Comparisons of the Anti-Inflammatory Activity of Dendropanax morbifera LEV Leaf Extract Contents Based on the Collection Season and Concentration of Ethanol as an Extraction Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Cell Culture
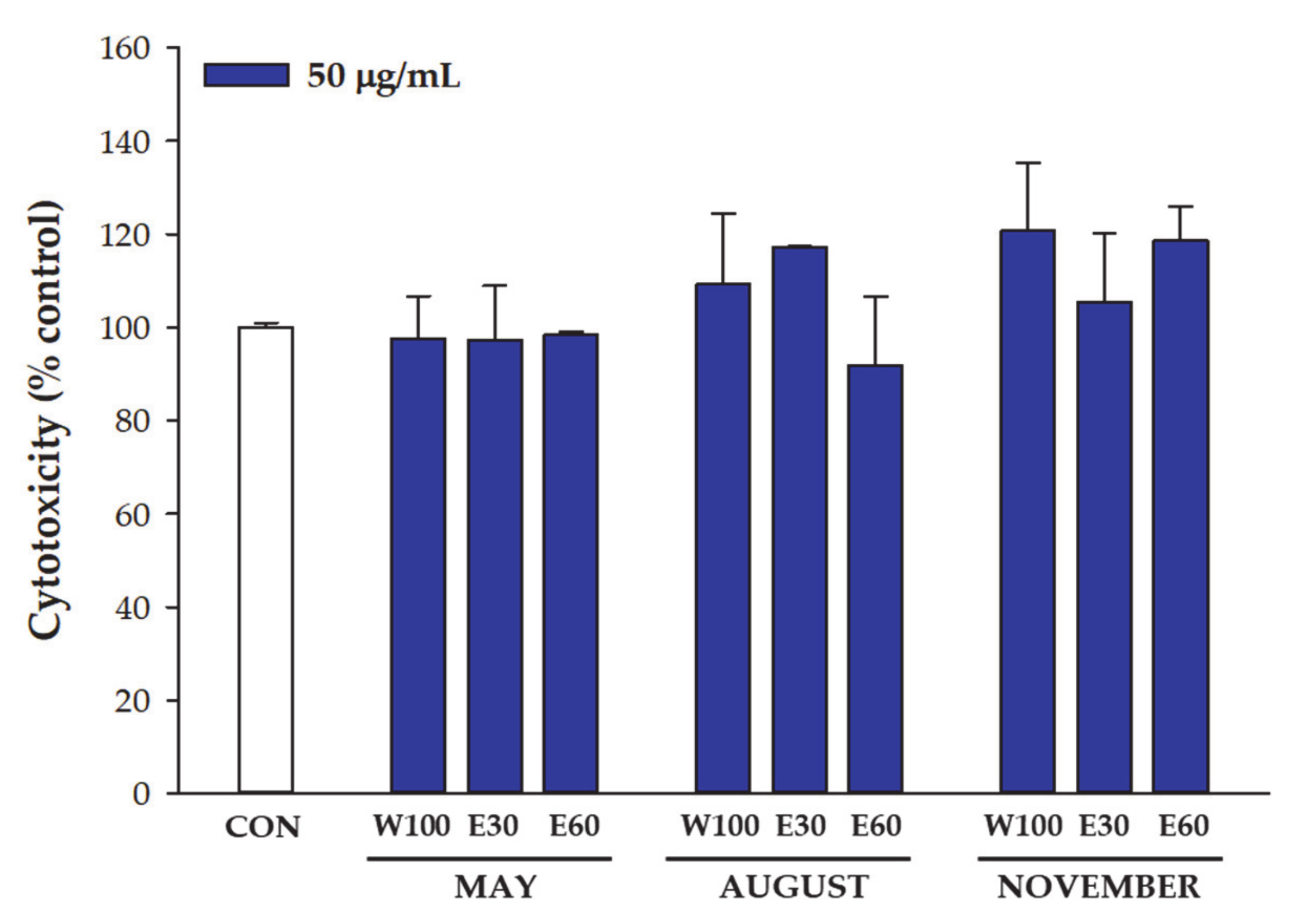
2.3. Cytotoxicity Assay
2.4. Measurement of Cytokines
2.5. Determination of Nitric Oxide (NO)
2.6. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.7. Protein Assay and Western Blotting
2.8. Amyrin Analysis
2.9. Analysis for the Identification of Selected Polyphenol Compounds
2.10. Statistical Analysis
3. Results
3.1. Inhibitory Effect of DM Extracts on Nitrite and PGE2 Production
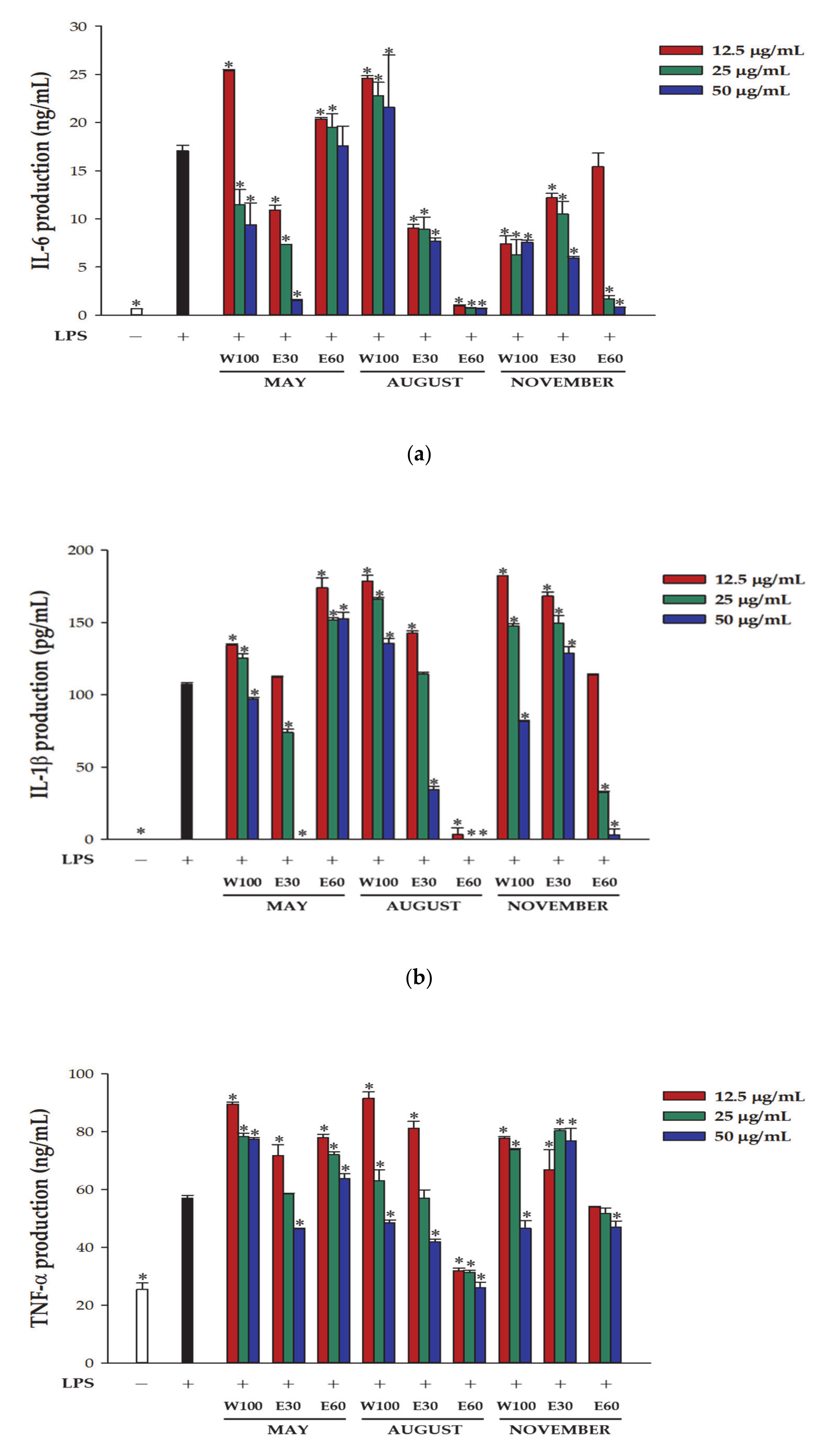
3.2. Inhibitory Effect of DM Extracts on Proinflammatory Cytokine Production
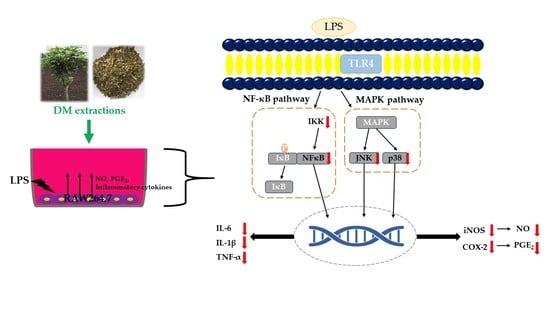
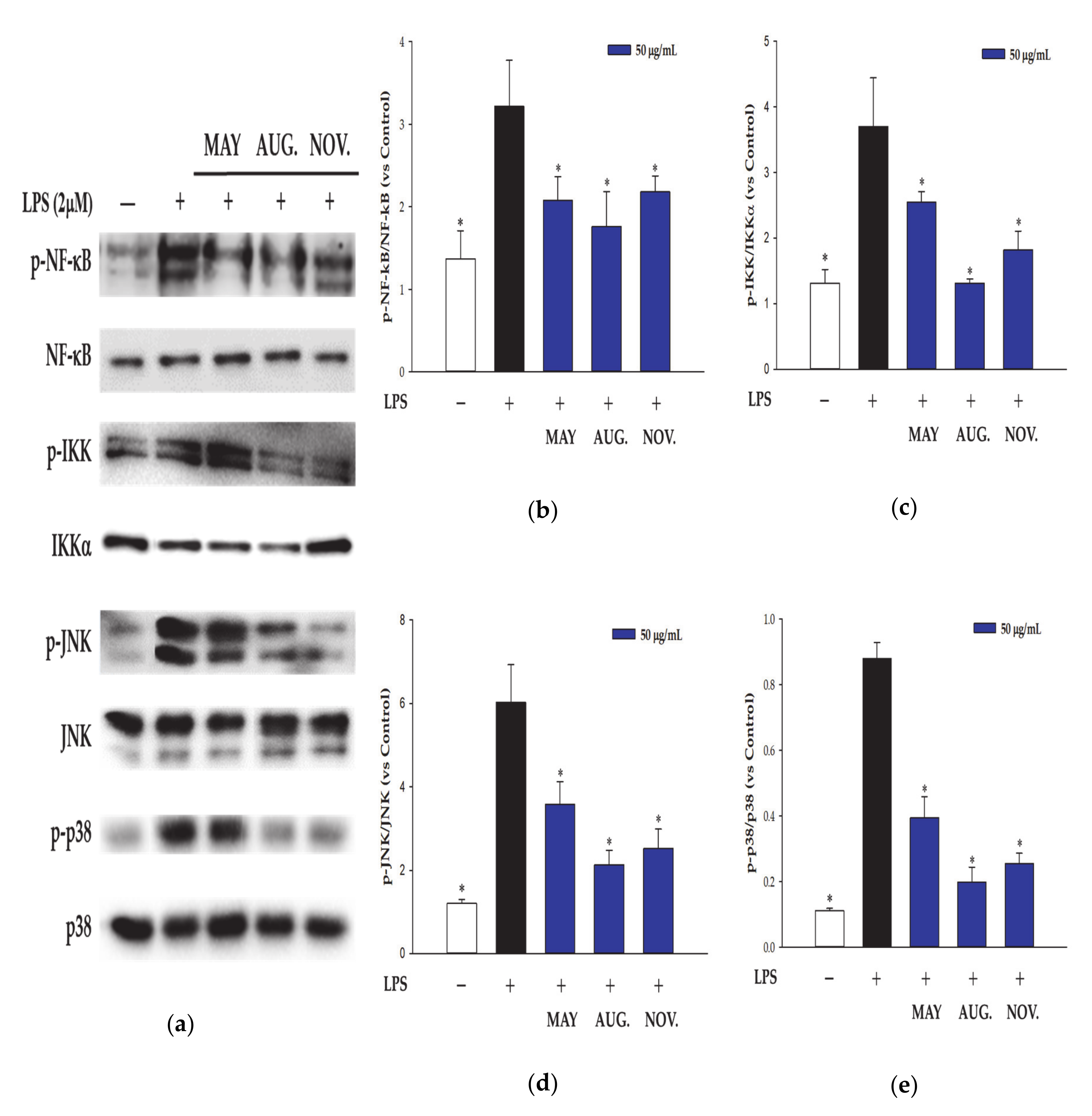
3.3. Effect of DM Extracts on the NF-κB and MAPK Signaling Pathway
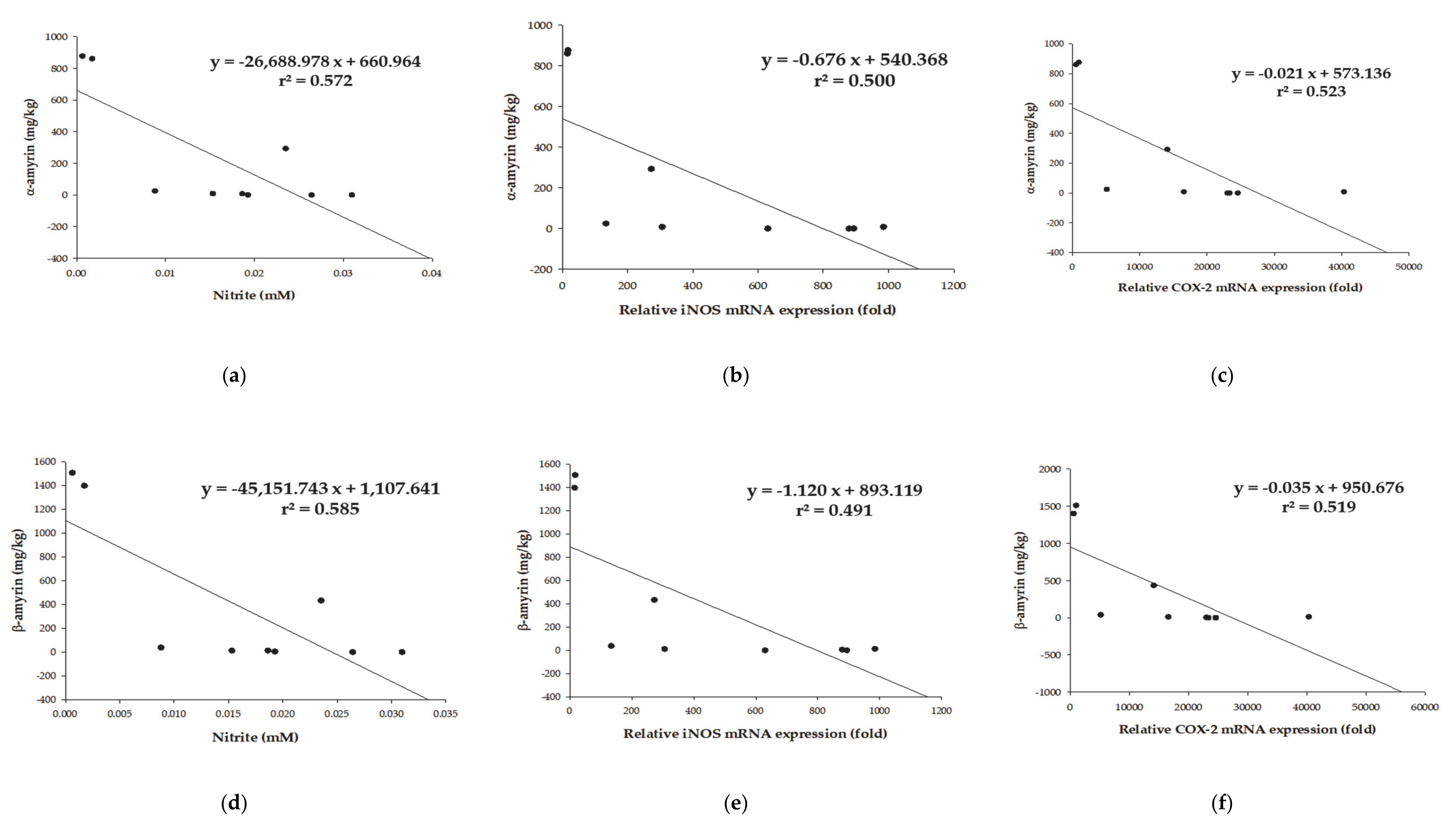
3.4. Chemical Characteristics for Each DM Extract
3.5. Content Changes Based on Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jönsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A Consideration of Biomarkers to be Used for Evaluation of Inflammation in Human Nutritional Studies. Br. J. Nutr. 2013, 109, S1–S34. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef]
- Ellulu, M.S. Obesity, cardiovascular disease, and role of vitamin C on inflammation: A review of facts and underlying mechanisms. Inflammopharmacology 2017, 25, 313–328. [Google Scholar] [CrossRef]
