Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
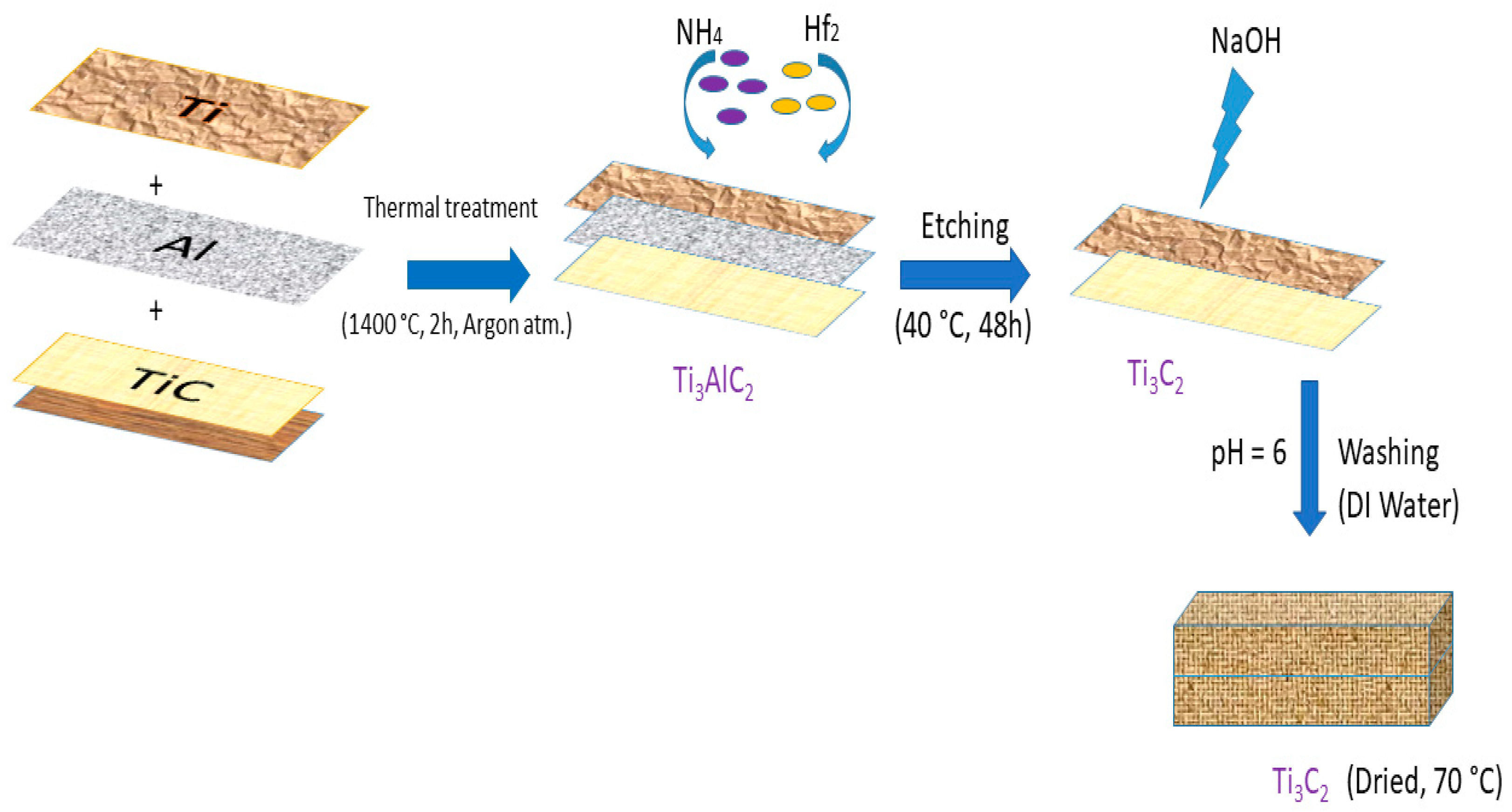
2.2. Synthesis and Characterization of MXene Nanoparticles
2.3. Preparation of Ionic Liquid-Based MXene Nanofluid
2.4. Optical Property and Stability Characterization
