Atmospheric Concentrations and Health Implications of PAHs, PCBs and PCDD/Fs in the Vicinity of a Heavily Industrialized Site in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling Procedure
2.2. Materials
2.3. Sample Extraction and Clean Up
2.4. Instrumental Analysis and Quality Assurance
2.5. Statistical Analysis
2.6. Health Risk Estimation
3. Results and Discussion
3.1. Results
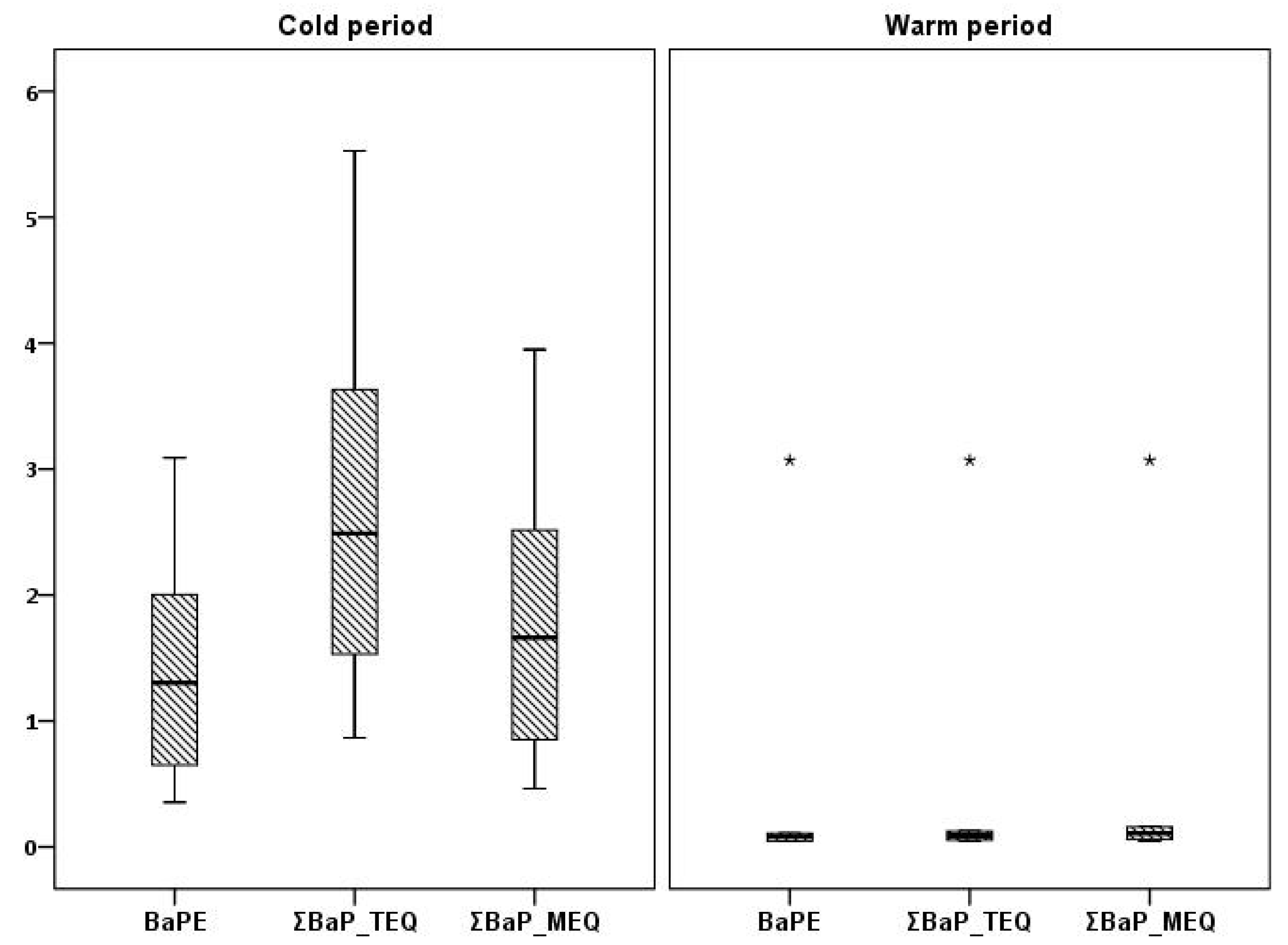
3.1.1. PAHs and Indpcbs
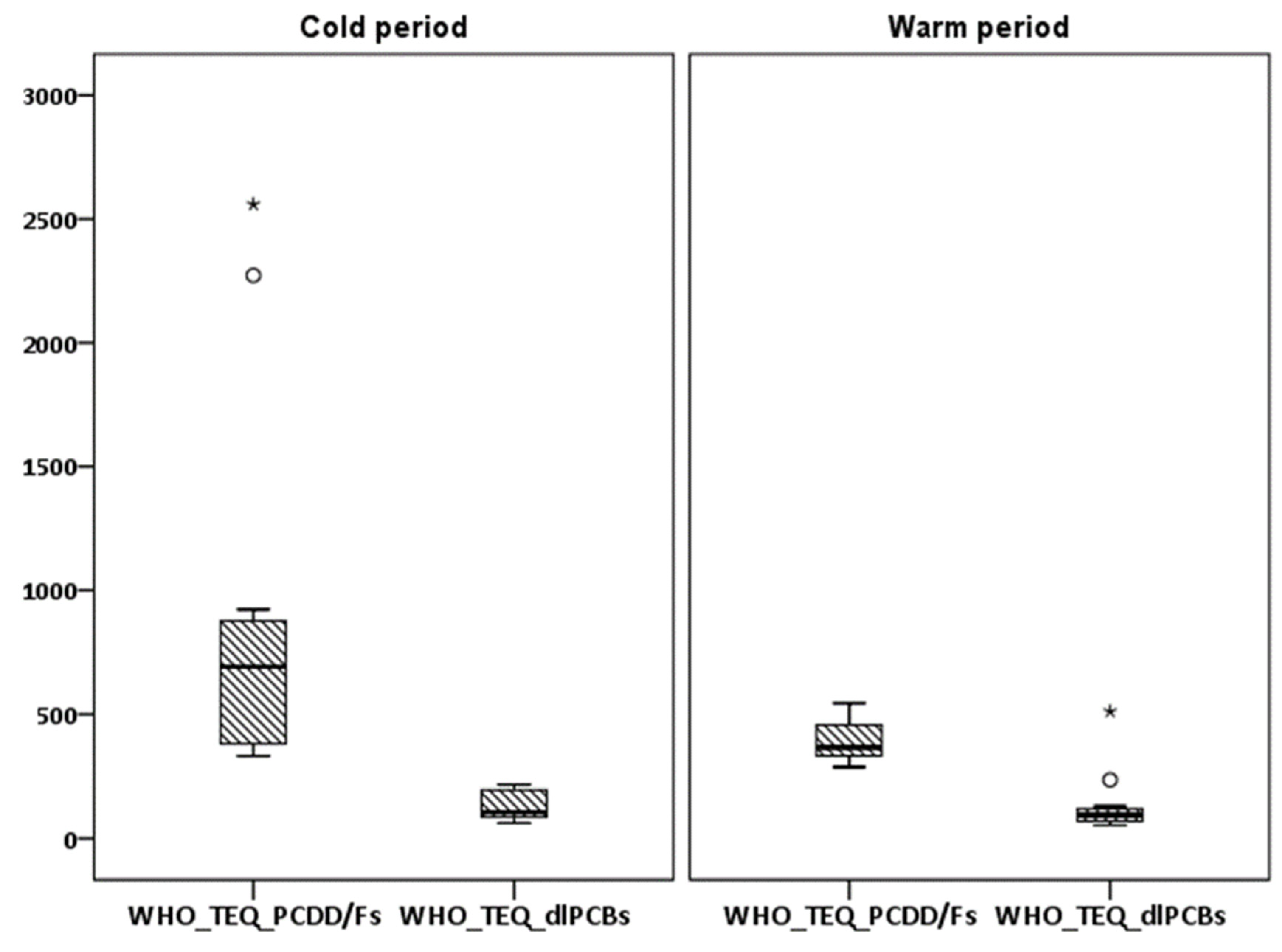
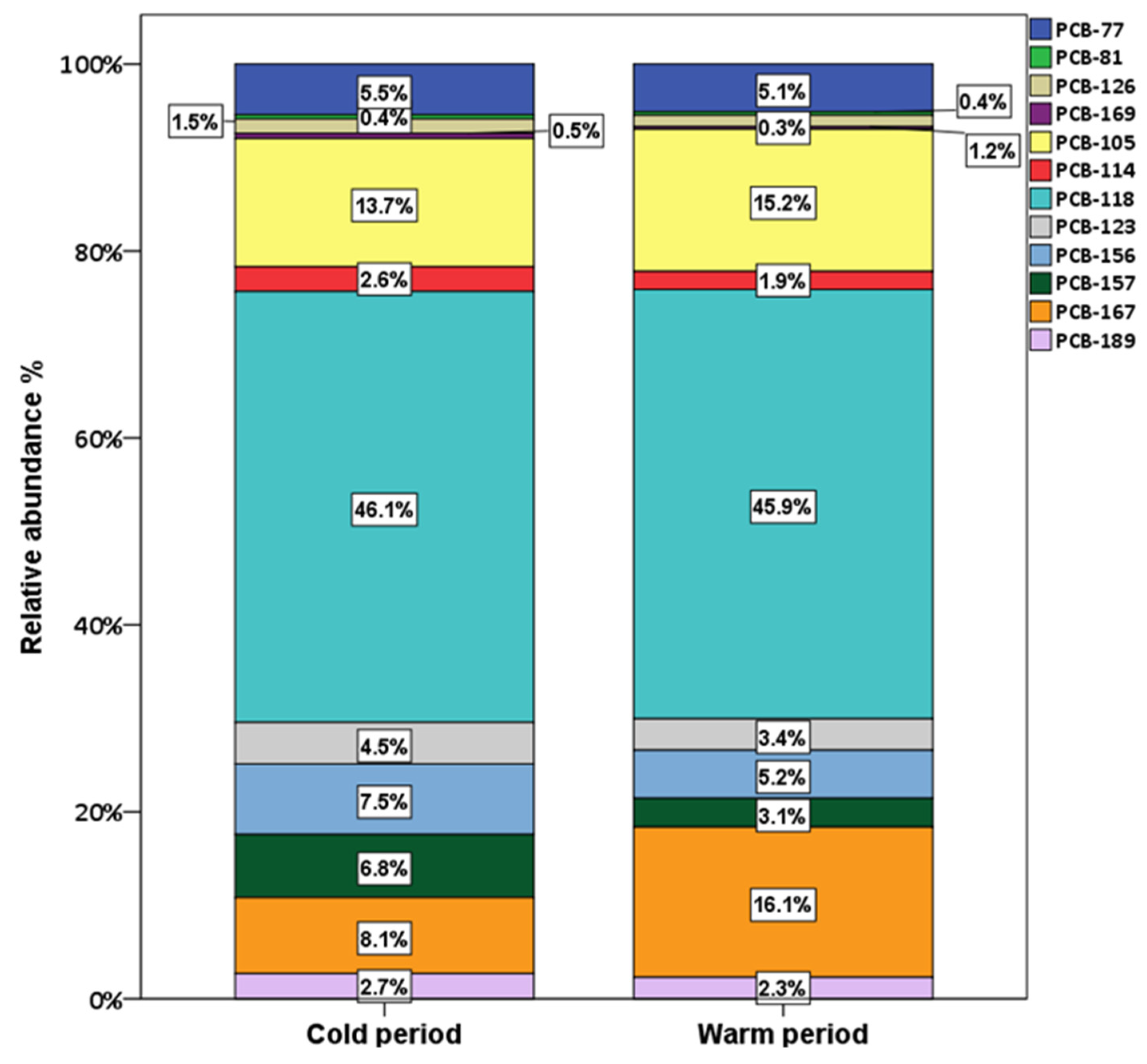
3.1.2. PCDD/Fs and dlPCBs
| Area | Area Description | Sampling (Year/Phase) | ΣPCDD/Fs (fg m−3) | WHO (TEQ fg m−3) | ΣdlPCBs | WHO (TEQ fg m−3) | ΣindPCBs (pg m−3) | ΣPAHs (ng m−3) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Eleusis, Greece | Industrial | 2018–2019/P | 145–3472 | 287–2560 | 975–4083 | 53.1–512 | 22.9–53.2 | 1.3–16.5 | This study |
| Athens, Greece | Background/Urban | 2000/P | 73–462 (P) | 73 (P + G) | 1.79–4.94 | [32] | |||
| Thessaloniki, Greece | Semirural/Urban | 1999/P | 150–12,890 | 4–119 | 0.5–29.2 | [53] | |||
| Porto, Portugal | Urban/Industrial | 1999–2004/P + G | 200–15,000 | 9.8–817 | [63] | ||||
| Rome, Italy | Urban | 2000–2001/P | 5.43–734 | 0.66–7.28 | 88.9–372 | [54] | |||
| Steel Complex, Korea | Industrial | 2006/G | 6100–61,800 | [61] | |||||
| Anshan, China | Industrial | 2008/G | 20–9790 | 4560 | 0.3–23 | [38] | |||
| Satellite cities of Seoul, Korea | Industrial | 2003–2009/P + G | 360–55,755 | 310–3143 | 678–40,968 | 200–1712 | [64] | ||
| Brescia, Italy | Urban/Industrial | /P | 380–11,390 | 10–190 | 11,600–708,400 | 4–130 | 92.7–8566 | [23] | |
| Brno, Czech Republic | Urban | 2009–2010/P | 4.3–661- | 14.2–614 | 0.04–14.4 | [56] | |||
| Aliaga, Turkey | Industrial | 2009–2010/G | 1.6–838 | [21] | |||||
| Shanghai, China | Industrial | 2013/P + G | 258–4928 | 9.28–423 | 340–7607 | 1.08–55.9 | [55] | ||
| Dunkirk, France | Industrial | 2008–2009/P | 718–1070 | 12,990–25,430 | 744–1747 | 0.29–1.95 | [31] | ||
| Istanbul, Turkey | Industrial | 2011–2012/P + G | 0.7–27.9 | 0.04–445 | [39] | ||||
| Umm Al-Aish, Kuwait | Industrial | 2014–2015/P + G | 31.2–516 | 3.9–36.8 | [20] | ||||
| Hochiminh, Vietnam | Urban | 2016–2017/P + G | 410–52,700 | 22.2–1530 | 1570–8300 | 1.77–44.1 | [62] | ||
| Tibet- Qinghai Plateau, China | Industrial | 2015/G– | 1180–2180 | 113–242 | 490–900 | 3.18–7.12 | [4] | ||
| Jiangsu, China | Industrial | 2018/P + G | 11,900–57,700 | 136–597 | 580–2710 | 0.64–11.3 | 11–18 | [16] |
3.2. Seasonal Variations and Congeners’ Contributions
3.3. Source Apportionment