- Poffenberger, M.C.; Straka, N.; El Warry, N.; Fang, D.; Shanina, I.; Horwitz, M.S. Lack of IL-6 during Coxsackievirus Infection Heightens the Early Immune Response Resulting in Increased Severity of Chronic Autoimmune Myocarditis. PLoS ONE 2009, 4, e6207. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kim, M.-O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.-S. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Leveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.-W.; Lee, S.-Y.; Kim, S.-G.; Heo, Y.-R.; Son, A.M.-K. Antimicrobial, Antioxidant and Cytotoxic Activities of Dendropanax morbifera Leveille extract for mouthwash and denture cleaning solution. J. Adv. Prosthodont. 2016, 8, 172–180. [Google Scholar] [CrossRef]
- Kim, W.; Kim, D.W.; Yoo, D.-Y.; Jung, H.Y.; Nam, S.M.; Kim, J.W.; Hong, S.-M.; Kim, D.-W.; Choi, J.H.; Moon, S.M.; et al. Dendropanax morbifera Leveille extract facilitates cadmium excretion and prevents oxidative damage in the hippocampus by increasing antioxidant levels in cadmium-exposed rats. BMC Complement. Altern. Med. 2014, 14, 428. [Google Scholar] [CrossRef]
- Cho, S.S.; Song, S.-H.; Choi, C.Y.; Park, K.-M.; Shim, J.-H.; Park, D.-H. Optimization of the Extraction Conditions and Biological Evaluation of Dendropanax morbifera H. Lev as an Anti-Hyperuricemic Source. Molecules 2018, 23, 3313. [Google Scholar] [CrossRef]
- Song, J.-H.; Kang, H.-B.; Kim, J.H.; Kwak, S.; Sung, G.-J.; Park, S.-H.; Jeong, J.-H.; Kim, H.; Lee, J.; Jun, W.; et al. Antiobesity and Cholesterol-Lowering Effects of Dendropanax morbifera Water Extracts in Mouse 3T3-L1 Cells. J. Med. Food 2018, 21, 793–800. [Google Scholar] [CrossRef]
