Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Implant Site Preparation for Standard Drills
2.2. Ultrasonic Implant Site Preparation
3. Results
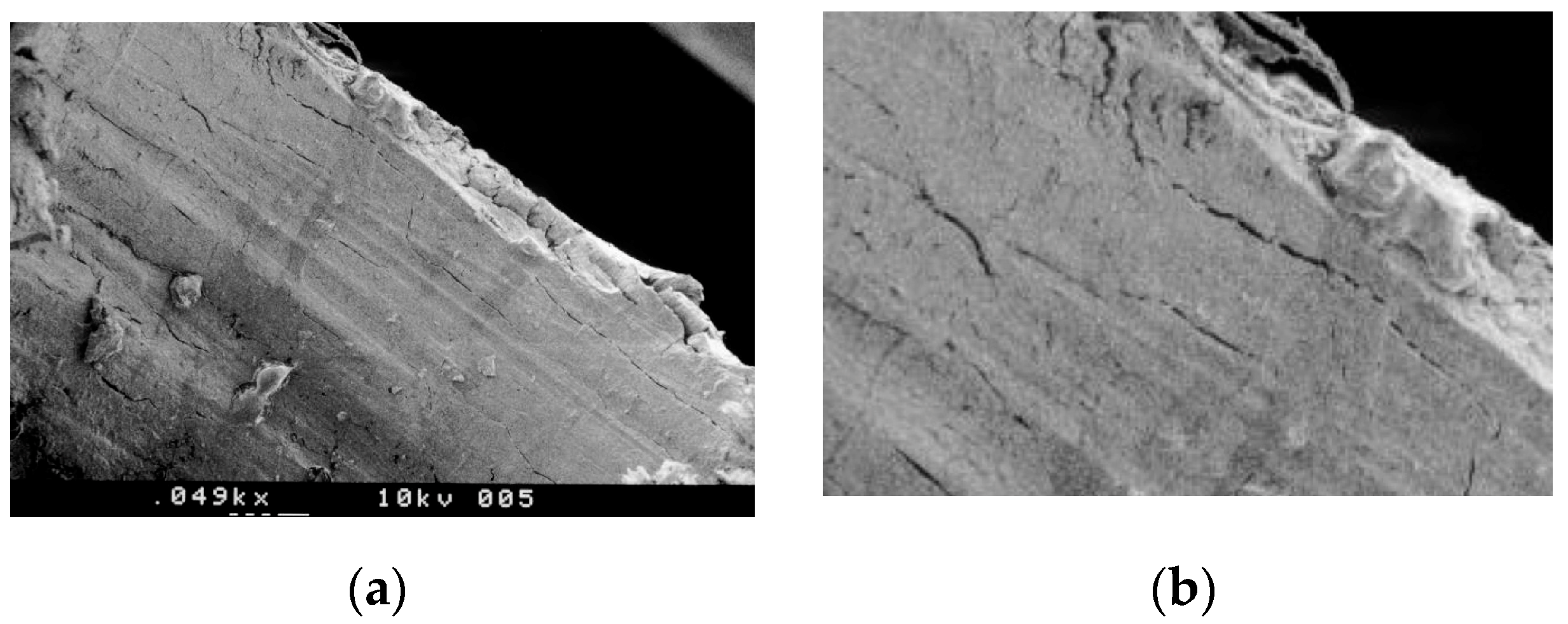
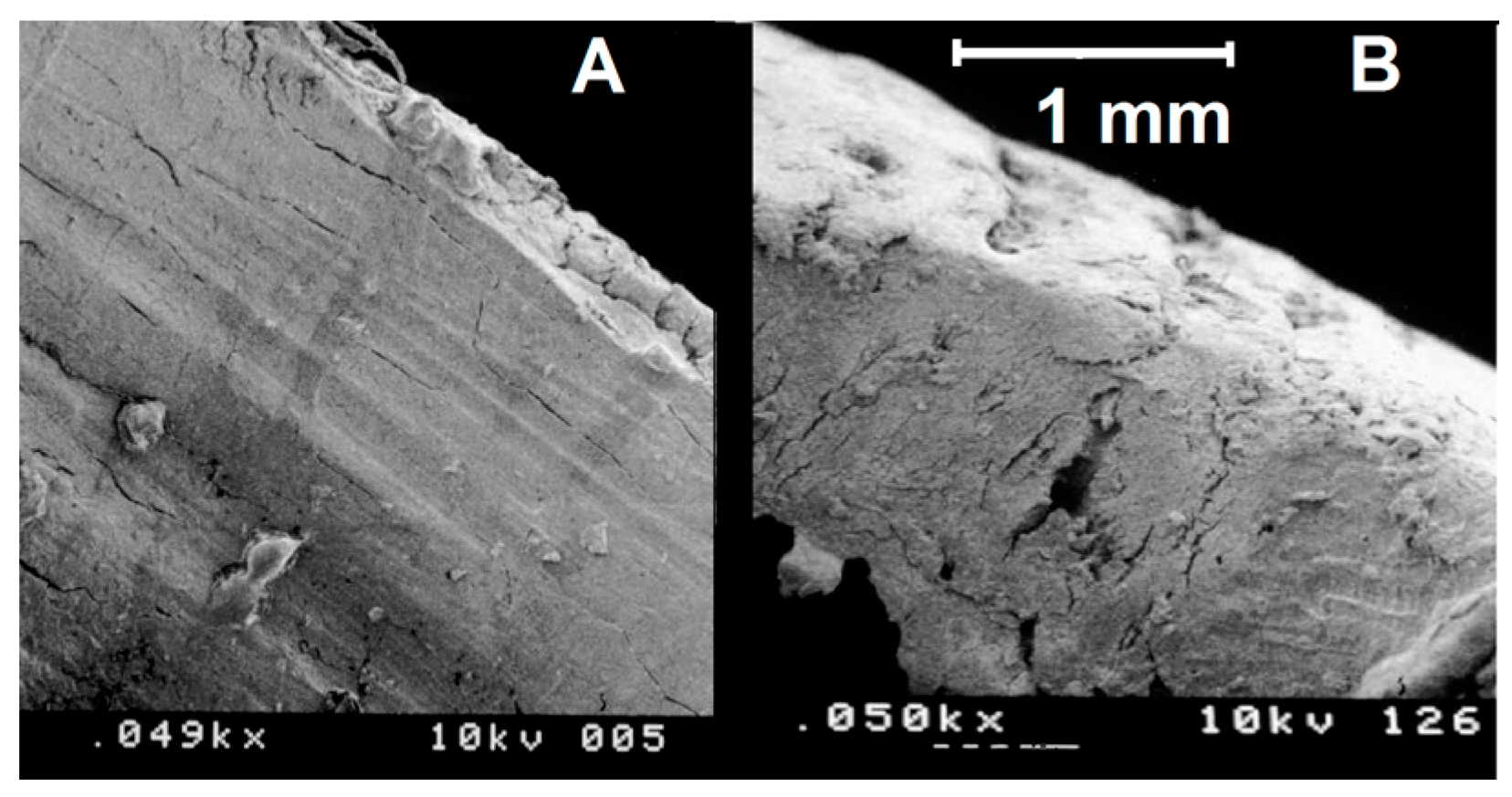
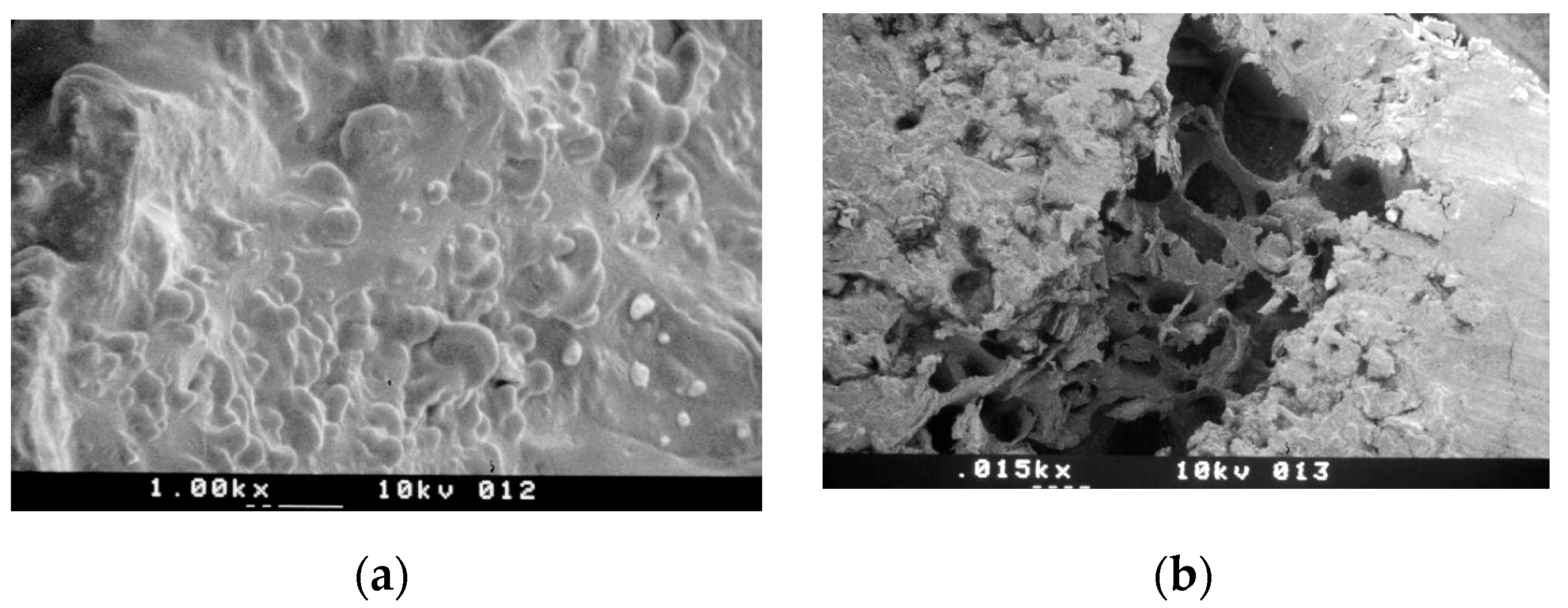
3.1. SEM Morphologic Analysis
3.1.1. Group A—Drill Implant Site Preparation
3.1.2. Group B—Ultrasonic Implant Site Preparation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, J.C.; Squillace, A.; Spagnuolo, G. Effect of different surface treatments on titanium dental implant micro-morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implants Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Trisi, P.; Rao, W.; Rebaudi, A. A histometric comparison of smooth and rough titanium implants in human low-density jawbone. Int. J. Oral Maxillofac. Implants 1999, 14, 689–698. [Google Scholar] [PubMed]
- Lamazza, L.; Lollobrigida, M.; Vozza, I.; Palmieri, L.; Stacchi, C.; Lombardi, T.; De Biase, A. Piezoelectric implant site preparation: Influence of handpiece movements on temperature elevation. Materials 2020, 13, 4072. [Google Scholar] [CrossRef]
- Rebaudi, A.; Laffi, N.; Benedicenti, S.; Angiero, F.; Romanos, G.E. Microcomputed tomographic analysis of bone reaction at insertion of orthodontic mini-implants in sheep. Int. J. Oral Maxillofac. Implants 2011, 26, 1233–1240. [Google Scholar]
- Amghar-Maach, S.; Sánchez-Torres, A.; Camps-Font, O.; Gay-Escoda, C. Piezoelectric surgery versus conventional drilling for implant site preparation: A meta-analysis. J. Prosthodont. Res. 2018, 62, 391–396. [Google Scholar] [CrossRef]
- Bernabeu-Mira, J.C.; Pellicer-Chover, H.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. In vitro study on bone heating during drilling of the implant site: Material, design and wear of the surgical drill. Materials 2020, 13, 1921. [Google Scholar] [CrossRef]
- Maglione, M.; Bevilacqua, L.; Dotto, F.; Costantinides, F.; Lorusso, F.; Scarano, A. Observational study on the preparation of the implant site with piezosurgery vs. drill: Comparison between the two methods in terms of postoperative pain, surgical times, and operational advantages. BioMed Res. Int. 2019, 2019, 8483658. [Google Scholar] [CrossRef]
