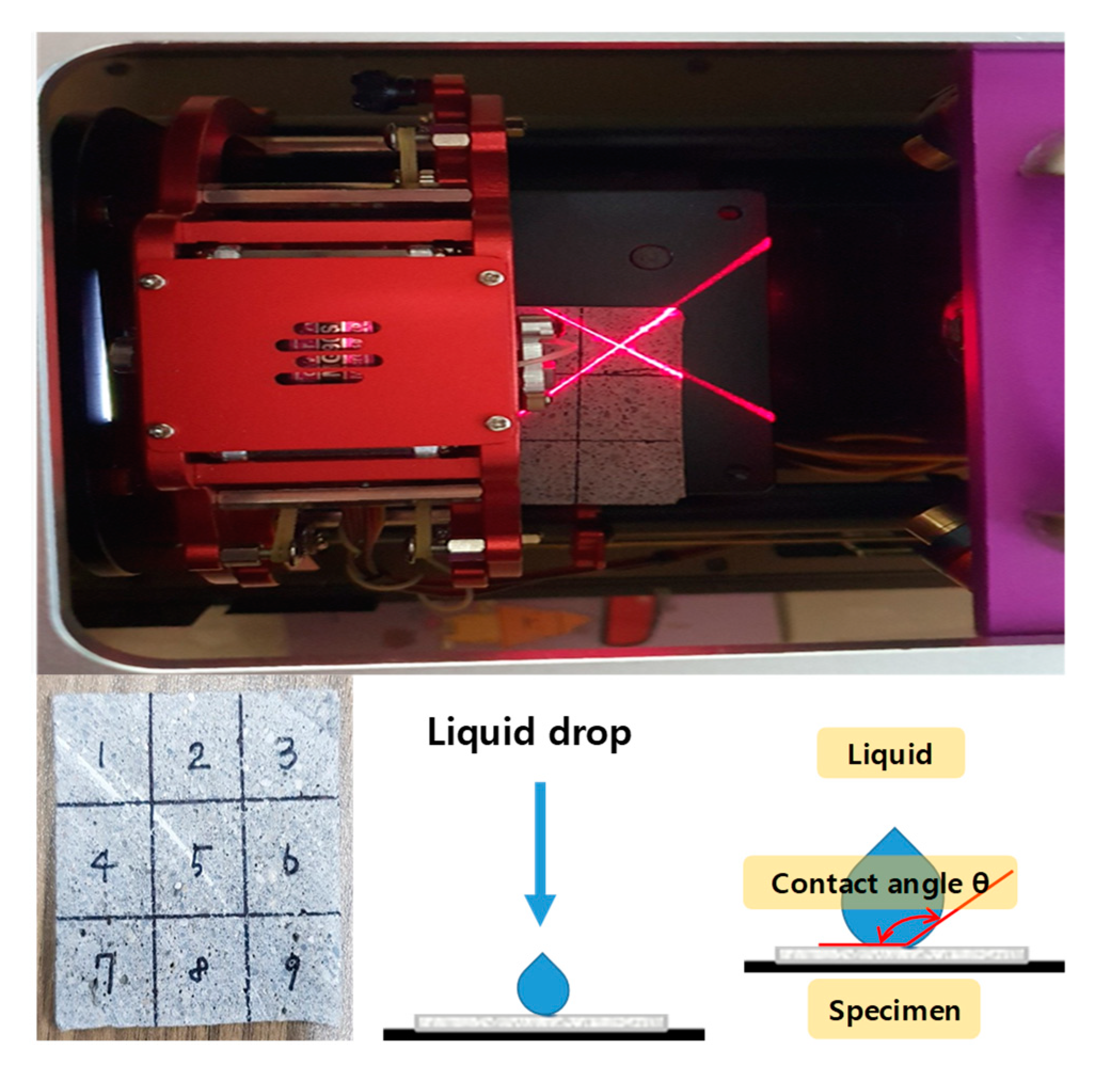

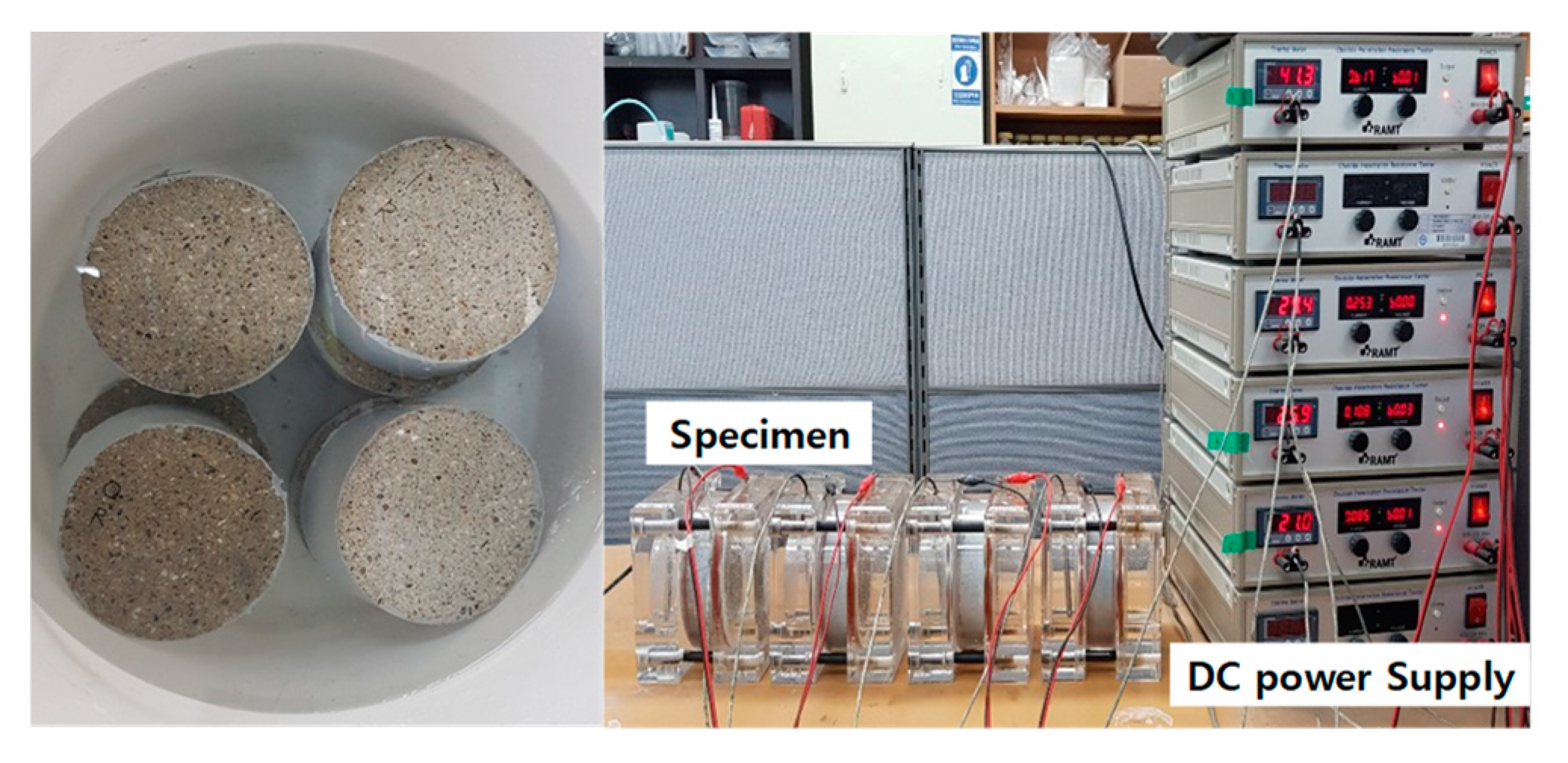





| Specimen | Experiment Level | ||
|---|---|---|---|
| Classifications | ||
| Method | Co | Water repellent coating | |
| In | Water repellent mortar inner | ||
| WR | Water repellent | ||
| ZWR | Natural Zeolite + water repellent | ||
| Impregnation | |||
| Curing Conditions | Under water, temperature 20 °C | ||
| Experiment | Physical test (compressive strength, air content, flow test, contact angle) | ||
| Durability test (water penetration, resistance chloride ion test, MIP) | |||
| Microstructures (scanning electron microscope (SEM)) | |||
| Name | Chemical Compositions (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | TiO2 | etc. | |
| OPC | 19.74 | 5.33 | 2.93 | 61.74 | 3.78 | 2.47 | 0.89 | 0.3 | 2.82 |
| FA | 52.6 | 21.4 | 9.20 | 5.01 | 2.01 | 0.27 | 1.13 | 8.38 | |
| SF | 92.3 | 1.4 | 0.6 | 0.5 | 0.3 | 4.9 | |||
| Name | Chemical Compositions (%) | ||||||
|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | Na2O | CEC | etc. | |
| Zeolite | 66.8 | 13.2 | 1.68 | 3.02 | 1.16 | 106 | 14.14 |