3.4. Health Risk Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El-Shahawi, M.; Hamza, A.; Bashammakh, A.; Al-Saggaf, W. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Sremacki, M.M.; Radonic, J.; Mihajlovic, I.; Obrovski, B.; Miloradov, M.V. Health risk assessment of PAHs, PCBs and OCPs in atmospheric air of municipal solid waste landfill in Novi Sad, Serbia. Sci. Total Environ. 2018, 644, 1201–1206. [Google Scholar] [CrossRef]
- Weber, R.; Watson, A.; Forter, M.; Oliaei, F. Review Article: Persistent organic pollutants and landfills—A review of past experiences and future challenges. Waste Manag. Res. 2011, 29, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, J.; Xu, C.; Zha, X.; Hua, Y.; Yang, L.; Jin, J. Preliminary investigation of polychlorinated dibenzo-p-dioxin and dibenzofuran, polychlorinated naphthalene, and dioxin-like polychlorinated biphenyl concentrations in ambient air in an industrial park at the northeastern edge of the Tibet–Qinghai Plateau, China. Sci. Total. Environ. 2019, 648, 935–942. [Google Scholar] [PubMed]
- [UNEP] United Nations Environment Program. Persistant Organic Pollutants (POPs). 2017. Available online: www.unenvironment.org/explore-topics/chemicals-waste/what-wedo/persistent-organic-pollutants/why-do-persistent-organic (accessed on 28 October 2020).
- Zheng, G.J.; Leung, A.O.; Jiao, L.P.; Wong, M.H. Polychlorinated dibenzo-p-dioxins and dibenzofurans pollution in China: Sources, environmental levels and potential human health impacts. Environ. Int. 2008, 34, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Kedikoglou, K.; Costopoulou, D.; Vassiliadou, I.; Bakeas, E.; Leondiadis, L. An effective and low cost carbon based clean-up method for PCDD/Fs and PCBs analysis in food. Chemosphere 2018, 206, 531–538. [Google Scholar] [CrossRef]
- Someya, M.; Ohtake, M.; Kunisue, T.; Subramanian, A.; Takahashi, S.; Chakraborty, P.; Ramachandran, R.; Tanabe, S. Persistent organic pollutants in breast milk of mothers residing around an open dumping site in Kolkata, India: Specific dioxin-like PCB levels and fish as a potential source. Environ. Int. 2010, 36, 27–35. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Haritash, A.; Kaushik, C. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- De Craemer, S.; Croes, K.; Van Larebeke, N.; Sioen, I.; Schoeters, G.; Loots, I.; Nawrot, T.; Nelen, V.; Campo, L.; Fustinoni, S.; et al. Investigating unmetabolized polycyclic aromatic hydrocarbons in adolescents’ urine as biomarkers of environmental exposure. Chemosphere 2016, 155, 48–56. [Google Scholar] [CrossRef]
- ATSDR Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; US Department of Health and Human Services: Atlanta, GA, USA, 1995.