- Marenzi, G.; Sammartino, J.C.; Scherillo, F.; Rengo, C.; De Rosa, A.; Graziano, V.; Spagnuolo, G. Comparative analysis of the chemical composition and microstructure conformation between different dental implant bone drills. Materials 2019, 12, 1866. [Google Scholar] [CrossRef]
- Tretto, P.H.W.; Fabris, V.; Cericato, G.O.; Sarkis-Onofre, R.; Bacchi, A. Does the instrument used for the implant site preparation influence the bone-implant interface? A systematic review of clinical and animal studies. Int. J. Oral Maxillofac. Surg. 2019, 48, 97–107. [Google Scholar] [CrossRef]
- Gürkan, A.; Tekdal, G.P.; Bostancı, N.; Belibasakis, G.N. Cytokine, chemokine, and growth factor levels in peri-implant sulcus during wound healing and osseointegration after piezosurgical versus conventional implant site preparation: Randomized, controlled, split-mouth trial. J. Periodontol. 2019, 90, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Sadr-Eshkevari, P.; Weuster, M.; Schmitz, I.; Prochnow, N.; Maurer, P. Material attrition and bone micromorphology after conventional and ultrasonic implant site preparation. Clin. Oral Implants Res. 2013, 24 (Suppl. A100), 110–114. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A.; Kaiser, A.; Prochnow, N.; Schmitz, I.; Hoffmann, E.; Maurer, P. Heat production during different ultrasonic and conventional osteotomy preparations for dental implants. Clin. Oral Implants Res. 2011, 22, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Vercellotti, T. Piezoelectric surgery in implantology: A case report-a new piezoelectric ridge expansion technique. Int. J. Periodontics Restor. Dent. 2000, 20, 358–365. [Google Scholar]
- Pereira, C.C.; Gealh, W.C.; Meorin-Nogueira, L.; Garcia-Júnior, I.R.; Okamoto, R. Piezosurgery applied to implant dentistry: Clinical and biological aspects. J. Oral Implantol. 2014, 40, 401–408. [Google Scholar] [CrossRef]
- Canullo, L.; Peñarrocha, D.; Peñarrocha, M.; Rocio, A.G.; Penarrocha-Diago, M. Piezoelectric vs. conventional drilling in implant site preparation: Pilot controlled randomized clinical trial with crossover design. Clin. Oral Implants Res. 2014, 25, 1336–1343. [Google Scholar] [CrossRef]