- Sun, S.; Kee, H.J.; Jin, L.; Ryu, Y.; Choi, S.Y.; Kim, G.R.; Jeong, M.H. Gentisic acid attenuates pressure overload-induced cardiac hypertrophy and fibrosis in mice through inhibition of the erk1/2 pathway. J. Cell. Mol. Med. 2018, 22, 5964–5977. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Kim, M.-Y.; Park, S.-D.; Park, W.-H.; Moon, H.-I. In vitro evaluation of the antiplasmodial activity of Dendropanax morbifera against chloroquine-sensitive strains of Plasmodium falciparum. Phytother. Res. 2009, 23, 1634–1637. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Song, H.-K.; Kim, S.-J.; Moon, H.-I. Anticomplement activity of polyacetylenes from leaves of Dendropanax morbiferaLeveille. Phytother. Res. 2010, 25, 784–786. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, D.-W.; Park, S.-E.; Lee, H.-J.; Kim, K.-M.; Kim, K.-J.; Kim, M.-K.; Kim, S.-J.; Kim, S. Anti-thrombotic effect of rutin isolated from Dendropanax morbifera Leveille. J. Biosci. Bioeng. 2015, 120, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Kim, K.-A.; Kim, E.-S.; Syed, A.S.; Kim, C.Y.; Lee, J.S.; Bae, O.-N. Potent Anti-inflammatory and Analgesic Actions of the Chloroform Extract of Dendropanax morbifera Mediated by the Nrf2/HO-1 Pathway. Biol. Pharm. Bull. 2016, 39, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jung, H.Y.; Yoo, D.Y.; Kim, W.; Kim, J.W.; Kwon, H.J.; Kim, D.W.; Yoon, Y.S.; Hwang, I.K.; Choi, J.H. Dendropanax