2.4.1. UV-Vis Spectroscopic Analysis of Ionic Liquid-Based MXene Nanofluid
2.4.2. Absorbance Analysis to Evaluate the Stability or Degradation of Nanofluids
2.4.3. Stability Analysis Using Visual Inspection Method
2.5. Electrical Conductivity and pH Measurement
3. Results and Discussion
3.1. Morphology and Chemical Composition
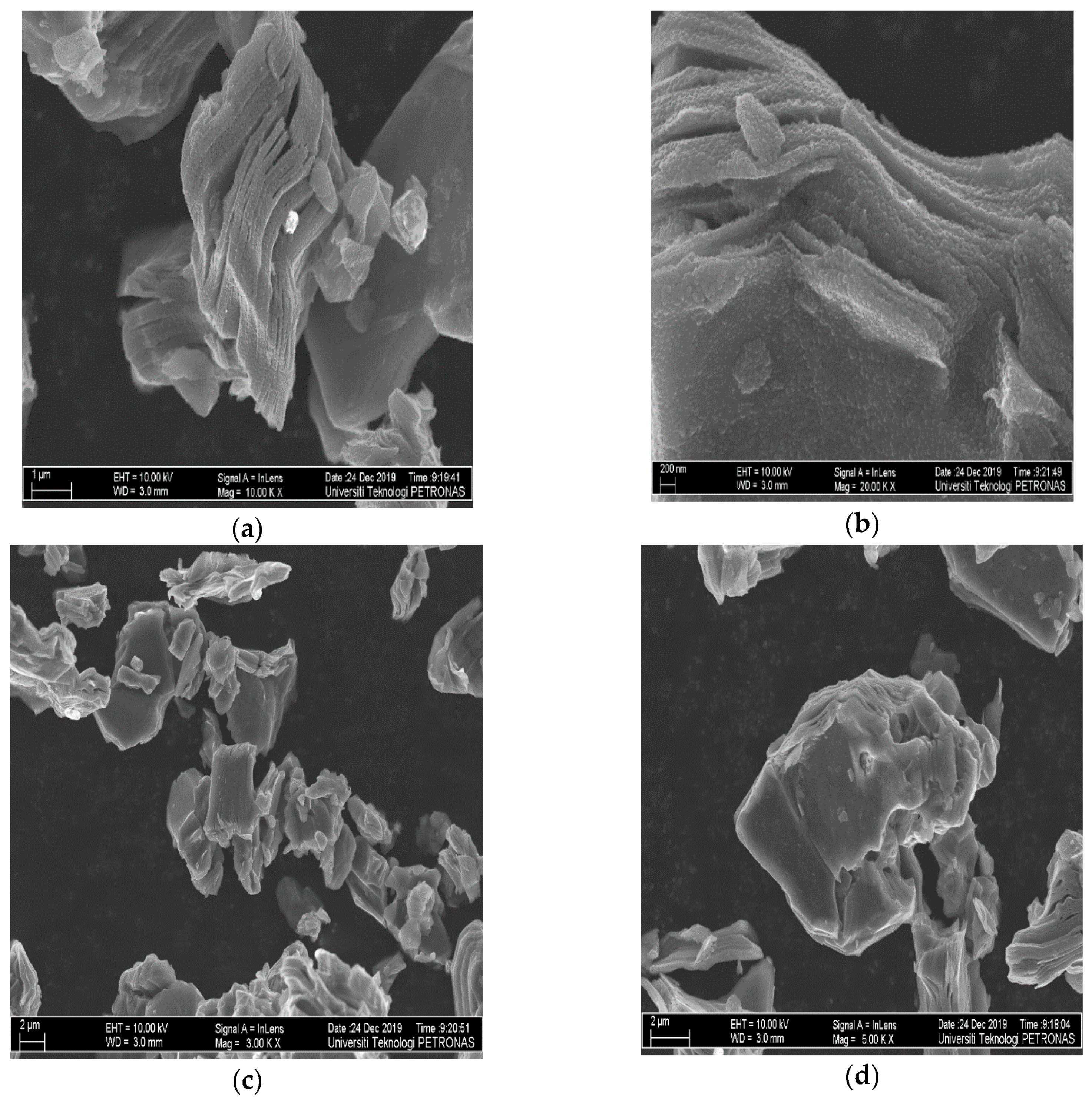
3.1.1. FESEM Analysis
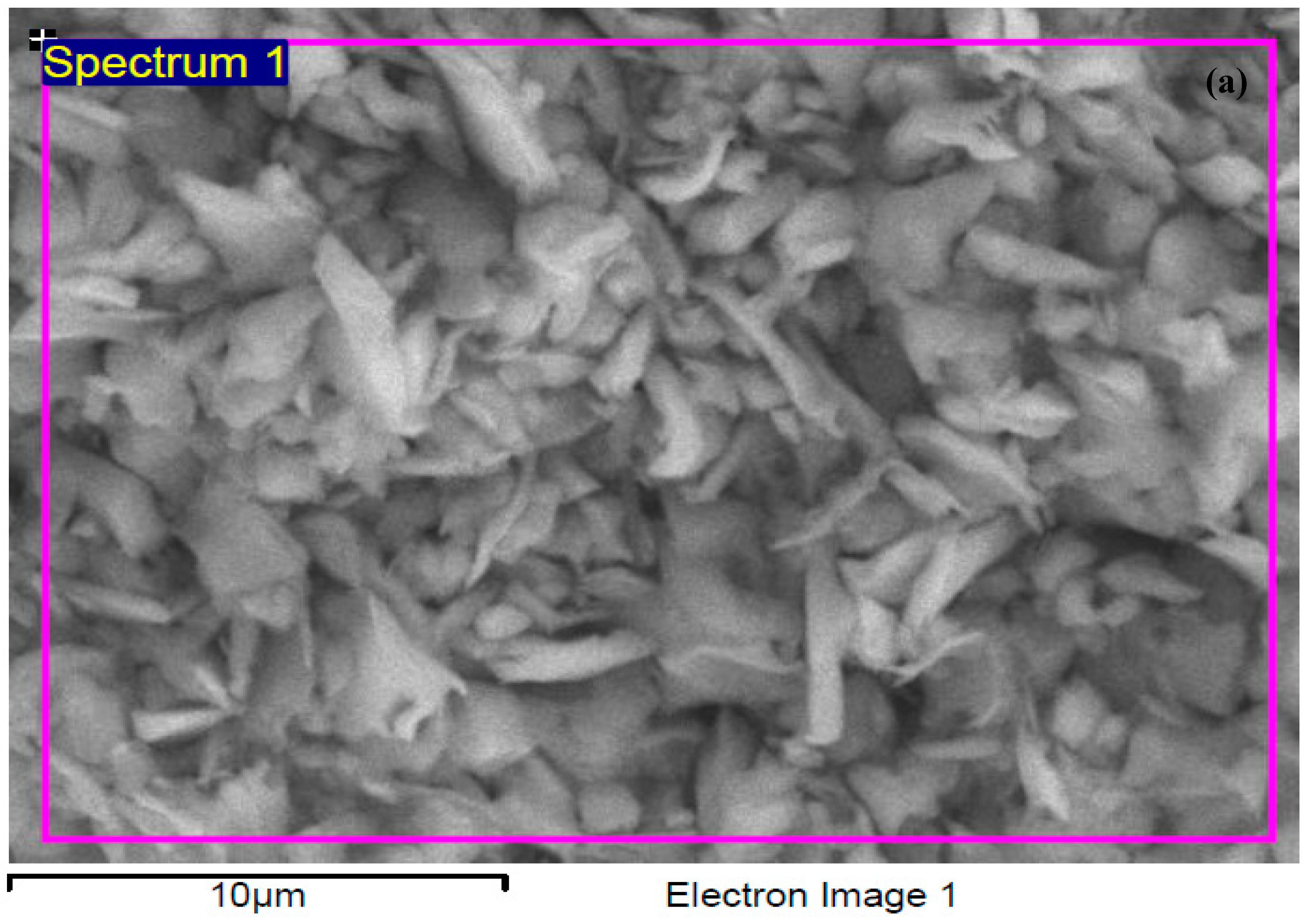
3.1.2. EDX Analysis
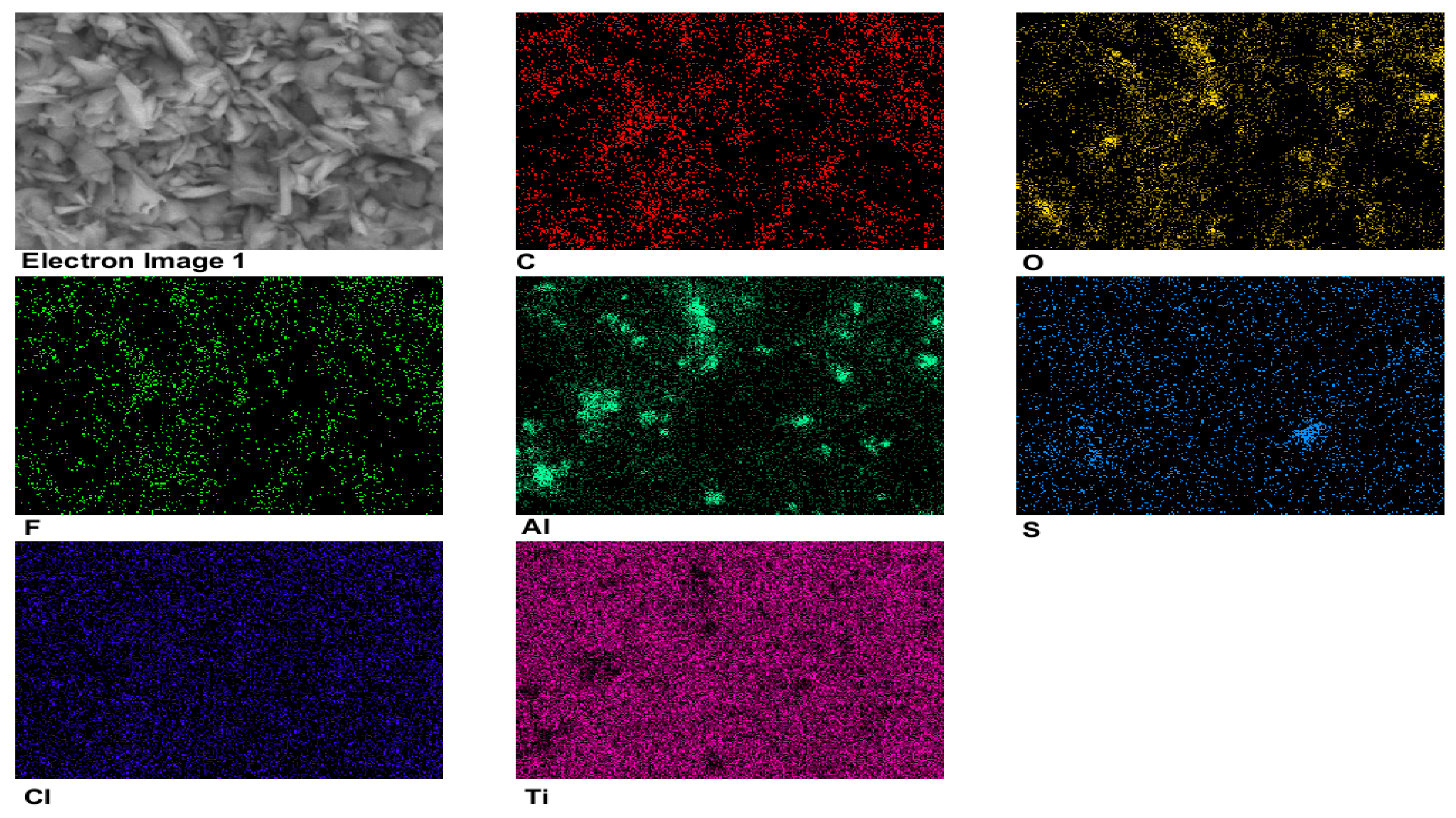
3.1.3. EDX Mapping
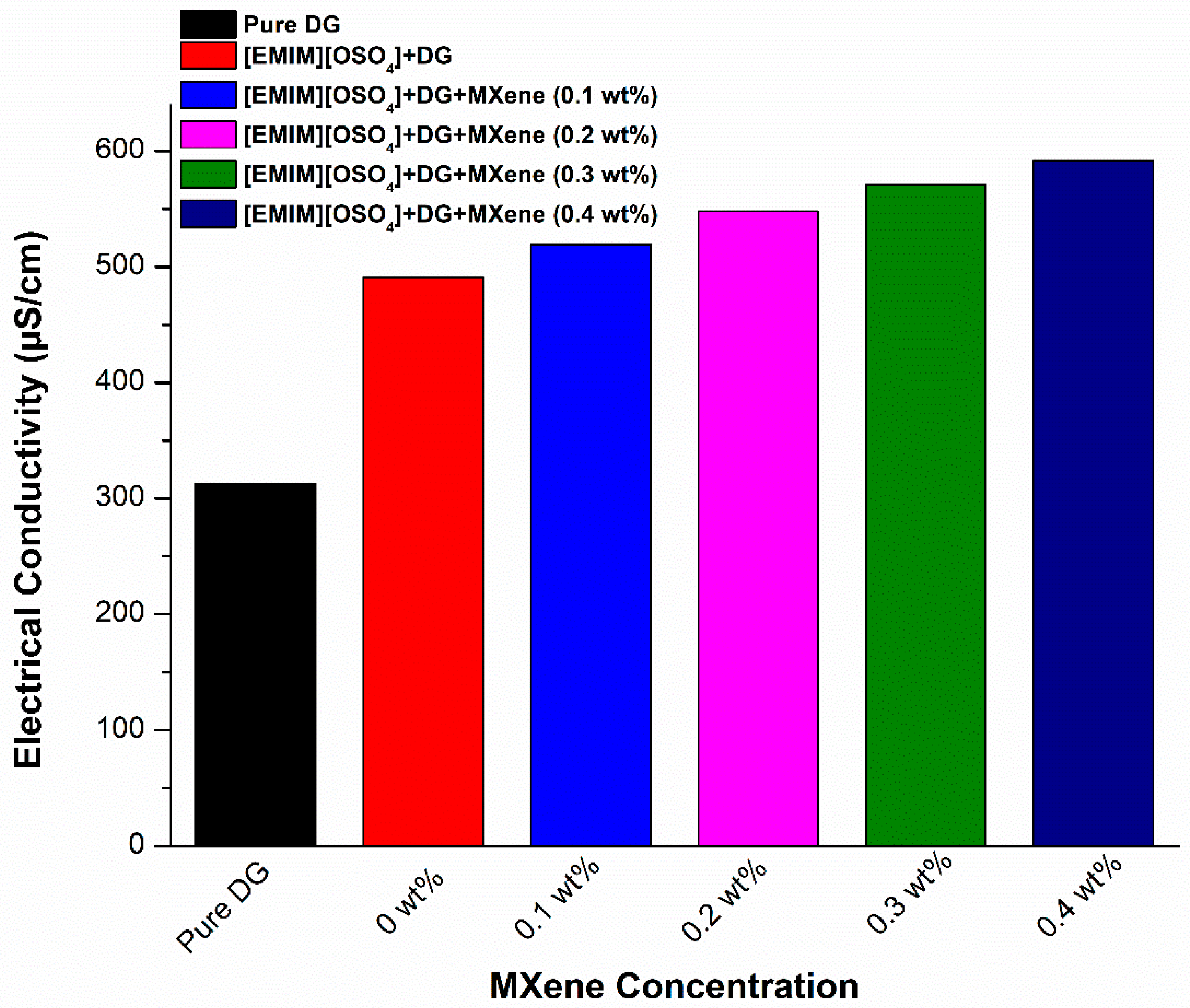
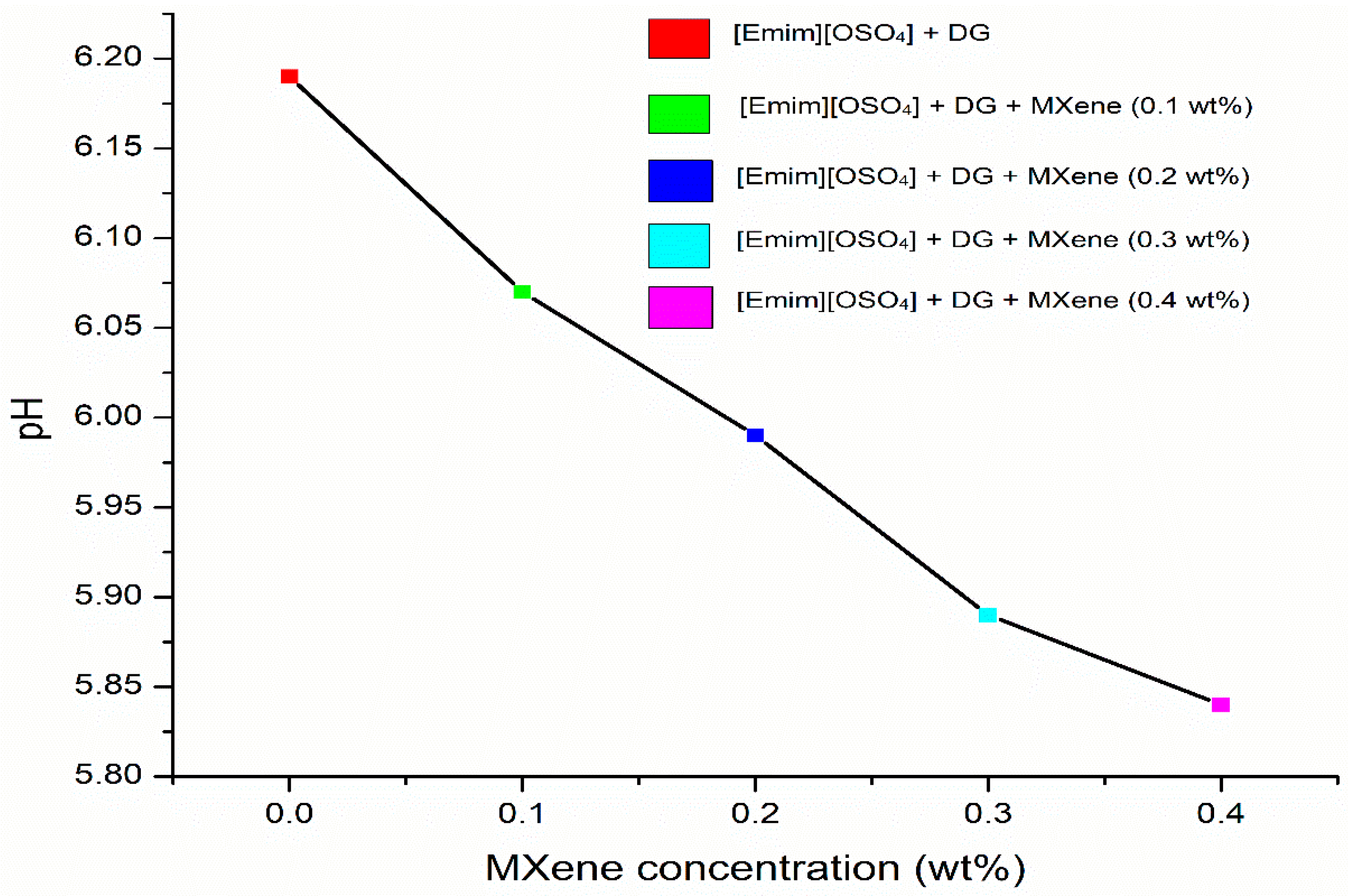