- Boström, C.-E.; Gerde, P.; Hanberg, A.; Jernström, B.; Johansson, C.; Kyrklund, T.; Rannug, A.; Törnqvist, M.; Victorin, K.; Westerholm, R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ. Health Perspect. 2002, 110 (Suppl. 3), 451–488. [Google Scholar] [CrossRef]
- IARC Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2010.
- Vassiliadou, I.; Papadopoulos, A.; Costopoulou, D.; Vasiliadou, S.; Christoforou, S.; Leondiadis, L. Dioxin contamination after an accidental fire in the municipal landfill of Tagarades, Thessaloniki, Greece. Chemosphere 2009, 74, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, J.; Xu, S.; Liu, G.-B.; Tu, Y.; Shi, P.; Li, A. Concentrations, Sources, and Potential Human Health Risks of PCDD/Fs, dl-PCBs, and PAHs in Rural Atmosphere Around Chemical Plants in Jiangsu Province, China. Bull. Environ. Contam. Toxicol. 2020, 104, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.-D.; Tsai, C.-L.; Hsu, Y.-C.; Chen, Y.-W.; Weng, Y.-M.; Chang, M.B. PCDD/Fs and dl-PCBs concentrations in water samples of Taiwan. Chemosphere 2017, 173, 603–611. [Google Scholar] [CrossRef]
- Wu, J.; Hu, J.; Wang, S.; Jin, J.; Wang, R.; Wang, Y.; Jin, J. Levels, sources, and potential human health risks of PCNs, PCDD/Fs, and PCBs in an industrial area of Shandong Province, China. Chemosphere 2018, 199, 382–389. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhan, J.; Liu, G.; Ren, Z.; Zheng, M.; Jin, R.; Yang, L.; Wang, M.; Jiang, X.; Zhang, X. Field study and theoretical evidence for the profiles and underlying mechanisms of PCDD/F formation in cement kilns co-incinerating municipal solid waste and sewage sludge. Waste Manag. 2017, 61, 337–344. [Google Scholar] [CrossRef]
- Martínez-Guijarro, K.; Ramadan, A.; Gevao, B. Atmospheric concentration of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dl-PCBs) at Umm-Al-Aish oil field-Kuwait. Chemosphere 2017, 168, 147–154. [Google Scholar] [CrossRef]
- Aydin, Y.M.; Kara, M.; Dumanoglu, Y.; Odabasi, M.; Elbir, T. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in ambient air of an industrial region in Turkey. Atmos. Environ. 2014, 97, 271–285. [Google Scholar] [CrossRef]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Colombo, A.; Benfenati, E.; Bugatti, S.G.; Lodi, M.; Mariani, A.; Musmeci, L.; Rotella, G.; Senese, V.; Ziemacki, G.; Fanelli, R. PCDD/Fs and PCBs in ambient air in a highly industrialized city in Northern Italy. Chemosphere 2013, 90, 2352–2357. [Google Scholar] [CrossRef]
- Frignani, M.; Bellucci, L.G.; Carraro, C.; Raccanelli, S. Polychlorinated biphenyls in sediments of the Venice Lagoon. Chemosphere 2001, 43, 567–575. [Google Scholar] [CrossRef]
- World Health Organization. Health Risks of Persistent Organic Pollutants from Long-Range Transboundary Air Pollution. 2003. Available online: http://www.euro.who.int (accessed on 28 October 2020).
- World Health Organization. Persistent Organic Pollutants: Impact on Child Health; WHO Press: Geneva, Switzerland, 2010; ISBN 978 92 4 150110 1. [Google Scholar]
- Koukoulakis, K.; Kanellopoulos, P.; Chrysochou, E.; Koukoulas, V.; Minaidis, M.; Maropoulos, G.; Nikoleli, G.-P.; Bakeas, V.B. Leukemia and PAHs levels in human blood serum: Preliminary results from an adult cohort in Greece. Atmos. Pollut. Res. 2020, 11, 1552–1565. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARC Monogr. Eval. Carcinog. Risks Chem. Hum. 2002, 82, i-vi+1-551. [Google Scholar]
- IARC. Benzo[a]pyrene. IARC Monogr. 100F. 2012. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100F-14.pdf (accessed on 28 October 2020).
- IARC. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Polychlorinated biphenyls and polybrominated biphenyls. IARC Monogr. Eval. Carcinog. Risks Chem. Hum. 2016, 107, 1–501. [Google Scholar]
- Cazier, F.; Genevray, P.; Dewaele, D.; Nouali, H.; Verdin, A.; LeDoux, F.; Hachimi, A.; Courcot, L.; Billet, S.; Bouhsina, S.; et al. Characterisation and seasonal variations of particles in the atmosphere of rural, urban and industrial areas: Organic compounds. J. Environ. Sci. 2016, 44, 45–56. [Google Scholar] [CrossRef]
- Mandalakis, M.; Tsapakis, M.; Tsoga, A.; Stephanou, E.G. Gas–particle concentrations and distribution of aliphatic hydrocarbons, PAHs, PCBs and PCDD/Fs in the atmosphere of Athens (Greece). Atmos. Environ. 2002, 36, 4023–4035. [Google Scholar] [CrossRef]
- Lee, R.G.M.; Jones, K.C. Gas–particle partitioning of atmospheric PCDD/Fs: Measurements and observations on modelling. Environ. Sci. Technol. 1999, 33, 3596–3604. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.G. Regional measurements of PCDD/PCDF concentrations in Korean atmosphere and comparison with gas–particle partitioning models. Chemosphere 2002, 49, 755–764. [Google Scholar] [CrossRef]
- Koukoulakis, K.; Chrysohou, E.; Kanellopoulos, P.; Karavoltsos, S.; Katsouras, G.; Dassenakis, M.; Nikolelis, D.; Bakeas, V.B. Trace elements bound to airborne PM10 in a heavily industrialized site nearby Athens: Seasonal patterns, emission sources, health implications. Atmos. Pollut. Res. 2019, 10, 1347–1356. [Google Scholar] [CrossRef]
- Kanellopoulos, P.G.; Verouti, E.; Chrysochou, E.; Koukoulakis, K.; Bakeas, V.B. Primary and secondary organic aerosol in an urban/industrial site: Sources, health implications and the role of plastic enriched waste burning. J. Environ. Sci. 2021, 99, 222–238. [Google Scholar] [CrossRef]
- Kanellopoulos, P.G.; Chrysochou, E.; Koukoulakis, K.; Vasileiadou, E.; Kizas, C.; Savvides, C.; Bakeas, V.B. Polar organic compounds in PM10 and PM2.5 atmospheric aerosols from a background Eastern Mediterranean site during the winter period: Secondary formation, distribution and source apportionment. Atmos. Environ. 2020, 237, 117622. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Zhang, Q.; Wang, P.; Yang, H.; Jiang, G.; Wei, F. Evaluation of atmospheric sources of PCDD/Fs, PCBs and PBDEs around a steel industrial complex in northeast China using passive air samplers. Chemosphere 2011, 84, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Ercan, Ö.; Dincer, F. Atmospheric concentrations of PCDD/Fs, PAHs, and metals in the vicinity of a cement plant in Istanbul. Air Qual. Atmos. Health 2015, 9, 159–172. [Google Scholar] [CrossRef]
- Jung, K.H.; Yan, B.; Chillrud, S.N.; Perera, F.P.; Whyatt, R.M.; Camann, D.; Kinney, P.L.; Miller, R.K. Assessment of Benzo(a)pyrene-equivalent Carcinogenicity and Mutagenicity of Residential Indoor versus Outdoor Polycyclic Aromatic Hydrocarbons Exposing Young Children in New York City. Int. J. Environ. Res. Public Health 2010, 7, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.M.; Teinilä, K.; Custódio, D.; Santos, A.G.; Xian, H.; Hillamo, R.; Alves, C.; De Andrade, J.B.; Da Rocha, G.O.; Kumar, P.; et al. Particulate pollutants in the Brazilian city of São Paulo: 1-year investigation for the chemical composition and source apportionment. Atmos. Chem. Phys. Discuss. 2017, 17, 11943–11969. [Google Scholar] [CrossRef]
- Manoli, E.; Kouras, A.; Karagkiozidou, O.; Argyropoulos, G.; Voutsa, D.; Samara, C. Polycyclic aromatic hydrocarbons (PAHs) at traffic and urban background sites of northern Greece: Source apportionment of ambient PAH levels and PAH-induced lung cancer risk. Environ. Sci. Pollut. Res. 2016, 23, 3556–3568. [Google Scholar] [CrossRef] [PubMed]
- US EPA. United States Environmental Protection Agency (USEPA) Risk-Based Concentration Table 2012. Available online: http://www.epa.gov/reg3hwmd/risk/human/index.htm (accessed on 28 October 2020).