| Name | Chemical Compositions (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| As | Cd | Hg | Pb | Cr | Cu | Ni | Zn | |
| Limit | 20 | 2 | 1 | 50 | 90 | 120 | 20 | 400 |
| Result | 0.64 | 7.87 | 4.37 | 3.75 | 23.81 | |||
| Name | Chemical Compositions (%) | |||||
|---|---|---|---|---|---|---|
| Color | Kind | Effective Ratio (%) | Diluent | Freeze Stability | pH | |
| Water Repellent | White | Silane–Siloxane | 50 | Water | - | 12 |
| Name | W/B (%) | Unit Weight (kg/m3) | |||||
|---|---|---|---|---|---|---|---|
| W | C | S | Carrier + Active Ingredient = Admixture | Water Repellent Active Ingredient (%) | |||
| Zeolite | Active Ingredient | ||||||
| OPC | 50 | 255 | 510 | 1530 | - | - | |
| Sand–Co | 255 | 510 | 1530 | - | 25.5 | 5% | |
| Silica–Co | 280.5 | 510 | 1530 | 25.5 | 25.5 | 5% | |
| FA–Co | 280.5 | 510 | 1530 | 25.5 | 25.5 | 5% | |
| WR–In | 229.5 | 510 | 1530 | - | 25.5 | 5% | |
| ZWR | 280.5 | 510 | 1530 | 25.5 | 25.5 | 5% | |
| Surface Contact Angle (θ) | Permeability |
|---|---|
| >130° | Very good repellency |
| 110–130° | Good repellency |