3.2. Effect of Ionic Liquid on the Electrical Conductivity and pH of MXene Nanofluid
3.3. UV-Vis Absorption Spectrum Analysis
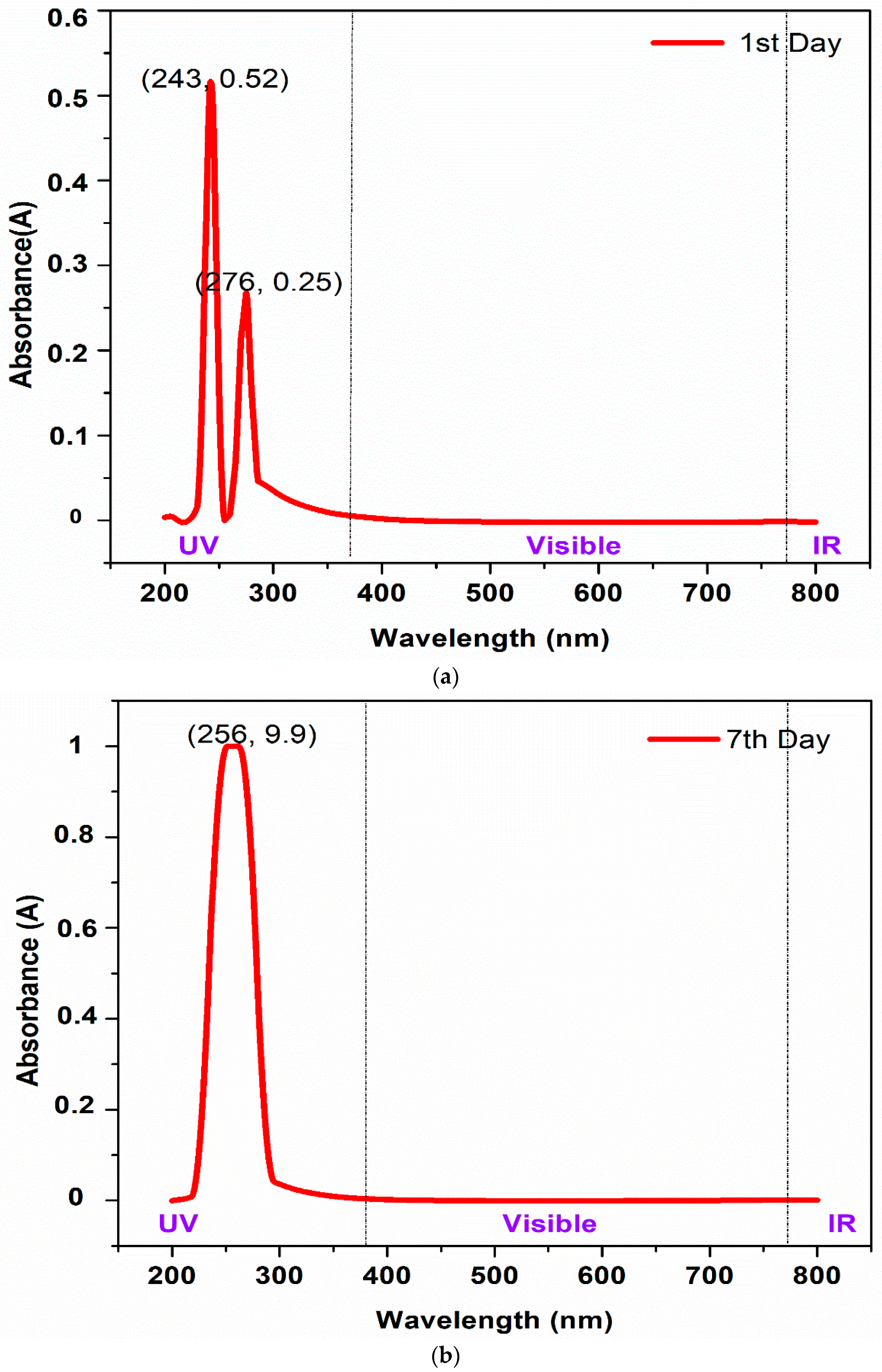
3.3.1. Light Absorption Spectra of Base Fluid ([Emim][OSO4] + DG)
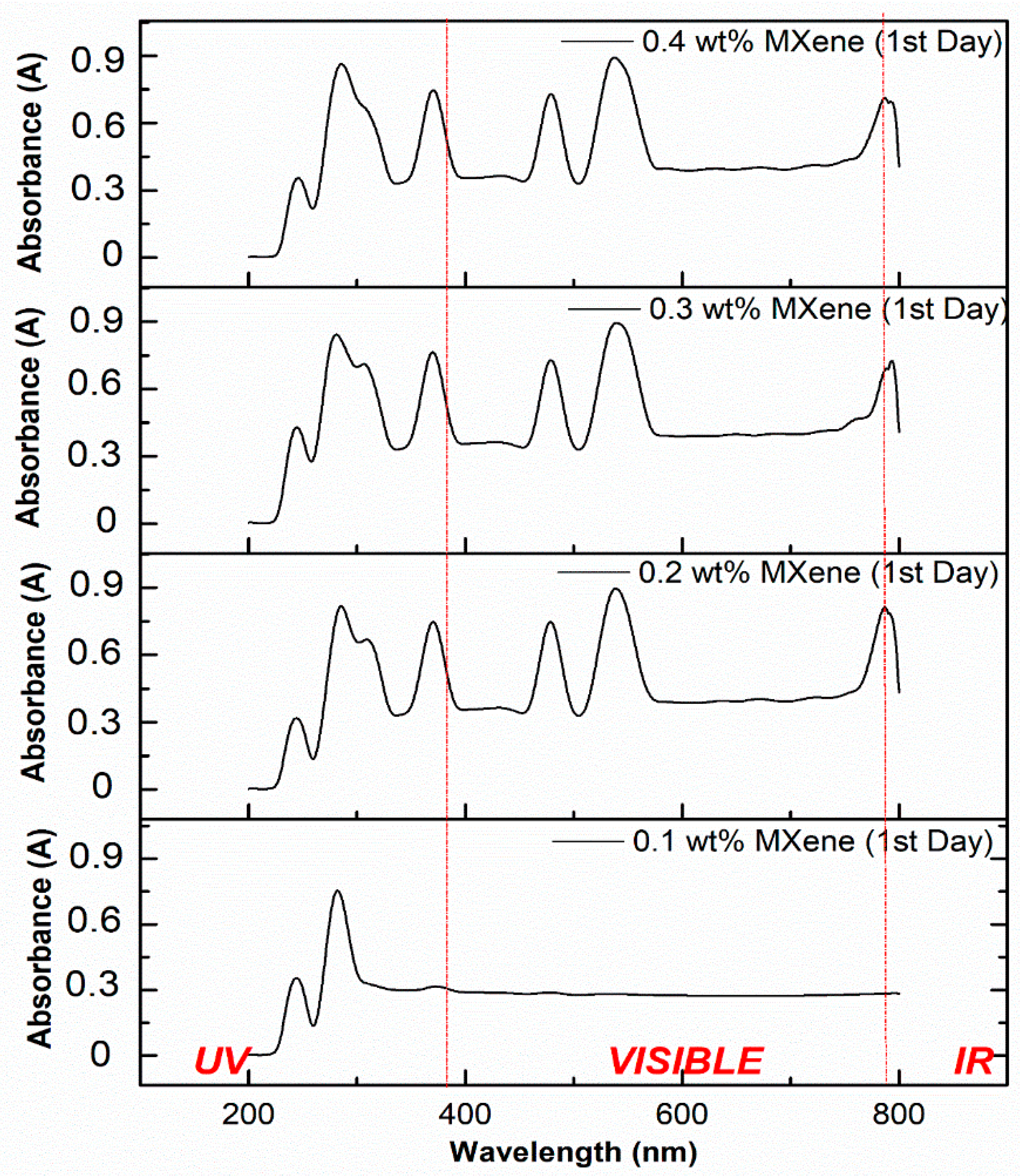
3.3.2. Light Absorption Spectra of the MXene Based Ionanofluid