- US EPA. Human Health Risk Assessment Protocol for Hazardous Waste Combustion Facilities; U.S. Environmental Protection Agency: Washington, DC, USA, 1998.
- Čupr, P.; Flegrová, Z.; Franců, J.; Landlová, L.; Klánová, J. Mineralogical, chemical and toxicological characterization of urban air particles. Environ. Int. 2013, 54, 26–34. [Google Scholar] [CrossRef]
- OEHHA. Air Toxics Hot Spots Program Risk Assessment Guidelines. Part II: Technical Support Document for Describing Available Cancer Potency Factors; Office of Environmental Health Hazard Assessment: Sacramento, CA, USA, 2002.
- Mantis, J.; Chaloulakou, A.; Samara, C. PM10-bound polycyclic aromatic hydrocarbons (PAHs) in the Greater Area of Athens, Greece. Chemosphere 2005, 59, 593–604. [Google Scholar] [CrossRef]
- Manoli, E.; Chelioti-Chatzidimitriou, A.; Karageorgou, K.; Kouras, A.; Voutsa, D.; Samara, C.; Kampanos, I. Polycyclic aromatic hydrocarbons and trace elements bounded to airborne PM10 in the harbor of Volos, Greece: Implications for the impact of harbor activities. Atmos. Environ. 2017, 167, 61–72. [Google Scholar] [CrossRef]
- Vasilakos, C.; Veros, D.; Michopoulos, J.; Maggos, T.; O’Connor, C. Estimation of selected heavy metals and arsenic in PM10 aerosols in the ambient air of the Greater Athens Area, Greece. J. Hazard. Mater. 2007, 140, 389–398. [Google Scholar] [CrossRef]
- Directive, C. 107/EC of the European Parliament and of the Council of 15 December 2004: Relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air (Fourth Daughter Directive). Off. J. Eur. Communities 2004, 26, 3–6. [Google Scholar]
- Directive, E. 50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off. J. Eur. Union 2008, 11, 1–44. [Google Scholar]
- Cheng, J.; Yuan, T.; Wu, Q.; Zhao, W.; Xie, H.; Ma, Y.; Ma, J.; Wang, W. PM10-bound Polycyclic Aromatic Hydrocarbons (PAHs) and Cancer Risk Estimation in the Atmosphere Surrounding an Industrial Area of Shanghai, China. Water Air Soil Pollut. 2007, 183, 437–446. [Google Scholar] [CrossRef]
- Kouimtzis, T.; Samara, C.; Voutsa, D.; Balafoutis, C.; Müller, L. PCDD/Fs and PCBs in airborne particulate matter of the greater Thessaloniki area, N. Greece. Chemosphere 2002, 47, 193–205. [Google Scholar] [CrossRef]
- Menichini, E.; Iacovella, N.; Monfredini, F.; Turrio-Baldassarri, L. Atmospheric pollution by PAHs, PCDD/Fs and PCBs simultaneously collected at a regional background site in central Italy and at an urban site in Rome. Chemosphere 2007, 69, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Die, Q.; Nie, Z.; Liu, F.; Tian, Y.; Fang, Y.; Gao, H.; Tian, S.; He, J.; Huang, Q. Seasonal variations in atmospheric concentrations and gas-particle partitioning of PCDD/Fs and dioxin-like PCBs around industrial sites in Shanghai, China. Atmos. Environ. 2015, 119, 220–227. [Google Scholar] [CrossRef]
- Degrendele, C.; Okonski, K.; Melymuk, L.; Landlová, L.; Kukučka, P.; Čupr, P.; Klánová, J. Size specific distribution of the atmospheric particulate PCDD/Fs, dl-PCBs and PAHs on a seasonal scale: Implications for cancer risks from inhalation. Atmos. Environ. 2014, 98, 410–416. [Google Scholar] [CrossRef]
- Muñoz-Arnanz, J.; Roscales, J.L.; Vicente, A.; Ros, M.; Barrios, L.; Morales, L.; Abad, E.; Jiménez, B. Assessment of POPs in air from Spain using passive sampling from2008 to 2015. Part II: Spatial and temporal observations of PCDD/Fs and dl-PCBs. Sci. Total Environ. 2018, 634, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R.; Jones, K.C. Dioxins and furans in air and deposition: A review of levels, behaviour and processes. Sci. Total Environ. 1998, 219, 53–81. [Google Scholar] [CrossRef]