- Fugito Junior, K.; Cortes, A.R.; de Carvalho Destro, R.; Yoshimoto, M. Comparative study on the cutting effectiveness and heat generation of rotary instruments versus piezoelectric surgery tips using scanning electron microscopy and thermal analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 345–350. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P.; Masciotra, L. Effect of 50 to 60 C heating on osseointegration of dental implants in dense bone: An in vivo histological study. Implants Dent. 2014, 23, 516–521. [Google Scholar] [CrossRef][Green Version]
- Lajolo, C.; Valente, N.A.; Romandini, W.G.; Petruzzi, M.; Verdugo, F.; D’Addona, A. Bone heat generated using conventional implant drills versus piezosurgery unit during apical cortical plate perforation. J. Periodontol. 2018, 89, 661–668. [Google Scholar] [CrossRef]
- Matthews, L.S.; Hirsch, C.J. Temperatures measured in human cortical bone when drilling. Bone Joint Surg. Am. 1972, 54, 297–308. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Vulpiani, M.P. Effect of temperature on the dental implant osseointegration development in low-density bone: An in vivo histological evaluation. Implants Dent. 2015, 24, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Andreana, S.; Spazzafumo, L.; Piemontese, M. An in vitro evaluation of heat production during osteotomy preparation for dental implants with compressive osteotomes. Implants Dent. 2013, 22, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Iezzi, G.; Perrotti, V.; Tetè, S.; Staiti, G.; Mortellaro, C.; Cappucci, C. Ultrasonic versus drills implant site preparation: A histologic analysis in bovine ribs. J. Craniofac. Surg. 2014, 25, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Sammartino, J.C.; Quaremba, G.; Graziano, V.; El Hassanin, A.; Qorri, M.E.; Sammartino, G.; Iorio-Siciliano, V. Clinical influence of micromorphological structure of dental implant bone drills. BioMed Res. Int. 2018, 2018, 8143962. [Google Scholar] [CrossRef]
- Di Fiore, A.; Sivolella, S.; Stocco, E.; Favero, V.; Stellini, E. Experimental analysis of temperature differences during implant site preparation: Continuous drilling technique versus intermittent drilling technique. J. Oral Implantol. 2018, 44, 46–50. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Quaranta, A.; Di Iorio, D.; Assenza, B.; Piattelli, A. Effects of bur wear during implant site preparation: An in vitro study. Int. J. Immunopathol. Pharmacol. 2007, 20 (Suppl. 1), 23–26. [Google Scholar] [CrossRef]