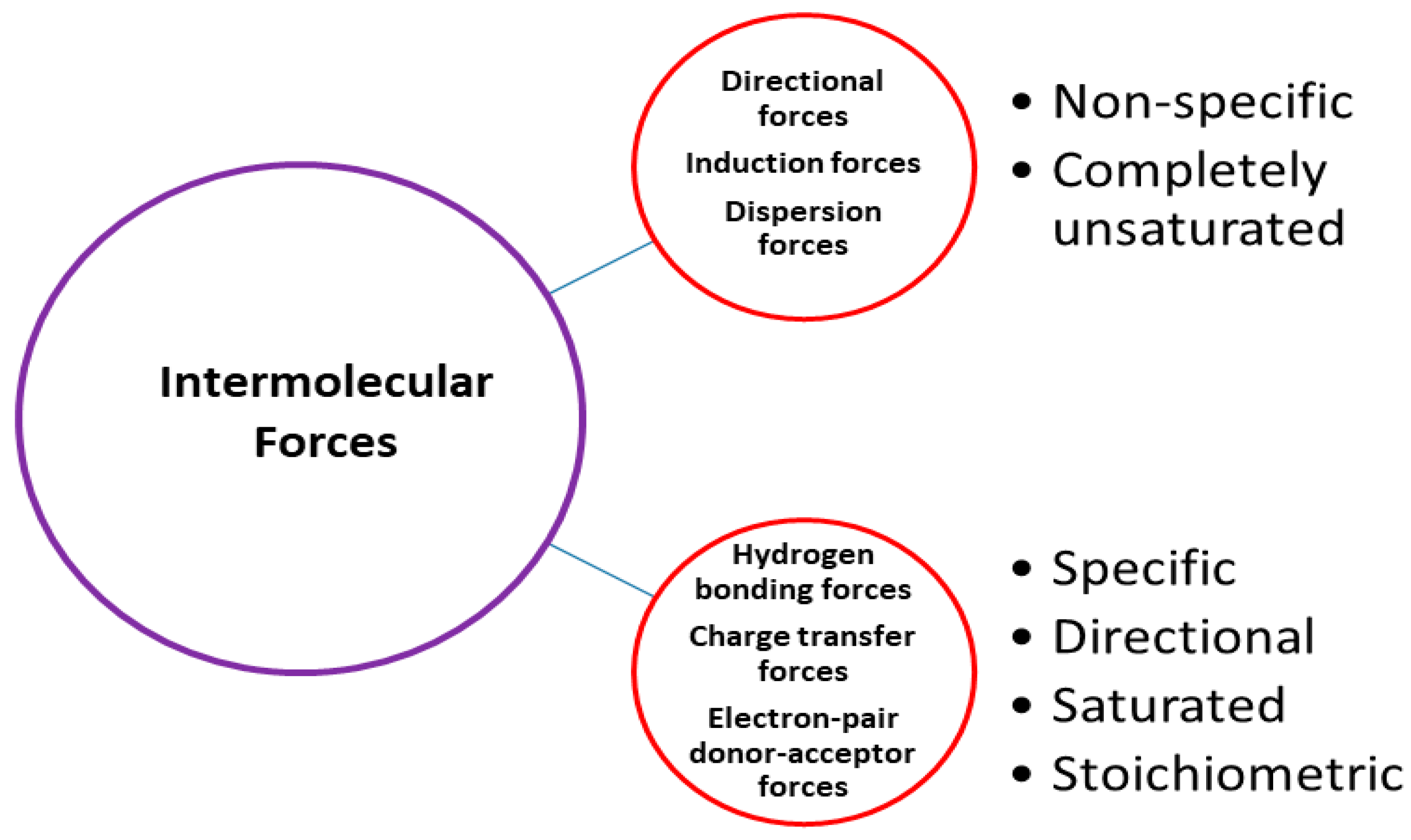
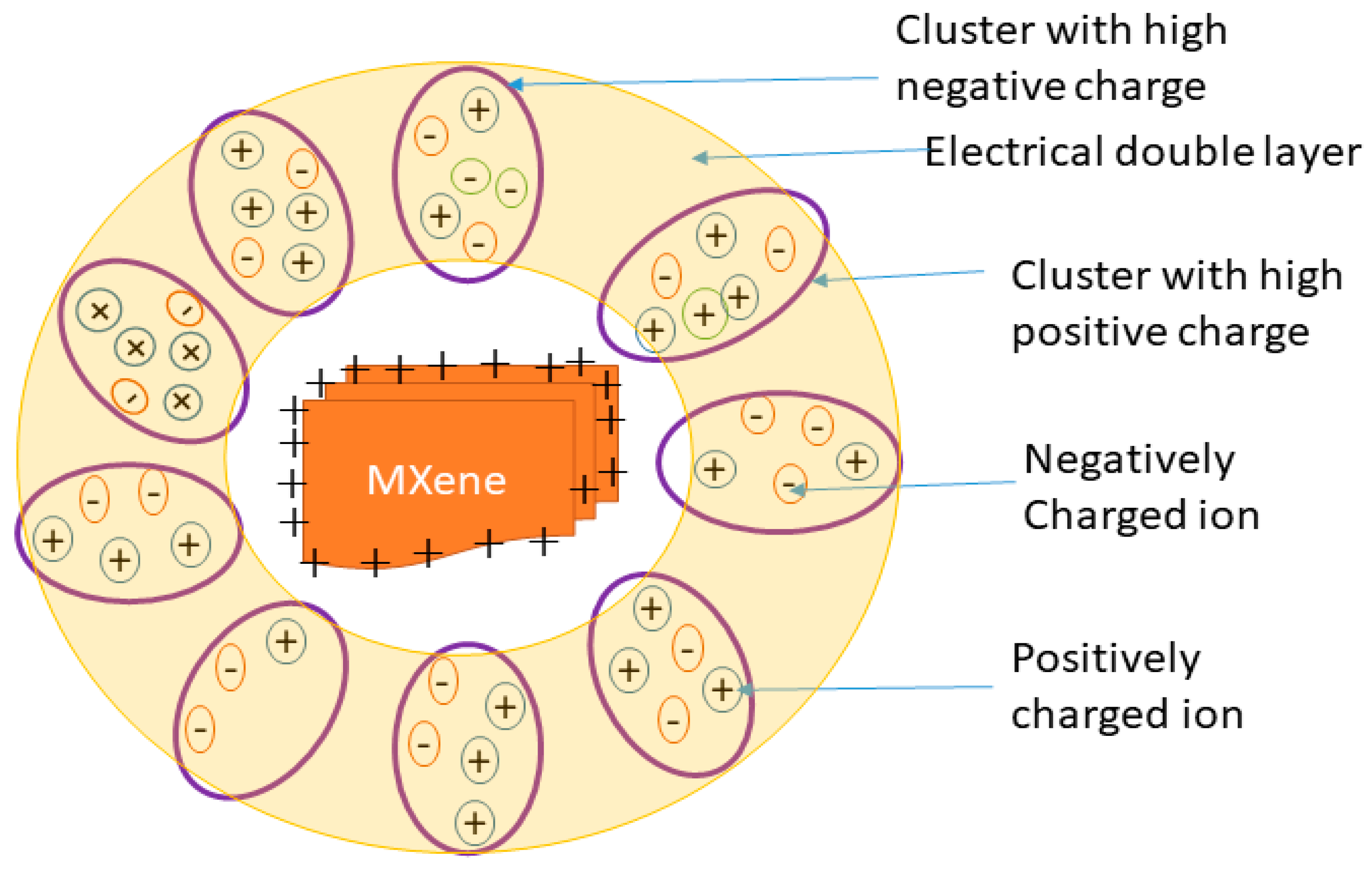
3.4. Solute–Solvent Interactions
3.5. Stability Analysis of Ionanofluids
3.5.1. Visual Inspection Analysis
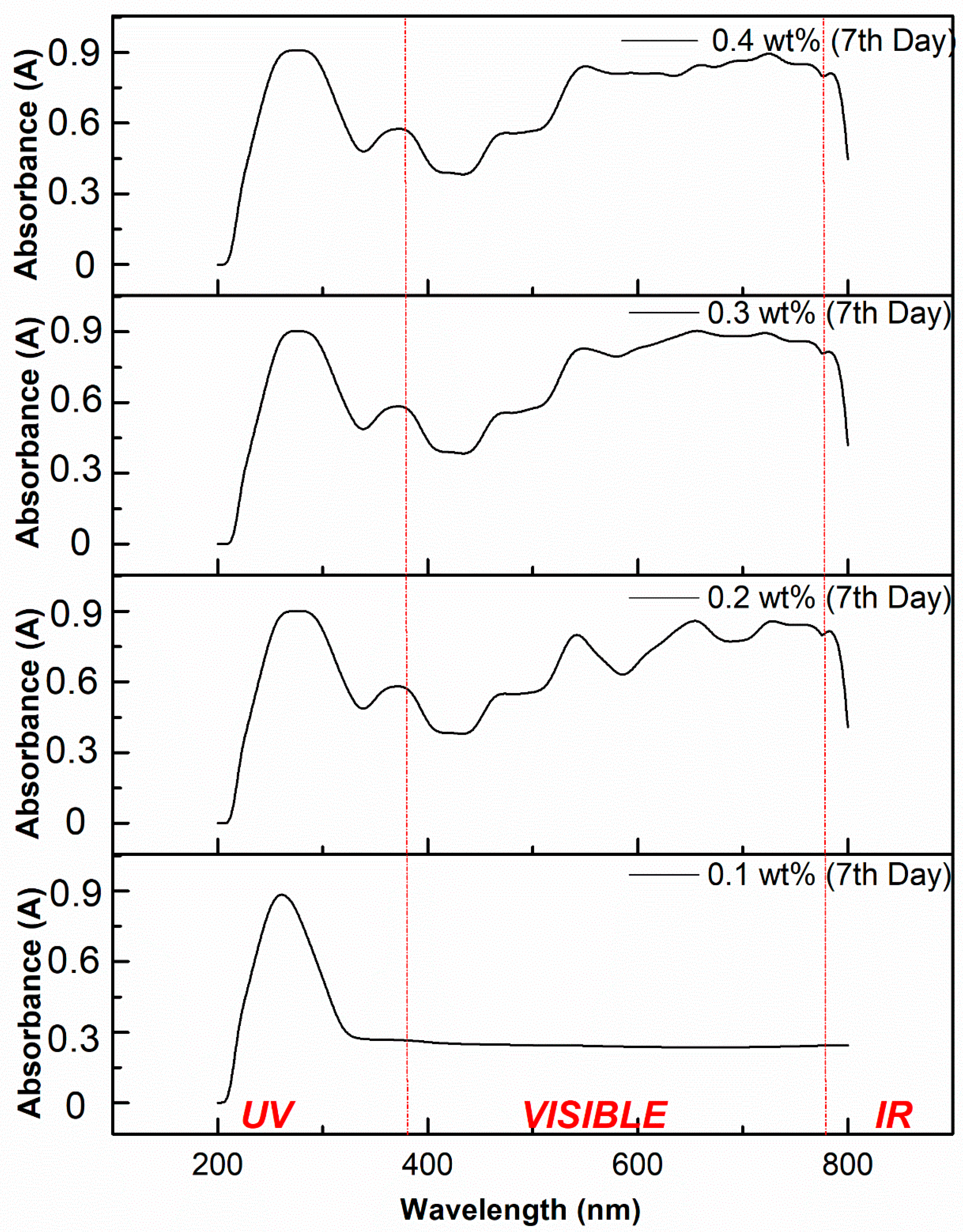
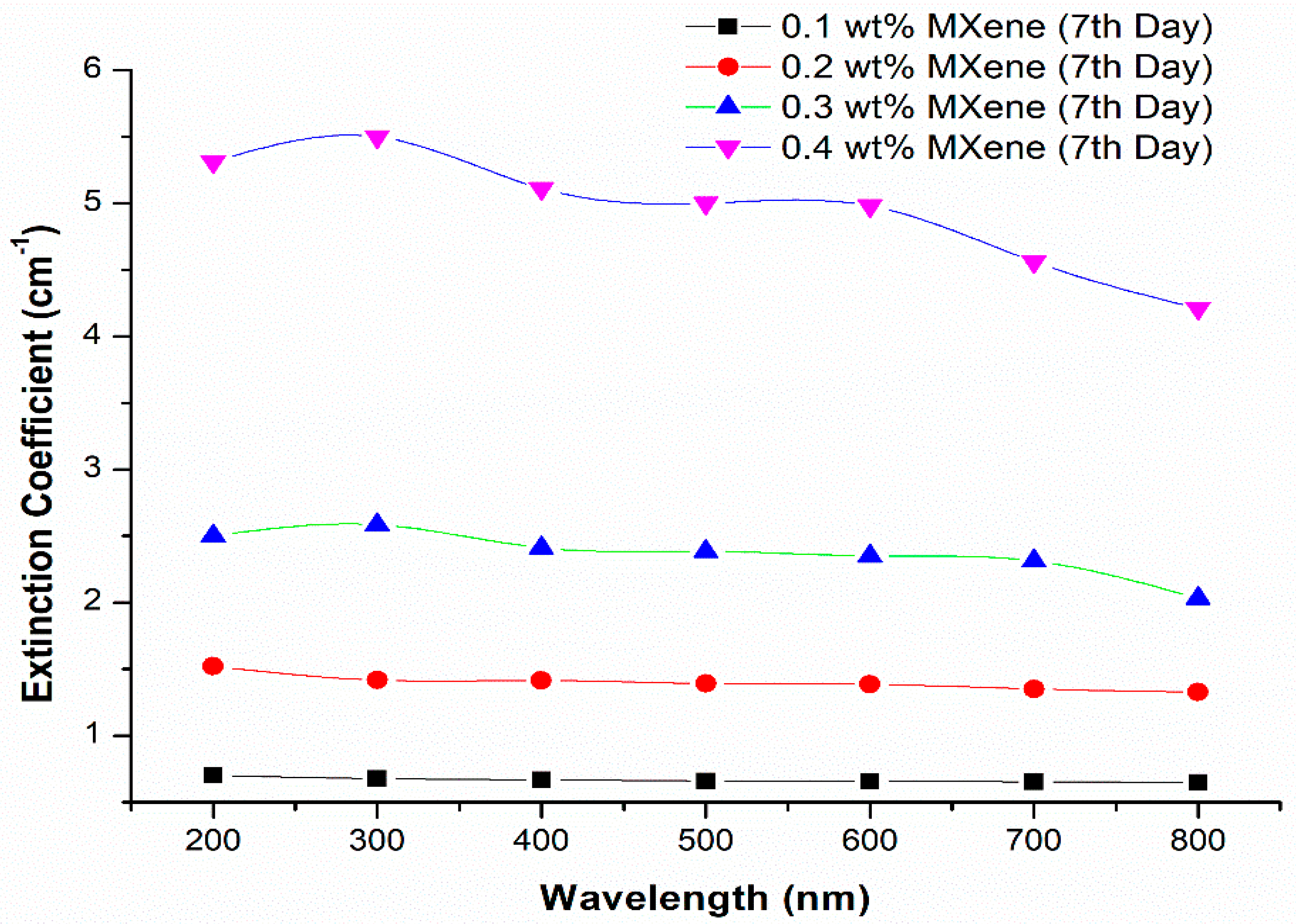