- Government of Germany Report of the Federal Immission Control Committee (in German). 2004. Available online: https://www.lai-immissionsschutz.de/servlet/is/20170/LAI_Schutz_Publikum_Schalleinwirkungen_Anhaenge.pdf?command=downloadContent&filename=LAI_Schutz_Publikum_Schalleinwirkungen_Anhaenge.pdf (accessed on 28 October 2020).
- Government of Japan. Information Brochure Dioxins. 2012. Available online: https://www.env.go.jp/en/chemi/dioxins/brochure2012.pdf (accessed on 28 October 2020).
- Choi, S.D.; Baek, S.Y.; Chang, Y.S. Atmospheric levels and distribution of dioxin-like polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in the vicinity of an iron and steel making plant. Atmos. Environ. 2008, 42, 2479–2488. [Google Scholar] [CrossRef]
- Trinh, M.M.; Tsai, C.L.; Hien, T.T.; Thuan, N.T.; Chi, K.H.; Lien, C.G.; Chang, M.B. Atmospheric concentrations and gas-particle partitioning of PCDD/Fs and dioxin-like PCBs around Hochiminh city. Chemosphere 2018, 202, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, M.; Pereira, M.; Borrego, C. Monitoring of ambient air PCDD/F levels in Portugal. Chemosphere 2007, 67, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Lee, M.; Kim, D.; Heo, J. Annual and seasonal variations in atmospheric PCDDs/PCDFs and dioxin-like PCBs levels in satellite cities of Seoul, Korea during 2003–2009. Atmos. Environ. 2013, 77, 222–230. [Google Scholar] [CrossRef]
- Finardi, S.; Radice, P.; Cecinato, A.; Gariazzo, C.; Gherardi, M.; Romagnoli, P. Seasonal variation of PAHs concentration and source attribution through diagnostic ratios analysis. Urban Clim. 2017, 22, 19–34. [Google Scholar] [CrossRef]
- Pateraki, S.; Fameli, K.M.; Assimakopoulos, V.; Bougiatioti, A.; Maggos, T.; Mihalopoulos, N. Levels, sources and health risk of PM2.5 and PM1-bound PAHs across the Greater Athens Area: The role of the type of environment and the meteorology. Atmosphere 2019, 10, 622. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Sweetman, A.J.; Jones, K.C. PAH molecular diagnostic ratios applied to atmospheric sources: A critical evaluation using two decades of source inventory and air concentration data from the UK. Environ. Sci. Technol. 2011, 45, 8897–8906. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. PAH diagnostic ratios for the identification of pollution emission sources. Environ. Pollut. 2012, 162, 110–119. [Google Scholar] [CrossRef]
- Duarte-Davidson, R.; Stewart, A.; Alcock, R.E.; Cousins, I.T.; Jones, K.C. Exploring the balance between sources, deposition, and the environmental burden of PCDD/Fs in the U.K. terrestrial environment: An aid to identifying uncertainties and research needs. Environ. Sci. Technol. 1997, 31, 1–11. [Google Scholar] [CrossRef]
- Motelay-Massei, A.; Harner, T.; Shoeib, M.; Diamond, M.; Stern, G.; Rosenberg, B. Using passive air samplers to assess urban–rural trends for persistent organic pollutants and polycyclic aromatic hydrocarbons. 2. Seasonal trends for PAHs, PCBs, and organochlorine pesticides. Environ. Sci. Technol. 2005, 39, 5763–5773. [Google Scholar] [CrossRef]
- Baek, S.Y.; Choi, S.D.; Park, H.; Kang, J.H.; Chang, Y.S. Spatial and seasonal distribution of polychlorinated biphenyls (PCBs) in the vicinity of an iron and steel making plant. Environ. Sci. Technol. 2010, 44, 3035–3040. [Google Scholar] [CrossRef]
- Cleverly, D.; Ferrario, J.; Byrne, C.; Riggs, K.; Joseph, D.; Hartford, P. A general indication of the contemporary background levels of PCDDs, PCDFs, and coplanar PCBs in the ambient air over rural and remote areas of the United States. Environ. Sci. Technol. 2007, 41, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Ho, K.F.; Wang, X.M.; Zou, S.C. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos. Environ. 2003, 37, 5307–5317. [Google Scholar] [CrossRef]