morbifera Leveille extract ameliorates D-galactose-induced memory deficits by decreasing inflammatory responses in the hippocampus. Lab. Anim. Res. 2017, 33, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Kim, K.S.; Lee, Y.C.; Moon, H.I.; Lee, J.H. Oleifolioside a, a new active compound, attenuates lps-stimulated inos and cox-2 expression through the downregulation of nf-kappab and mapk activities in raw 264.7 macrophages. Evid. Based Complement. Alternat. Med. 2012, 2012, 637512. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.C.; Kim, S.H.; Kim, M.Y.; Kim, O.T.; Kim, K.S.; Hwang, B. Seasonal variations in yields of Hwangchil lacquer and major sesquiterpene compounds from selected superior individuals of Dendropanax morbifera Lev. J. Plant Biol. 2003, 46, 38–40. [Google Scholar] [CrossRef]
- Youn, J.S.; Kim, Y.-J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2018, 28, 201–207. [Google Scholar] [CrossRef]
- Shao, H.J.; Jeong, J.B.; Kim, K.J.; Lee, S.H. Anti-inflammatory activity of mushroom-derived hispidin through blocking of nf-kappab activation. J. Sci. Food Agric. 2015, 95, 2482–2486. [Google Scholar] [CrossRef]
- Sun, P.; Zhou, K.; Wang, S.; Li, P.; Chen, S.; Lin, G.; Zhao, Y.; Wang, T. Involvement of mapk/nf-kappab signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PLoS ONE 2013, 8, e69424. [Google Scholar]