| 90–110° | Slight wetting |
| 30–90° | Pronounced wetting |
| <30° | Surface completely wet |
| Coulombs (C) | Permeability |
|---|---|
| >4000 | High |
| 2000–4000 | Normal |
| 1000–2000 | Low |
| 100–1000 | Very low |
| <100 | Negligible |
| Name | Flow (mm) | Air Content (%) |
|---|---|---|
| OPC | 115 | 5 |
| Sand–Co | 180 | 11 |
| Silica–Co | 170 | 9.5 |
| FA–Co | 170 | 11 |
| WR–In | 160 | 12 |
| ZWR | 110 | 7.5 |
| Name | Compressive Strength (MPa) | Normalized Values OPC 28 Days (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 7 Days | 28 Days | ||||||||
| 1st | 2nd | 3rd | Average | 1st | 2nd | 3rd | Average | ||
| OPC | 26 | 27.5 | 28 | 27 | 36 | 35 | 37 | 36 | 100 |
| Sand–Co | 8 | 4 | 5 | 5 | 11 | 6 | 7 | 8 | 22.2 |
| Silica–Co | 13 | 19 | 15 | 16 | 15 | 21 | 17 | 18 | 50 |
| FA–Co | 16 | 12.5 | 13 | 14 | 18 | 12 | 16 | 15 | 41.6 |
| WR–In | 10 | 10 | 13 | 11 | 7 | 9 | 13 | 10 | 27.7 |
| ZWR | 25 | 25 | 23 | 24 | 30 | 29 | 32 | 30 | 83.3 |
| Name | Contact Angle (°) 28 Days | Normalized Values OPC 28 Days (%) | |||
|---|---|---|---|---|---|
| Measurement Average | |||||