- Sienra, M.D.R.; Rosazza, N.G.; Préndez, M. Polycyclic aromatic hydrocarbons and their molecular diagnostic ratios in urban atmospheric respirable particulate matter. Atmos. Res. 2005, 75, 267–281. [Google Scholar] [CrossRef]
- Xu, M.X.; Yan, J.H.; Lu, S.Y.; Li, X.D.; Chen, T.; Ni, M.J.; Dai, H.F.; Wang, F.; Cen, K.F. Concentrations, profiles, and sources of atmospheric PCDD/Fs near a municipal solid waste incinerator in Eastern China. Environ. Sci. Technol. 2009, 43, 1023–1029. [Google Scholar] [CrossRef]
- Yang, H.H.; Chen, C.M. Emission inventory and sources of polycyclic aromatic hydrocarbons in the atmosphere at a suburban area in Taiwan. Chemosphere 2004, 56, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Masiol, M.; Hofer, A.; Squizzato, S.; Piazza, R.; Rampazzo, G.; Pavoni, B. Carcinogenic and mutagenic risk associated to airborne particle-phase polycyclic aromatic hydrocarbons: A source apportionment. Atmos. Environ. 2012, 60, 375–382. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Karakitsios, S.P.; Zikopoulos, D.; Nikolaki, S.; Kermenidou, M. Lung cancer risk from PAHs emitted from biomass combustion. Environ. Res. 2015, 137, 147–156. [Google Scholar] [CrossRef]

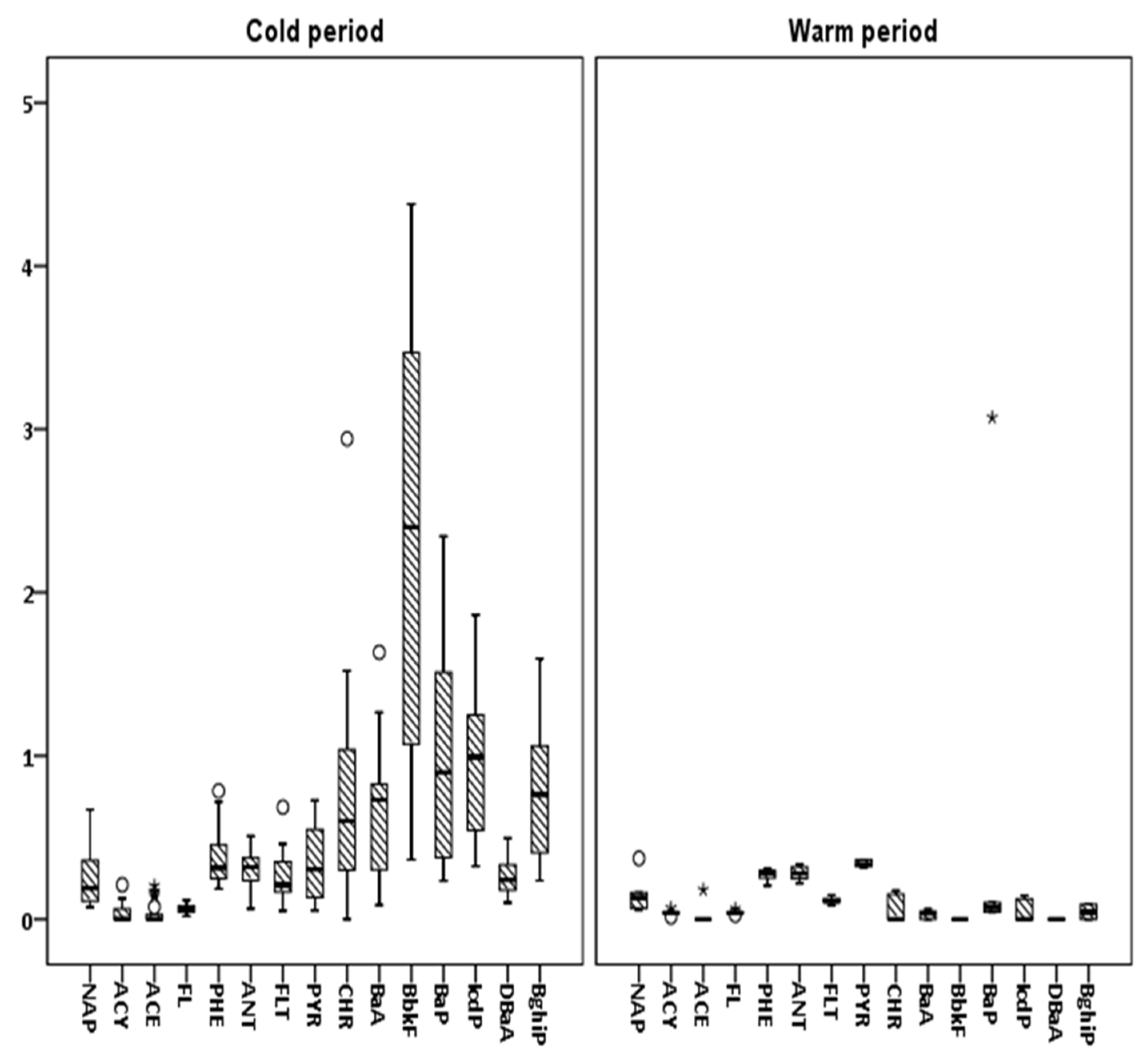
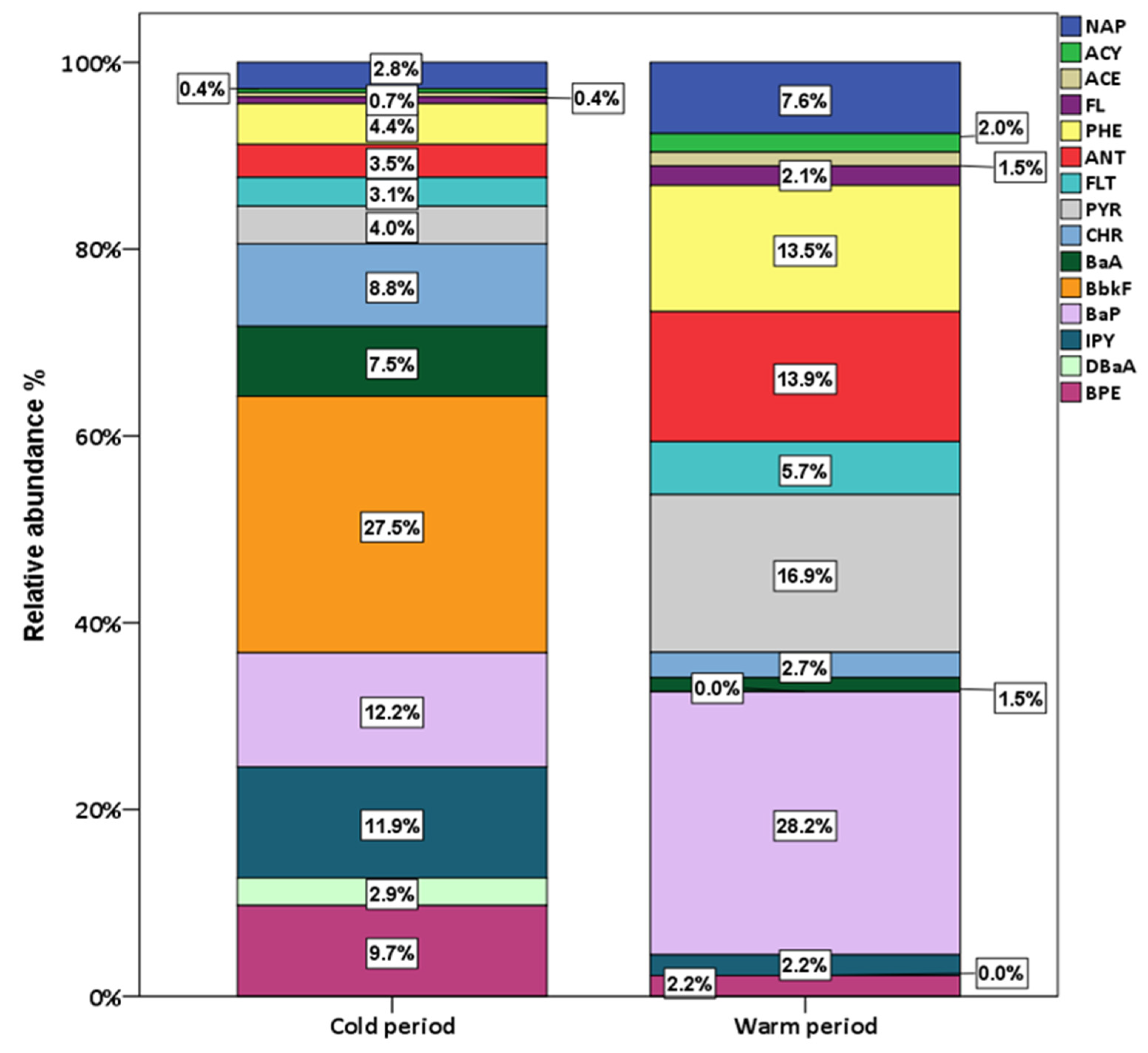
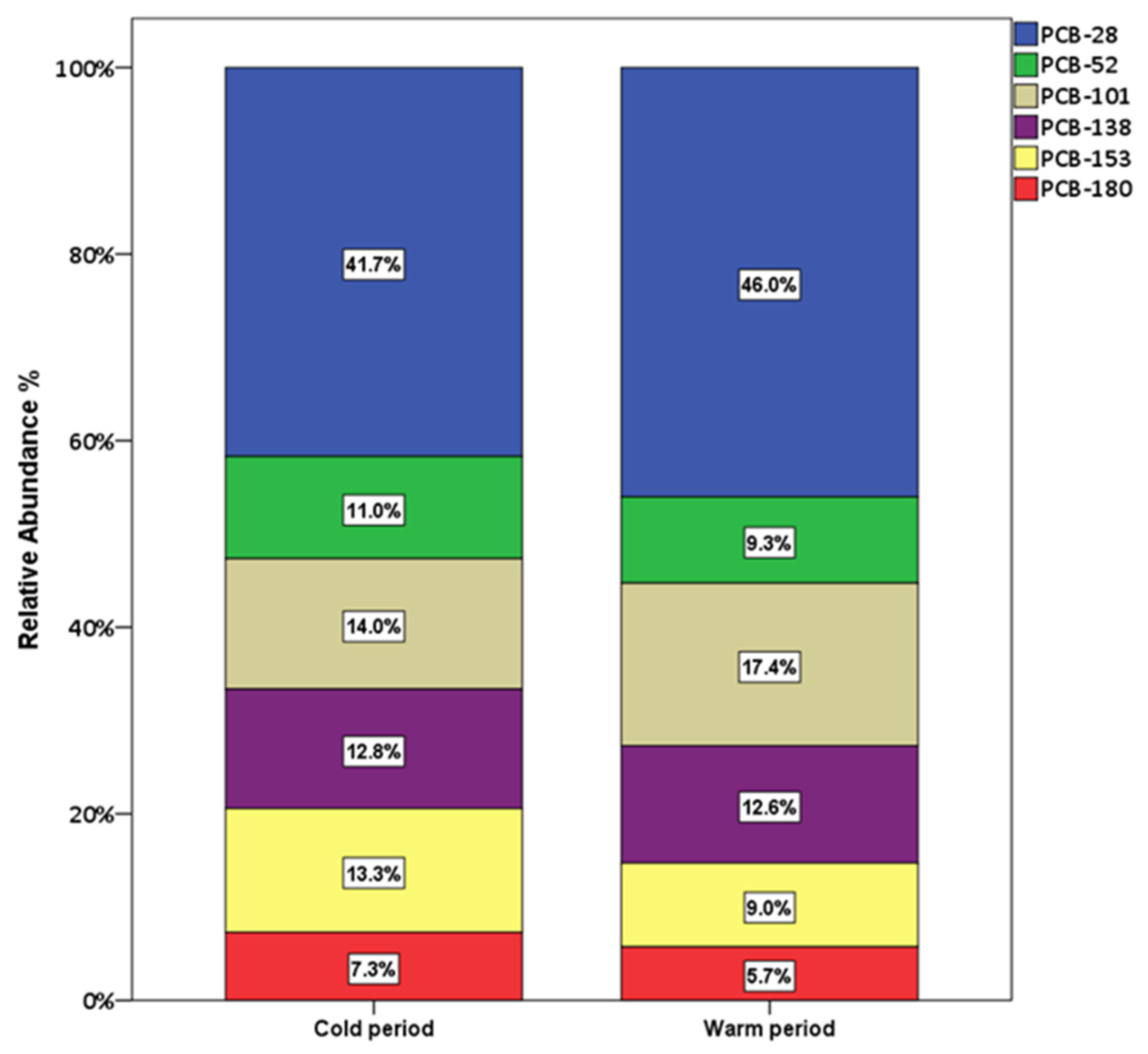
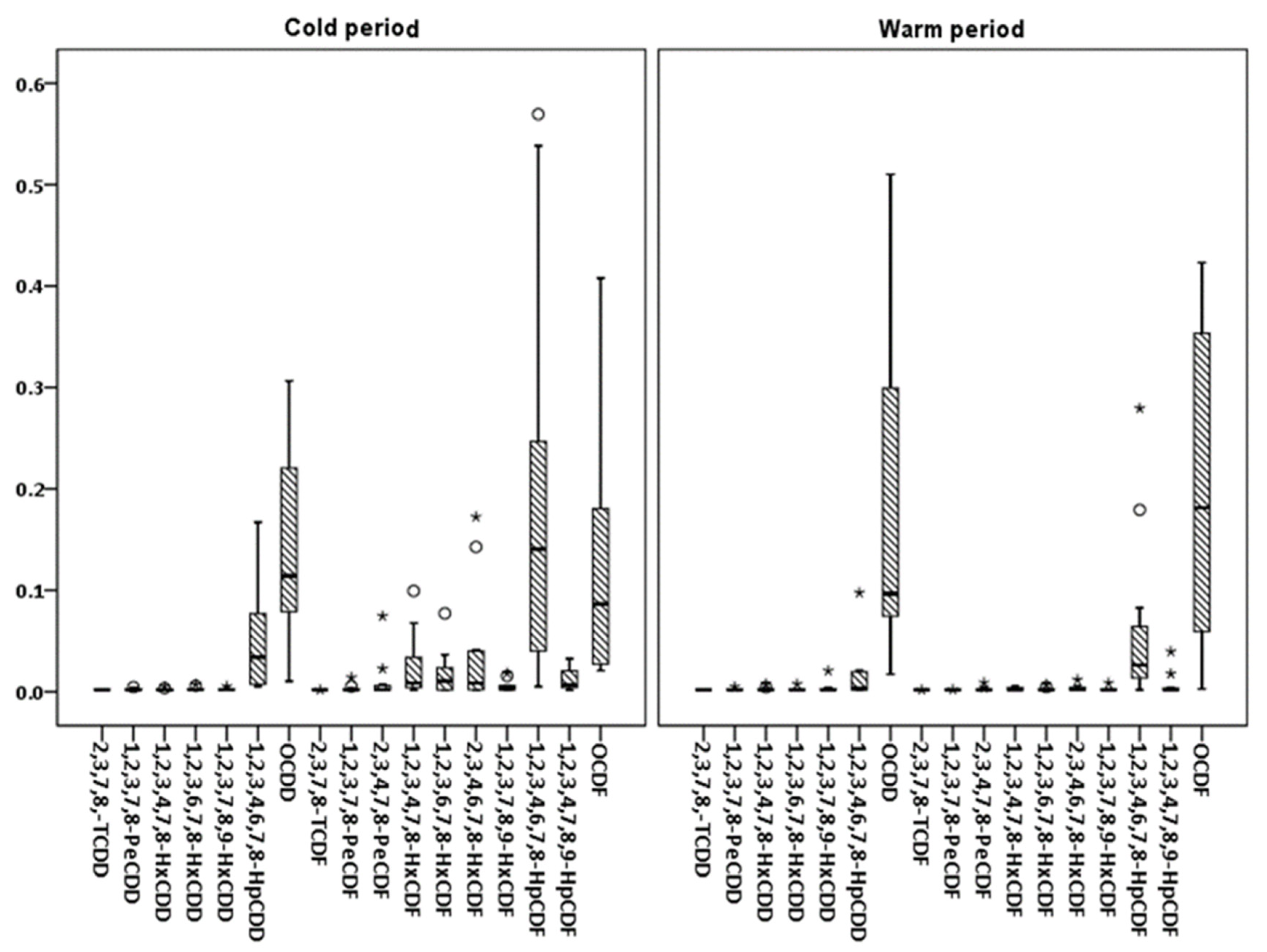

| NAP | ACY | ACE | FL | PHE | ANT | FLT | PYR | CHR | BaA | BbkF | BaP | IPY | DBaA | BPE | ΣPAHs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Sampling Campaign (N = 30) | ||||||||||||||||
| Average | 0.22 | 0.03 | 0.03 | 0.06 | 0.35 | 0.29 | 0.23 | 0.34 | 0.60 | 0.50 | 1.82 | 0.93 | 0.80 | 0.20 | 0.65 | 7.07 |
| Median | 0.17 | 0.007 | 0.0001 | 0.06 | 0.30 | 0.30 | 0.18 | 0.34 | 0.49 | 0.39 | 1.54 | 0.66 | 0.69 | 0.19 | 0.59 | 6.12 |
| Range | 0.05–0.67 | 0.0002–0.21 | 0.0001–0.20 | 0.02–0.12 | 0.19–0.79 | 0.06–0.51 | 0.05–0.69 | 0.05–0.73 | 0.00009–2.94 | 0.0002–1.63 | 0.0001–4.38 | 0.04–3.07 | 0.00005–1.86 | 0.00002–0.50 | 0.00003–1.59 | 1.27–16.5 |
| Cold Period (N = 15) | ||||||||||||||||
| Average | 0.24 | 0.03 | 0.04 | 0.06 | 0.37 | 0.30 | 0.26 | 0.34 | 0.74 | 0.63 | 2.32 | 1.03 | 1.00 | 0.25 | 0.82 | 8.44 |
| Median | 0.19 | 0.002 | 0.0001 | 0.06 | 0.31 | 0.32 | 0.21 | 0.30 | 0.60 | 0.73 | 2.39 | 0.90 | 0.99 | 0.24 | 0.76 | 8.88 |
| Range | 0.07–0.67 | 0.002–0.21 | 0.0001–0.20 | 0.02–0.12 | 0.19–0.79 | 0.06–0.51 | 0.05–0.69 | 0.05–0.73 | 0.00009–2.94 | 0.09–1.63 | 0.36–4.38 | 0.23–2.34 | 0.32–1.86 | 0.10–0.50 | 0.24–1.59 | 2.88–16.5 |
| Warm Period (N = 15) | ||||||||||||||||
| Average | 0.15 | 0.04 | 0.03 | 0.04 | 0.27 | 0.28 | 0.11 | 0.34 | 0.05 | 0.03 | 0.0001 | 0.57 | 0.05 | 0.00002 | 0.04 | 2.01 |
| Median | 0.13 | 0.03 | 0.0001 | 0.04 | 0.28 | 0.28 | 0.11 | 0.34 | 0.00009 | 0.04 | 0.0001 | 0.07 | 0.00005 | 0.00002 | 0.04 | 1.60 |
| Range | 0.05–0.37 | 0.01–0.07 | 0.0001–0.18 | 0.02–0.06 | 0.21–0.31 | 0.22–0.33 | 0.09–0.15 | 0.32–0.36 | 0.00009–0.17 | 0.0002–0.06 | 0.0001–0.0001 | 0.04–3.07 | 0.0005–0.14 | <0.0002 | 0.00003–0.09 | 1.27–4.46 |
| PCB-28 | PCB-52 | PCB-101 | PCB-138 | PCB-153 | PCB-180 | ΣPCBs | |
|---|---|---|---|---|---|---|---|
| Overall Sampling Campaign (N = 30) | |||||||
| Average | 7.96 | 1.82 | 2.86 | 2.30 | 2.01 | 1.18 | 18.1 |
| Median | 8.25 | 1.71 | 2.56 | 2.26 | 1.83 | 1.11 | 17.4 |
| Range | 4.53–11.7 | 1.09–2.94 | 1.34–5.78 | 1.68–3.80 | 1.18–3.30 | 0.66–1.87 | 11.4–26.6 |
| Cold Period (N = 15) | |||||||
| Average | 7.36 | 1.93 | 2.47 | 2.26 | 2.34 | 1.29 | 17.6 |
| Median | 7.49 | 1.86 | 2.53 | 2.35 | 2.30 | 1.21 | 17.1 |
| Range | 4.53–9.37 | 1.09–2.94 | 1.63–3.37 | 1.72–3.07 | 1.56–3.30 | 0.80–1.87 | 11.6–22.4 |
| Warm Period (N = 15) | |||||||
| Average | 8.56 | 1.72 | 3.24 | 2.34 | 1.67 | 1.07 | 18.6 |
| Median | 8.46 | 1.62 | 2.80 | 2.13 | 1.58 | 0.94 | 17.6 |