- Van Ommen, B.; Keijer, J.; Heil, S.G.; Kaput, J. Challenging homeostasis to define biomarkers for nutrition related health. Mol. Nutr. Food Res. 2009, 53, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, O. Culinary plants and their potential impact on metabolic overload. Ann. N. Y. Acad. Sci. 2011, 1229, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Moezizadeh, M.; Javand, F.; Tabatabaei, F.S. Effects of extracts of Salvadora persica on proliferation and viability of human dental pulp stem cells. J. Conserv. Dent. 2015, 18, 315–320. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Russell, R.M. Biological functions of plant pigment phytochemicals in humans. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 4023–4045. [Google Scholar]
- Mestas, J.; Hughes, C.C.W. Of Mice and Not Men: Differences between Mouse and Human Immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. The Antioxidant and Anti-Inflammatory Properties of Rice Bran Phenolic Extracts. Foods 2020, 9, 829. [Google Scholar] [CrossRef]
- Cho, B.O.; So, Y.; Jin, C.H.; Nam, B.M.; Yee, S.T.; Jeong, I.Y. 3-deoxysilybin exerts anti-inflammatory effects by suppressing nf-kappab activation in lipopolysaccharide-stimulated raw264.7 macrophages. Biosci. Biotechnol. Biochem. 2014, 78, 2051–2058. [Google Scholar] [CrossRef]
- Hobbs, S.; Reynoso, M.; Geddis, A.V.; Mitrophanov, A.Y.; Matheny, R.W., Jr. Lps-stimulated nf-kappab p65 dynamic response marks the initiation of tnf expression and transition to il-10 expression in raw 264.7 macrophages. Physiol. Rep. 2018, 6, e13914. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The nf-kappab family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta Bioenerg. 