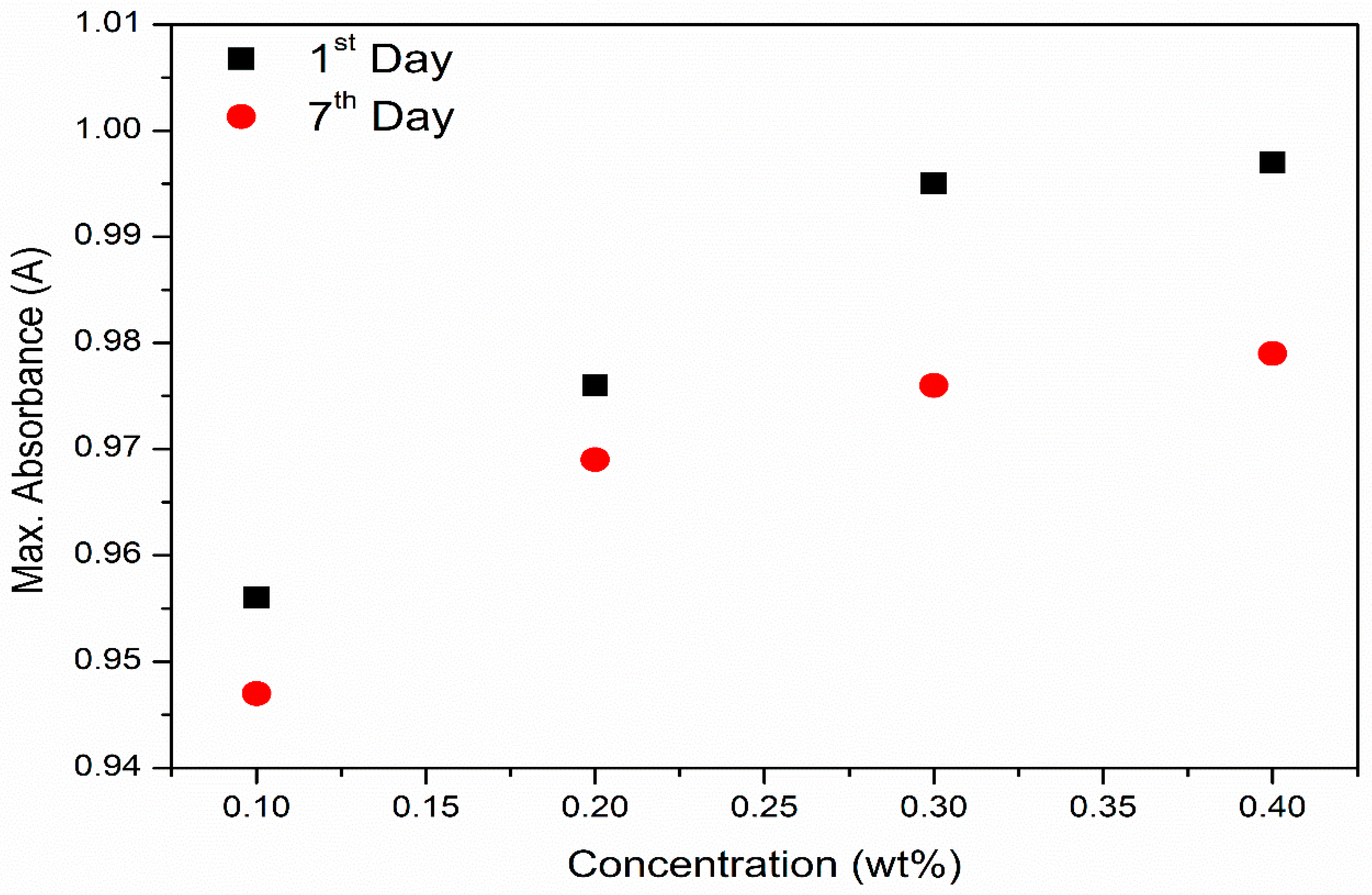
3.5.2. Stability Analysis Using Light Absorbance
4. Conclusions
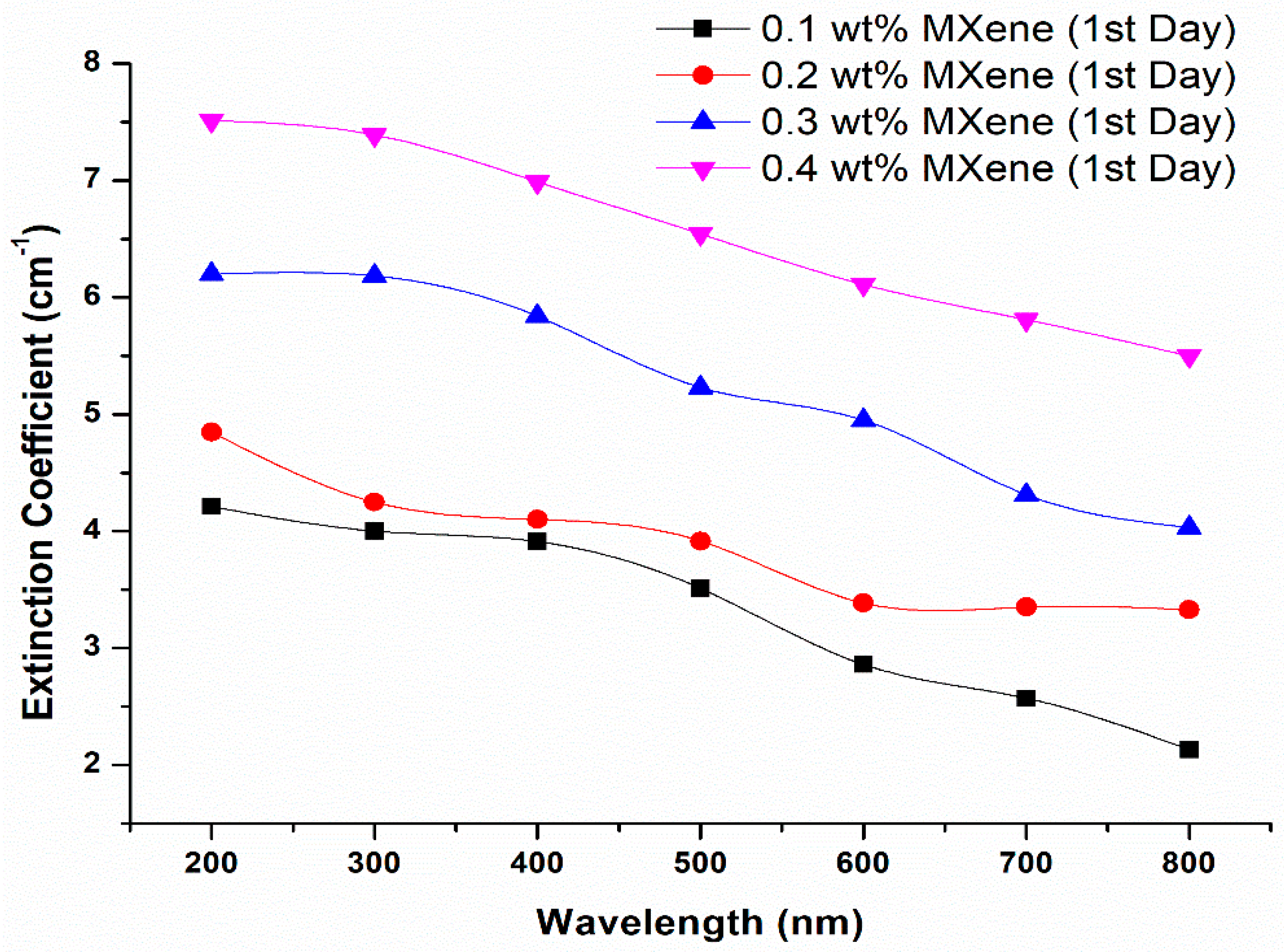
- The concentration of 2D MXene nanoparticles impacted a significant effect on the absorbance spectra
- With the increase in the nanoparticle loading, the absorbance capacity of the ionanofluid increased
- High extinction coefficient was obtained for fluid containing less concentration of MXene
- High absorption peaks were found on both days, which meant that the light absorbance potential over the measured wavelengths showed great stability.