| Range | 4.60–11.7 | 1.10–2.32 | 1.34–5.78 | 1.68–3.80 | 1.18–2.33 | 0.66–1.73 | 11.4–26.6 |
| 2,3,7,8-ΤCDD | 1,2,3,7,8-PeCDD | 1,2,3,4,7,8-HxCDD | 1,2,3,6,7,8-HxCDD | 1,2,3,7,8,9-HxCDD | 1,2,3,4,6,7,8-HpCDD | OCDD | ΣPCDDs | 2,3,7,8-TCDF | 1,2,3,7,8-PeCDF | 2,3,4,7,8-PeCDF | 1,2,3,4,7,8-HxCDF | 1,2,3,6,7,8-HxCDF | 2,3,4,6,7,8-HxCDF | 1,2,3,7,8,9-HxCDF | 1,2,3,4,6,7,8-HpCDF | 1,2,3,4,7,8,9-HpCDF | OCDF | ΣPCDFs | WHO-TEQ PCDD/Fs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Sampling Campaign (N = 30) | ||||||||||||||||||||
| Average | 1.81 | 2.35 | 2.58 | 2.69 | 3.19 | 33.2 | 172 | 217 | 1.83 | 2.64 | 6.93 | 13.8 | 10.4 | 21.6 | 4.25 | 129 | 9.93 | 279 | 479 | 656 |
| Median | 1.81 | 1.81 | 1.81 | 1.81 | 1.81 | 10.8 | 111 | 167 | 1.81 | 1.81 | 1.81 | 3.93 | 1.92 | 2.88 | 2.14 | 55.1 | 3.89 | 94.0 | 325 | 444 |
| Range | <1.81 | 1.81–4.75 | 1.81–7.70 | 1.81–7.53 | 1.81–7.53 | 1.81–167 | 10.4–510 | 29.8–528 | 1.81–2.07 | 1.81–14.0 | 1.81–74.5 | 1.81–99.3 | 1.81–77.2 | 1.81–172 | 1.81–18.3 | 1.81–569 | 1.81–39.6 | 2.75–2912 | 52.8–2945 | 287–2560 |
| Cold Period (N = 15) | ||||||||||||||||||||
| Average | 1.81 | 2.53 | 2.41 | 2.96 | 2.45 | 50.2 | 151 | 427 | 1.83 | 3.43 | 11.2 | 24.8 | 18.0 | 39.6 | 5.84 | 193 | 12.8 | 128 | 439 | 914 |
| Median | 1.81 | 1.81 | 1.81 | 2.07 | 2.07 | 34.0 | 114 | 327 | 1.81 | 1.81 | 2.07 | 8.50 | 10.5 | 8.59 | 3.28 | 141 | 6.84 | 86.4 | 311 | 692 |
| Range | <1.81 | 1.81–4.67 | 1.81–5.01 | 1.81–7.35 | 1.81–5.36 | 5.43–167 | 10.4–306 | 157–861 | 1.81–2.07 | 1.81–74.5 | 1.81–74.5 | 1.81–99.3 | 1.81–77.2 | 1.81–172 | 1.81–18.3 | 5.06–569 | 1.81–32.7 | 20.8–408 | 52.9–1176 | 332–2560 |
| Warm Period (N = 15) | ||||||||||||||||||||
| Average | 1.81 | 2.17 | 2.75 | 2.42 | 3.93 | 16.3 | 192 | 221 | 1.83 | 1.85 | 2.66 | 2.79 | 2.78 | 3.57 | 2.66 | 64.1 | 7.06 | 429 | 519 | 398 |
| Median | 1.81 | 1.81 | 1.81 | 1.81 | 1.81 | 3.51 | 96.6 | 170 | 1.81 | 1.81 | 1.81 | 1.81 | 1.81 | 2.02 | 1.81 | 26.3 | 1.81 | 181 | 339 | 366 |
| Range | <1.81 | 1.81–4.75 | 1.81–7.70 | 1.81–7.53 | 1.81–20.6 | 1.81–97.6 | 17.2–510 | 29.8–527 | 1.81–2.03 | 1.81–2.21 | 1.81–8.85 | 1.81–5.36 | 1.81–7.15 | 1.81–11.8 | 1.81–8.66 | 1.81–279 | 1.81–39.6 | 2.75–2912 | 63–2945 | 287–544 |
| PCB-77 | PCB-81 | PCB-126 | PCB-169 | PCB-105 | PCB-114 | PCB-118 | PCB-123 | PCB-156 | PCB-157 | PCB-167 | PCB-189 | ΣdlPCBs | WHO-TEQ dlPCBs | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non- Ortho PCBs | Mono- Ortho PCBs | |||||||||||||
| Overall Sampling Campaign (N = 30) | ||||||||||||||
| Average | 85.8 | 6.28 | 22.4 | 6.37 | 238 | 36.3 | 752 | 62.4 | 101 | 75.7 | 208 | 40.5 | 1634 | 137 |
| Median | 72.9 | 5.45 | 17.1 | 5.25 | 186 | 36.3 | 594 | 60.8 | 92.9 | 74.5 | 156 | 36.3 | 1438 | 105 |
| Range | 41.2–268 | 1.81–19.0 | 8.26–86.6 | 1.81–22.0 | 113–634 | <36.3 | 453–2029 | 36.3–143 | 36.0–259 | 36.3–188 | 36.3–969 | 36.3–116 | 975–4083 | 53.1–512 |
| Cold Period (N = 15) | ||||||||||||||
| Average | 75.4 | 5.72 | 21.4 | 7.51 | 190 | 36.3 | 637 | 61.5 | 104 | 93.3 | 112 | 37.4 | 1382 | 136 |
| Median | 74.0 | 5.61 | 17.2 | 6.40 | 184 | 36.3 | 586 | 63.7 | 93.5 | 87.1 | 74.3 | 36.3 | 1290 | 105 |
| Range | 41.2–113 | 1.99–9.33 | 8.47–35.6 | 1.81–14.7 | 146–287 | <36.3 | 505–871 | 41.0–94.0 | 36.0–195 | 36.3–188 | 36.3–324 | 36.3–43.9 | 1092–1893 | 62.5–217 |
| Warm Period (N = 15) | ||||||||||||||
| Average | 96.3 | 6.80 | 23.3 | 5.24 | 287 | 36.3 | 867 | 63.3 | 97.2 | 58.0 | 303 | 43.5 | 1887 | 139 |
| Median | 71.6 | 5.16 | 16.0 | 2.76 | 237 | 36.3 | 815 | 42.5 | 85.1 | 51.6 | 238 | 36.3 | 1592 | 93.9 |
| Range | 50.8–268 | 1.81–19.0 | 8.26–86.6 | 1.81–22.0 | 113–634 | <36.3 | 453–2029 | 36.3–143 | 37.6–259 | 36.3–81.2 | 36.3–969 | 36.3–116 | 975–4083 | 53.1–512 |
| Petrogenic Origin | Pyrogenic Origin | This Study | |||
|---|---|---|---|---|---|