2007, 1773, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Jin, Z.; Wang, J.; Xu, X. Nitrite oxide and inducible nitric oxide synthase were regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J. Ethnopharmacol. 2008, 118, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Poteser, M.; Wakabayashi, I. Serum albumin induces iNOS expression and NO production in RAW 267.4 macrophages. Br. J. Pharmacol. 2004, 143, 143–151. [Google Scholar] [CrossRef]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264.7 cells. Acta Biochim. Biophys. Sin. 2019, 51, 365–374. [Google Scholar] [CrossRef]
- Chun, K.-S.; Surh, Y.-J. Signal transduction pathways regulating cyclooxygenase-2 expression: Potential molecular targets for chemoprevention. Biochem. Pharmacol. 2004, 68, 1089–1100. [Google Scholar] [CrossRef]
- Da Silva Júnior, W.F.; Pinheiro, J.G.D.O.; De Menezes, D.L.B.; E Silva, N.E.d.S.; De Almeida, P.D.O.; Lima, E.S.; Da Veiga Júnior, V.F.; De Azevedo, E.P.; De Lima, Á.A.N. Development, physicochemical characterization and in vitro anti-inflammatory activity of solid dispersions of α,β amyrin isolated from protium oilresin. Molecules 2017, 22, 1512. [Google Scholar] [CrossRef]
- Da Silva Júnior, W.F.; Bezerra de Menezes, D.L.; de Oliveira, L.C.; Koester, L.S.; de Almeida, P.D.O.; Lima, E.S.; de Azevedo, E.P.; da Veiga Junior, V.F.; de Lima, Á.A.N. Inclusion complexes of β and HPβ-cyclodextrin with α,β amyrin and in vitro anti-inflammatory activity. Biomolecules 2019, 9, 241. [Google Scholar] [CrossRef]