- Oliveira, N.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Thermal changes and drill wear in bovine bone during implant site preparation. A comparative in vitro study: Twisted stainless steel and ceramic drills. Clin. Oral Implants Res. 2012, 23, 963–969. [Google Scholar] [CrossRef]
- Chacon, G.E.; Bower, D.L.; Larsen, P.E.; McGlumphy, E.A.; Beck, F.M. Heat production by 3 implant drill systems after repeated drilling and sterilization. J. Oral Maxillofac. Surg. 2006, 64, 265–269. [Google Scholar] [CrossRef]
- Mozzati, M.; Gallesio, G.; Pol, R.; Cipollina, A.; Giacalone, A.; Mucciacito, N.; Galli, M.; Karanxha, L.; Greco Lucchina, A.; Del Fabbro, M. Implant site preparation using ultrasonic technique vs. Conventional drilling: Three-year follow-up of a split-mouth study. J. Biol. Regul. Homeost. Agents 2019, 33 (Suppl. 2), 49–58. [Google Scholar]
- Semez, G.I.; Luca, R.E.; Cescato, A.; Faoro, V.; Mocuţa, D.E.; Todea, D.C. Effect of the laser beam on implant site preparation: A preliminary pilot study. Rom. J. Morphol. Embryol. 2018, 59, 861–867. [Google Scholar]
- Schwarz, F.; Olivier, W.; Herten, M.; Sager, M.; Chaker, A.; Becker, J. Influence of implant bed preparation using an Er:YAG laser on the osseointegration of titanium implants: A histomorphometrical study in dogs. J. Oral Rehabil. 2007, 34, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rebaudi, A.; Cella, R.; Cecchini, G.; Trisi, P. Pre-operative evaluation of bone quality/density evaluation using novel CT/microCT-based Hard-Normal-Soft classification system. Int. J. Oral Maxillofac. Implants 2010, 25, 31–41. [Google Scholar]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implants Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Paolantoni, G.; Marenzi, G.; Blasi, A.; Mignogna, J.; Sammartino, G. Findings of a four-year randomized controlled clinical trial comparing two-piece and one-piece zirconia abutments supporting single prosthetic restorations in maxillary anterior region. BioMed Res. Int. 2016, 2016, 8767845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coyac, B.R.; Salvi, G.; Leahy, B.; Li, Z.; Salmon, B.; Hoffmann, W.; Helms, J.A. A novel system exploits bone debris for implant osseointegration. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Noetzel, N.; Fienitz, T.; Kreppel, M.; Zirk, M.; Safi, A.F.; Rothamel, D. Osteotomy speed, heat development, and bone structure influence by various piezoelectric systems-an in vitro study. Clin. Oral Investig. 2019, 23, 4029–4041. [Google Scholar] [CrossRef]
- Lamazza, L.; Garreffa, G.; Laurito, D.; Lollobrigida, M.; Palmieri, L.; De Biase, A. Temperature values variability in piezoelectric implant site preparation: Differences between cortical and corticocancellous bovine bone. BioMed Res. Int. 2016, 2016, 6473680. [Google Scholar] [CrossRef][Green Version]
- Vercellotti, T.; Stacchi, C.; Russo, C.; Rebaudi, A.; Vincenzi, G.; Pratella, U.; Baldi, D.; Mozzati, M.; Monagheddu, C.; Sentineri, R.; et al. Ultrasonic implant site preparation using piezosurgery: A multicenter case series study analyzing 3,579 implants with a 1- to 3-year follow-up. Int. J. Periodontics Restor. Dent. 2014, 34, 11–18. [Google Scholar] [CrossRef]
- Preti, G.; Martinasso, G.; Peirone, B.; Navone, R.; Manzella, C.; Muzio, G.; Russo, C.; Canuto, R.A.; Schierano, G. Cytokines and growth factors involved in the osseointegration of oral titanium implants positioned using piezoelectric bone surgery versus a drill technique: A pilot study in minipigs. J. Periodontol. 2007, 78, 716–722. [Google Scholar] [CrossRef]
- Sirolli, M.; Mafra, C.E.; Santos, R.A.; Saraiva, L.; Holzhausen, M.; César, J.B. Influence of piezosurgery on bone healing around titanium implants: A histological study in rats. Braz. Dent. J. 2016, 27, 278–283. [Google Scholar] [CrossRef]
- Rastelli, C.; Falisi, G.; Gatto, R.; Galli, M.; Saccone, E.; Severino, M.; DI Paolo, C. Implant stability in different techniques of surgical sites preparation: An in vitro study. Oral Implantol. 2014, 7, 33–39. [Google Scholar] [CrossRef] [PubMed]
- El-Kholey, K.E.; Elkomy, A. Does the drilling technique for implant site preparation enhance implant success in low-density bone? A systematic review. Implants Dent. 2019, 28, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Marzook, H.A.M.; Yousef, E.A.; Denewar, M.; Farahat, M.R.L. In-vitro assessment of bone viability with different implant drill speeds. Br. J. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kucukguven, M.B.; Topaloglu, G.; Isıkhan, S.Y.; Tosun, E.; Saysel, M.Y. In vitro evaluation of the primary stability of short implants in different surgical techniques. Int. J. Oral Maxillofac. Implants 2020, 35, 700–706. [Google Scholar] [CrossRef]