| Mean | Cold | Warm | |||
| ANT/(ANT + PHE) | <0.1 | >0.1 | 0.46 | 0.45 | 0.51 |
| BaA/(BaA + CHR) | <0.2 | >0.35 | 0.56 | 0.51 | 0.62 |
| FLT/(FLT + PYR) | <0.4 | >0.4 | 0.46 | 0.47 | 0.45 |
| IPY/(IPY + BPE) | <0.2 | >0.2 | 0.55 | 0.56 | 0.52 |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| Explained Variance | 36.2% | 24.3% | 22.9% |
| NAP | −0.399 | 0.329 | −0.213 |
| ACY | −0.223 | −0.258 | 0.114 |
| ACE | 0.330 | 0.307 | −0.205 |
| FL | −0.198 | 0.210 | 0.763 |
| PHE | −0.027 | −0.048 | 0.805 |
| ANT | 0.439 | −0.077 | −0.260 |
| FLT | 0.116 | 0.204 | 0.893 |
| PYR | 0.132 | 0.370 | 0.848 |
| CHR | 0.755 | 0.420 | 0.525 |
| BaA | 0.729 | 0.398 | 0.501 |
| BbkF | 0.759 | 0.182 | 0.491 |
| BaP | 0.805 | 0.026 | −0.005 |
| IPY | 0.821 | 0.124 | 0.448 |
| DBaA | 0.534 | 0.340 | 0.748 |
| BPE | 0.808 | 0.112 | 0.433 |
| 1,2,3,7,8- PeCDD | 0.314 | 0.287 | 0.056 |
| 1,2,3,4,7,8- HxCDD | 0.785 | −0.119 | 0.444 |
| 1,2,3,6,7,8- HxCDD | −0.362 | −0.029 | 0.338 |
| 1,2,3,7,8,9- HxCDD | −0.173 | −0.388 | 0.345 |
| 1,2,3,4,6,7,8- HpCDD | 0.768 | 0.268 | 0.015 |
| OCDD | 0.131 | 0.064 | −0.580 |
| 2,3,7,8- TCDF | 0.664 | −0.362 | −0.080 |
| 1,2,3,7,8- PeCDF | 0.788 | −0.227 | −0.023 |
| 2,3,4,7,8- PeCDF | 0.772 | 0.499 | −0.174 |
| 1,2,3,4,7,8- HxCDF | 0.815 | −0.070 | −0.161 |
| 1,2,3,6,7,8- HxCDF | 0.743 | 0.554 | −0.018 |
| 2,3,4,6,7,8- HxCDF | 0.879 | 0.234 | −0.135 |
| 1,2,3,7,8,9- HxCDF | 0.622 | 0.531 | −0.044 |
| 1,2,3,4,6,7,8- HpCDF | 0.889 | 0.180 | −0.104 |
| 1,2,3,4,7,8,9- HpCDF | 0.497 | −0.106 | 0.182 |
| OCDF | −0.087 | 0.040 | −0.452 |
| PCB-77 | 0.270 | 0.113 | 0.082 |
| PCB-81 | −0.069 | −0.251 | 0.010 |
| PCB-126 | 0.079 | 0.779 | 0.223 |
| PCB-169 | 0.132 | 0.600 | 0.570 |
| PCB-105 | −0.049 | 0.313 | −0.360 |
| PCB-118 | −0.062 | 0.583 | −0.388 |
| PCB-123 | −0.201 | 0.686 | −0.184 |
| PCB-156 | 0.169 | 0.720 | 0.128 |
| PCB-157 | 0.286 | 0.706 | 0.234 |
| PCB-167 | −0.067 | −0.489 | −0.055 |
| PCB-189 | −0.082 | 0.455 | 0.294 |
| PCB-28 | 0.108 | 0.384 | −0.630 |
| PCB-52 | 0.238 | 0.717 | −0.269 |
| PCB-101 | −0.266 | 0.473 | −0.472 |
| PCB-138 | −0.010 | 0.838 | −0.236 |
| PCB-153 | 0.163 | 0.832 | 0.216 |
| PCB-180 | −0.238 | 0.651 | 0.299 |
| Cancer Risk | |
|---|---|
| ΣRiskPAHs | 2.4 × 10−5 |
| ΣRiskPCDD/Fs | 9.9 × 10−6 |
| ΣRiskdlPCBs | 1.6 × 10−6 |
| Total Cancer Risk | 3.6 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koukoulakis, K.G.; Kanellopoulos, P.G.; Chrysochou, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Bakeas, E. Atmospheric Concentrations and Health Implications of PAHs, PCBs and PCDD/Fs in the Vicinity of a Heavily Industrialized Site in Greece. Appl. Sci. 2020, 10, 9023. https://doi.org/10.3390/app10249023
Koukoulakis KG, Kanellopoulos PG, Chrysochou E, Costopoulou D, Vassiliadou I, Leondiadis L, Bakeas E. Atmospheric Concentrations and Health Implications of PAHs, PCBs and PCDD/Fs in the Vicinity of a Heavily Industrialized Site in Greece. Applied Sciences. 2020; 10(24):9023. https://doi.org/10.3390/app10249023
Chicago/Turabian StyleKoukoulakis, Konstantinos G., Panagiotis George Kanellopoulos, Eirini Chrysochou, Danae Costopoulou, Irene Vassiliadou, Leondios Leondiadis, and Evangelos Bakeas. 2020. "Atmospheric Concentrations and Health Implications of PAHs, PCBs and PCDD/Fs in the Vicinity of a Heavily Industrialized Site in Greece" Applied Sciences 10, no. 24: 9023. https://doi.org/10.3390/app10249023
APA StyleKoukoulakis, K. G., Kanellopoulos, P. G., Chrysochou, E., Costopoulou, D., Vassiliadou, I., Leondiadis, L., & Bakeas, E. (2020). Atmospheric Concentrations and Health Implications of PAHs, PCBs and PCDD/Fs in the Vicinity of a Heavily Industrialized Site in Greece. Applied Sciences, 10(24), 9023. https://doi.org/10.3390/app10249023