- Melo, C.M.; Morais, T.C.; Tome, A.R.; Brito, G.A.C.; Chaves, M.H.; Rao, V.S.; Santos, F.A. Anti-inflammatory effect of α,β-amyrin, a triterpene from Protium heptaphyllum, on cerulein-induced acute pancreatitis in mice. Inflamm. Res. 2011, 60, 673–681. [Google Scholar] [CrossRef]
- Pinto, S.A.H.; Pinto, L.M.S.; Cunha, G.M.A.; Chaves, M.H.; Santos, F.A.; Rao, V. Anti-inflammatory effect of α,β-amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology 2007, 16, 48–52. [Google Scholar] [CrossRef] [PubMed]


| Month | EtOH (%) | α-Amyrin (mg/kg) | β-Amyrin (mg/kg) | Chlorogenic Acid (mg/g) | 4-Methylcatechol (mg/g) | p-Coumaric Acid (mg/g) | Rutin (mg/g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May | 0 | N.D. * | N.D. * | 39.61 | ± | 0.52 | 0.08 | ± | 0.05 | 2.77 | ± | 0.07 | 127.90 | ± | 0.02 | ||||
| 30 | 24.39 | ± | 1.31 | 37.86 | ± | 1.13 | 71.44 | ± | 1.48 | 0.38 | ± | 0.13 | 5.21 | ± | 0.12 | 167.00 | ± | 3.05 | |
| 60 | 292.97 | ± | 11.83 | 433.76 | ± | 11.21 | 76.71 | ± | 0.16 | 0.68 | ± | 0.09 | 5.56 | ± | 0.02 | 169.36 | ± | 0.61 | |
| August | 0 | N.D. * | N.D. * | 60.40 | ± | 0.07 | 0.21 | ± | 0.04 | 5.38 | ± | 0.02 | 105.31 | ± | 2.20 | ||||
| 30 | 8.28 | ± | 0.88 | 11.82 | ± | 0.21 | 66.98 | ± | 4.21 | 0.71 | ± | 0.03 | 6.59 | ± | 0.15 | 119.35 | ± | 2.47 | |
| 60 | 876.70 | ± | 18.20 | 1508.87 | ± | 20.21 | 79.43 | ± | 0.11 | 2.00 | ± | 0.08 | 9.76 | ± | 0.43 | 134.54 | ± | 0.51 | |
| November | 0 | 8.17 | ± | 0.47 | 12.30 | ± | 0.60 | 25.65 | ± | 1.44 | N.D. * | 2.20 | ± | 0.00 | 43.45 | ± | 0.22 | ||