| 1st | 2nd | 3rd | Total | ||
| OPC | - | 20 | - | 20 | 100 |
| Sand–Co | 85 | 80 | 72 | 79 | 395 |
| Silica–Co | 20 | - | - | 20 | 100 |
| FA–Co | 74 | 60 | 69 | 68 | 340 |
| WR–In | 136 | 130 | 133 | 133 | 665 |
| ZWR | 122 | 124 | 128 | 124 | 620 |
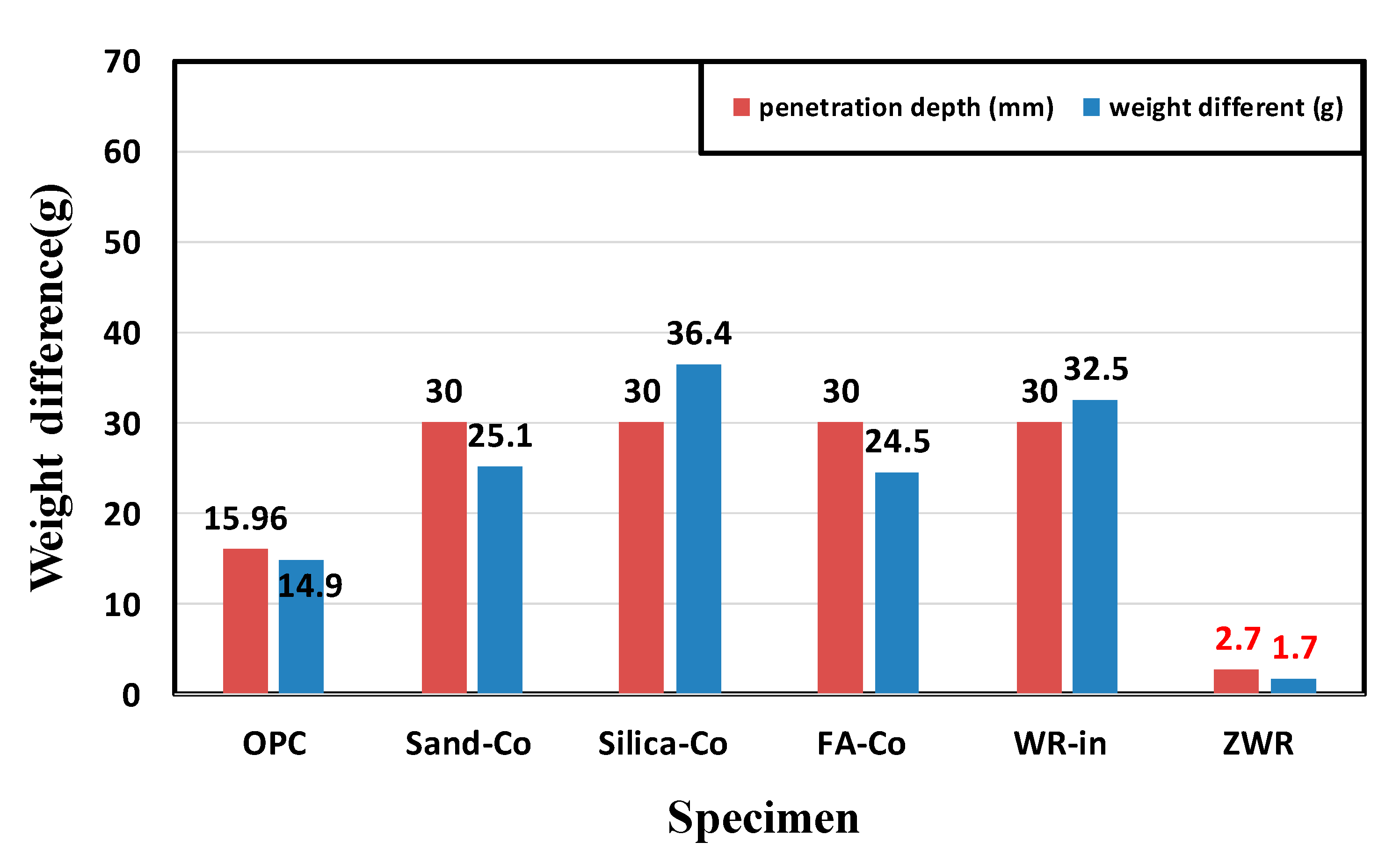
| Name. | Penetration Depth (mm) | Weight Difference (g) | Normalized Values OPC 28 Days Penetration Depth (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| - | 1st | 2nd | 3rd | Average | 1st | 2nd | 3rd | Average | - |
| OPC | 18.13 | 15.34 | 14.41 | 15.96 | 13.1 | 14.5 | 12.7 | 14.09 | 100 |
| Sand–Co | 30 | 30 | 30 | All penetration | 25.1 | 22.6 | 27.8 | 25.1 | 188 |
| Silica–Co | 30 | 30 | 30 | All penetration | 38.3 | 34.6 | 36.4 | 36.4 | 188 |
| FA–Co | 30 | 30 | 30 | All penetration | 27.5 | 21.7 | 24.3 | 24.5 | 188 |
| WR–In | 30 | 30 | 30 | All penetration | 31.5 | 31.6 | 34.3 | 32.5 | 188 |
| ZWR | 2.9 | 2.8 | 2.3 | 2.7 | 2.1 | 1.6 | 1.5 | 1.7 | 16.91 |
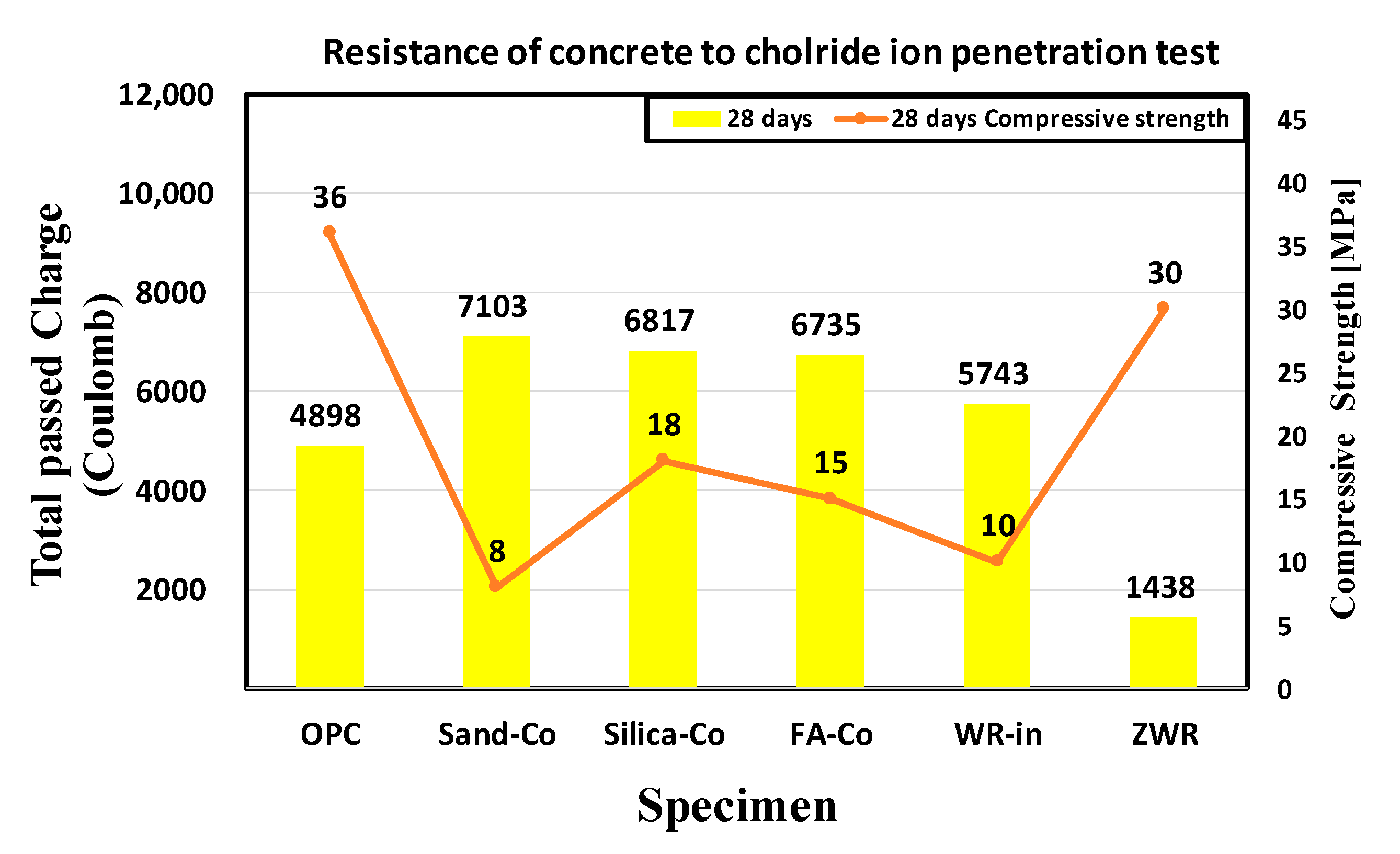
| Name | Coulombs | Penetration | Normalized Values OPC 28 Days Coulombs (%) | |||
|---|---|---|---|---|---|---|
| - | 1st | 2nd | 3rd | Average | ||
| OPC | 4298 | 5663 | 4735 | 4898 | High | 100.0 |
| Sand–Co | 6550 | 7414 | 7346 | 7103 | High | 145.0 |
| Silica–Co | 6289 | 6918 | 7244 | 6817 | High | 139.1 |
| FA–Co | 6641 | 6469 | 7094 | 6735 | High | 137.5 |
| WR–In | 6310 | 5242 | 5676 | 5743 | High | 117.2 |
| ZWR | 1382 | 1518 | 1416 | 1438 | Low | 29.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.B.; Lee, H.S. Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It. Appl. Sci. 2020, 10, 9097. https://doi.org/10.3390/app10249097
Yoon CB, Lee HS. Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It. Applied Sciences. 2020; 10(24):9097. https://doi.org/10.3390/app10249097
Chicago/Turabian StyleYoon, Chang Bok, and Han Seung Lee. 2020. "Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It" Applied Sciences 10, no. 24: 9097. https://doi.org/10.3390/app10249097
APA StyleYoon, C. B., & Lee, H. S. (2020). Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It. Applied Sciences, 10(24), 9097. https://doi.org/10.3390/app10249097