- Slight aggregation of nanoparticles was observed from day 5, which was monitored via a visual inspection method
- Stability analysis through absorbance spectra illustrated that the prepared ionanofluids were stable for 0.1 to 0.4 wt% MXene nanoparticle concentration
- Electrical conductivity improved with the addition of MXene nanoparticles, resulting in a maximum electrical conductivity of 571 μS/cm at 0.4 wt%.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adhami Dehkordi, R.; Hemmat Esfe, M.; Afrand, M. Effects of functionalized single walled carbon nanotubes on thermal performance of antifreeze: An experimental study on thermal conductivity. Appl. Therm. Eng. 2017, 120, 358–366. [Google Scholar] [CrossRef]
- Lee, Y.; Jeong, H.; Park, J.-T.; Delgado, A.; Kim, S. Experimental Investigation on Evaluation of Thermal Performance of Solar Heating System Using Al2O3 Nanofluid. Appl. Sci. 2020, 10, 5521. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Du, Z.; Xu, X.; Zhang, Z.; Fang, X. A highly stable hydroxylated graphene/ethylene glycol-water nanofluid with excellent extinction property at a low loading for direct absorption solar collectors. Thermochim. Acta 2020, 684, 178487. [Google Scholar] [CrossRef]
- Gheymasi, A.N.; Rajabi, Y.; Zare, E.N. Nonlinear optical properties of poly(aniline-co-pyrrole)@ ZnO-based nanofluid. Opt. Mater. 2020, 102, 109835. [Google Scholar] [CrossRef]
- Aguilar, T.; Sani, E.; Mercatelli, L.; Carrillo-Berdugo, I.; Torres, E.; Navas, J. Exfoliated graphene oxide-based nanofluids with enhanced thermal and optical properties for solar collectors in concentrating solar power. J. Mol. Liq. 2020, 306, 112862. [Google Scholar] [CrossRef]
- Li, X.; Zeng, G.; Lei, X. The stability, optical properties and solar-thermal conversion performance of SiC-MWCNTs hybrid nanofluids for the direct absorption solar collector (DASC) application. Sol. Energy Mater. Sol. Cells 2020, 206, 110323. [Google Scholar] [CrossRef]
- Valizade, M.; Heyhat, M.M.; Maerefat, M. Experimental comparison of optical properties of nanofluid and metal foam for using in direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2019, 195, 71–80. [Google Scholar] [CrossRef]
- Di Rosa, D.; Wanic, M.; Fal, J.; Żyła, G.; Mercatelli, L.; Sani, E. Optical and dielectric properties of ethylene glycol-based nanofluids containing nanodiamonds with various purities. Powder Technol. 2019, 356, 508–516. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; He, G.; Ye, Z.; Fang, X.; Zhang, Z. Radiative properties of ionic liquid-based nanofluids for medium-to-high-temperature direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2014, 130, 521–528. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Das, L.; Habib, K.; Saidur, R.; Aslfattahi, N.; Yahya, S.M.; Rubbi, F. Improved Thermophysical Properties and Energy Efficiency of Aqueous Ionic Liquid/MXene Nanofluid in a Hybrid PV/T Solar System. Nanomaterials 2020, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, A.S.; Tan, K.H.; Aslfattahi, N.; Arifutzzaman, A.; Saidur, R.; Al-Sulaiman, F.A. Optical, stability and energy performance of water-based MXene nanofluids in hybrid PV/thermal solar systems. Sol. Energy 2020, 204, 32–47. [Google Scholar] [CrossRef]
- Bao, Z.; Bing, N.; Zhu, X.; Xie, H.; Yu, W. Ti3C2Tx MXene Contained Nanofluids with High Thermal Conductivity, Super Colloidal Stability and Low Viscosity. Chem. Eng. J. 2020, 126390. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Irshad, K.; Hussein, O.A. An investigation of thermal stability and heat capacity of imidazolium based ionic liquids at elevated temperature. IOP Conf. Ser. Mater. Sci. Eng. 2020, 863, 012026. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M.; et al. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J.; Liu, Q.; Liu, G.; Xiong, J.; Yang, J.; Jin, W. Ultrathin two-dimensional MXene membrane for pervaporation desalination. J. Memb. Sci. 2018, 548, 548–558. [Google Scholar] [CrossRef]
- Ding, L.; Wei, Y.; Wang, Y.; Chen, H.; Caro, J.; Wang, H. A Two-Dimensional Lamellar Membrane: MXene Nanosheet Stacks. Angew. Chem. 2017, 129, 1851–1855. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Dybko, A.; Jastrzębska, A.M.; Wojciechowski, T.; Rozmysłowska, A.; Chudy, M.; Grabowska-Jadach, I.; Ziemkowska, W.; Brzózka, Z.; et al. 2D Ti2C (MXene) as a novel highly efficient and selective agent for photothermal therapy. Mater. Sci. Eng. C 2019, 98, 874–886. [Google Scholar] [CrossRef]
- Keaveney, S.T.; Haines, R.S.; Harper, J.B. Reactions in Ionic Liquids. In Encyclopedia of Physical Organic Chemistry; American Cancer Society: Atlanta, GA, USA, 2017; pp. 1–54. ISBN 9781118468586. [Google Scholar]
- Duan, H. Analysis on the extinction properties of nanofluids for direct solar absorption. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 120, 114046. [Google Scholar] [CrossRef]
- Chen, W.; Zou, C.; Li, X. Application of large-scale prepared MWCNTs nanofluids in solar energy system as volumetric solar absorber. Sol. Energy Mater. Sol. Cells 2019, 200, 109931. [Google Scholar] [CrossRef]
- Tan, J.; Xie, Y.; Wang, F.; Jing, L.; Ma, L. Investigation of optical properties and radiative transfer of TiO2 nanofluids with the consideration of scattering effects. Int. J. Heat Mass Transf. 2017, 115, 1103–1112. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Wilfred, C.D.; Saidur, R.; Saha, B.B. Comparative evaluation on the thermal properties and stability of MWCNT nanofluid with conventional surfactants and ionic liquid. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]

| Time | Concentration (Wt%) | |||
|---|---|---|---|---|
| 0.1 | 0.2 | 0.3 | 0.4 | |
| Preparation Day |  | |||
| Day 1 |  | |||
| Day 2 |  | |||
| Day 3 |  | |||
| Day 4 |  | |||
| Day 5 |  | |||
| Day 6 |  | |||
| Day 7 |  | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakthavatchalam, B.; Habib, K.; Saidur, R.; Aslfattahi, N.; Rashedi, A. Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Appl. Sci. 2020, 10, 8943. https://doi.org/10.3390/app10248943
Bakthavatchalam B, Habib K, Saidur R, Aslfattahi N, Rashedi A. Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Applied Sciences. 2020; 10(24):8943. https://doi.org/10.3390/app10248943
Chicago/Turabian StyleBakthavatchalam, Balaji, Khairul Habib, R. Saidur, Navid Aslfattahi, and A. Rashedi. 2020. "Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids" Applied Sciences 10, no. 24: 8943. https://doi.org/10.3390/app10248943
APA StyleBakthavatchalam, B., Habib, K., Saidur, R., Aslfattahi, N., & Rashedi, A. (2020). Investigation of Electrical Conductivity, Optical Property, and Stability of 2D MXene Nanofluid Containing Ionic Liquids. Applied Sciences, 10(24), 8943. https://doi.org/10.3390/app10248943