- Li, Z.; Arioka, M.; Liu, Y.; Aghvami, M.; Tulu, S.; Brunski, J.B.; Helms, J.A. Effects of condensation and compressive strain on implant primary stability: A longitudinal, in vivo, multiscale study in mice. Bone Jt. Res. 2020, 9, 60–70. [Google Scholar] [CrossRef]
- Falisi, G.; Severino, M.; Rastelli, C.; Bernardi, S.; Caruso, S.; Galli, M.; Lamazza, L.; Di Paolo, C. The effects of surgical preparation techniques and implant macro-geometry on primary stability: An in vitro study. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e201–e206. [Google Scholar] [CrossRef]
- Nóbrega, A.R.; Norton, A.; Silva, J.A.; Silva, J.P.; Branco, F.M.; Anitua, E. Osteotome versus conventional drilling technique for implant site preparation: A comparative study in the rabbit. Int. J. Periodontics Restor. Dent. 2012, 32, e109–e115. [Google Scholar]
- Möhlhenrich, S.C.; Kniha, K.; Heussen, N.; Hölzle, F.; Modabber, A. Effects on primary stability of three different techniques for implant site preparation in synthetic bone models of different densities. Br. J. Oral Maxillofac. Surg. 2016, 54, 980–986. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebaudi, A.; Rebaudi, F.; Barberis, F.; Sammartino, G.; Marenzi, G. Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison. Appl. Sci. 2020, 10, 8909. https://doi.org/10.3390/app10248909
Rebaudi A, Rebaudi F, Barberis F, Sammartino G, Marenzi G. Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison. Applied Sciences. 2020; 10(24):8909. https://doi.org/10.3390/app10248909
Chicago/Turabian StyleRebaudi, Alberto, Federico Rebaudi, Fabrizio Barberis, Gilberto Sammartino, and Gaetano Marenzi. 2020. "Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison" Applied Sciences 10, no. 24: 8909. https://doi.org/10.3390/app10248909
APA StyleRebaudi, A., Rebaudi, F., Barberis, F., Sammartino, G., & Marenzi, G. (2020). Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison. Applied Sciences, 10(24), 8909. https://doi.org/10.3390/app10248909