| 30 | N.D. * | 4.94 | ± | 0.59 | 41.76 | ± | 0.81 | 0.12 | ± | 0.06 | 4.70 | ± | 0.06 | 47.88 | ± | 0.22 | |||
| 60 | 860.65 | ± | 35.97 | 1399.34 | ± | 59.38 | 42.87 | ± | 1.53 | 0.08 | ± | 0.05 | 3.92 | ± | 0.11 | 72.03 | ± | 2.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.J.; Youn, J.S.; Kim, Y.-J.; Kim, J.Y. Comparisons of the Anti-Inflammatory Activity of Dendropanax morbifera LEV Leaf Extract Contents Based on the Collection Season and Concentration of Ethanol as an Extraction Solvent. Appl. Sci. 2020, 10, 8756. https://doi.org/10.3390/app10238756
Kim KJ, Youn JS, Kim Y-J, Kim JY. Comparisons of the Anti-Inflammatory Activity of Dendropanax morbifera LEV Leaf Extract Contents Based on the Collection Season and Concentration of Ethanol as an Extraction Solvent. Applied Sciences. 2020; 10(23):8756. https://doi.org/10.3390/app10238756
Chicago/Turabian StyleKim, Kyeong Jin, Ji Sun Youn, Young-Jun Kim, and Ji Yeon Kim. 2020. "Comparisons of the Anti-Inflammatory Activity of Dendropanax morbifera LEV Leaf Extract Contents Based on the Collection Season and Concentration of Ethanol as an Extraction Solvent" Applied Sciences 10, no. 23: 8756. https://doi.org/10.3390/app10238756
APA StyleKim, K. J., Youn, J. S., Kim, Y.-J., & Kim, J. Y. (2020). Comparisons of the Anti-Inflammatory Activity of Dendropanax morbifera LEV Leaf Extract Contents Based on the Collection Season and Concentration of Ethanol as an Extraction Solvent. Applied Sciences, 10(23), 8756. https://doi.org/10.3390/app10238756