Multi-Element Determination of Toxic and Nutrient Elements by ICP-AES after Dispersive Solid-Phase Extraction with Modified Graphene Oxide
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Preparation of Graphite Oxide
2.3. Preparation of RGO
2.4. Preparation of NaGO
2.5. Instrumentation
2.6. Validation
2.7. Sample Preparation
2.8. d-SPE Process
3. Results and Discussions
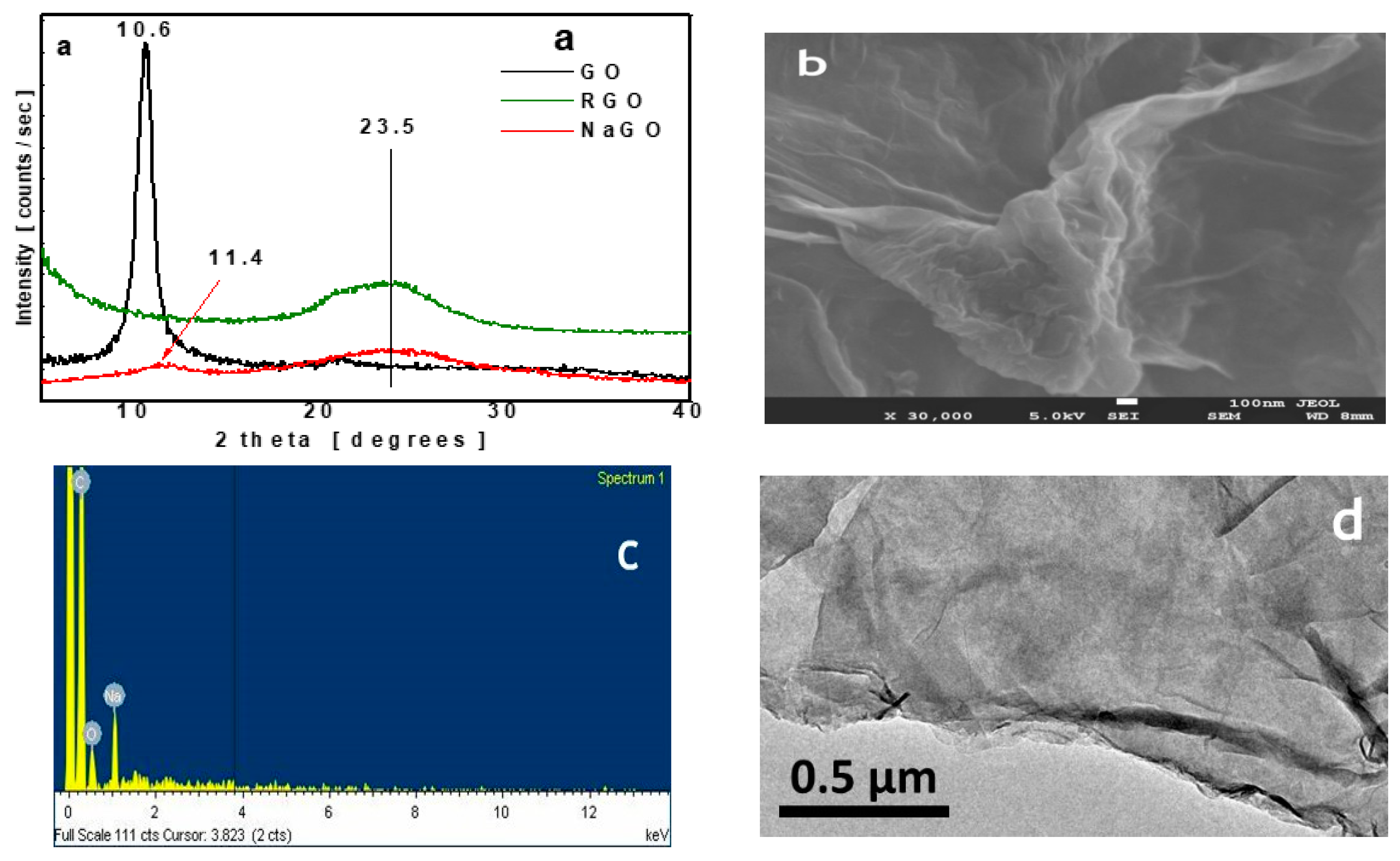
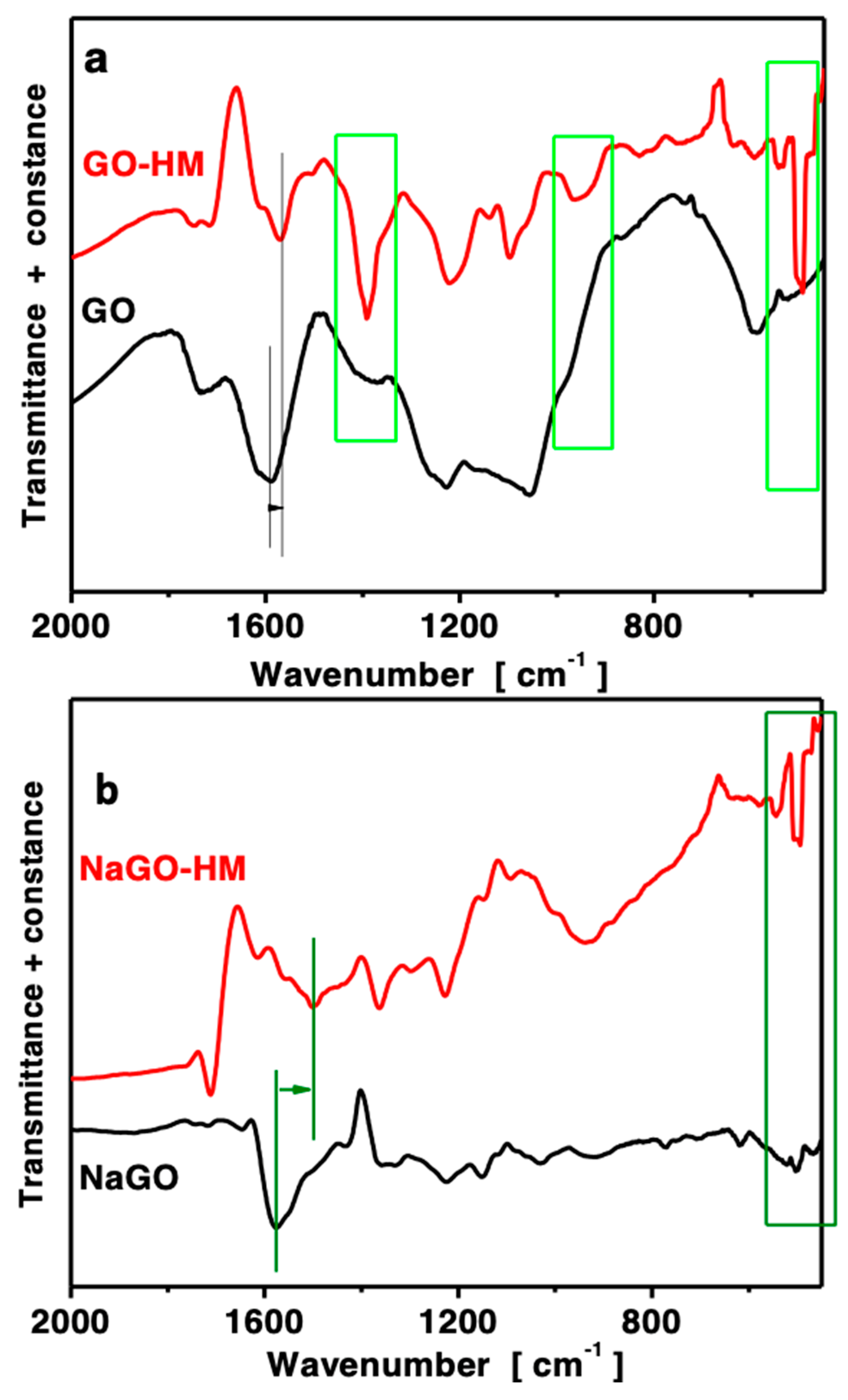
3.1. Material Characterization
3.2. Optimization of Dispersive Solid-Phase Extraction Procedure
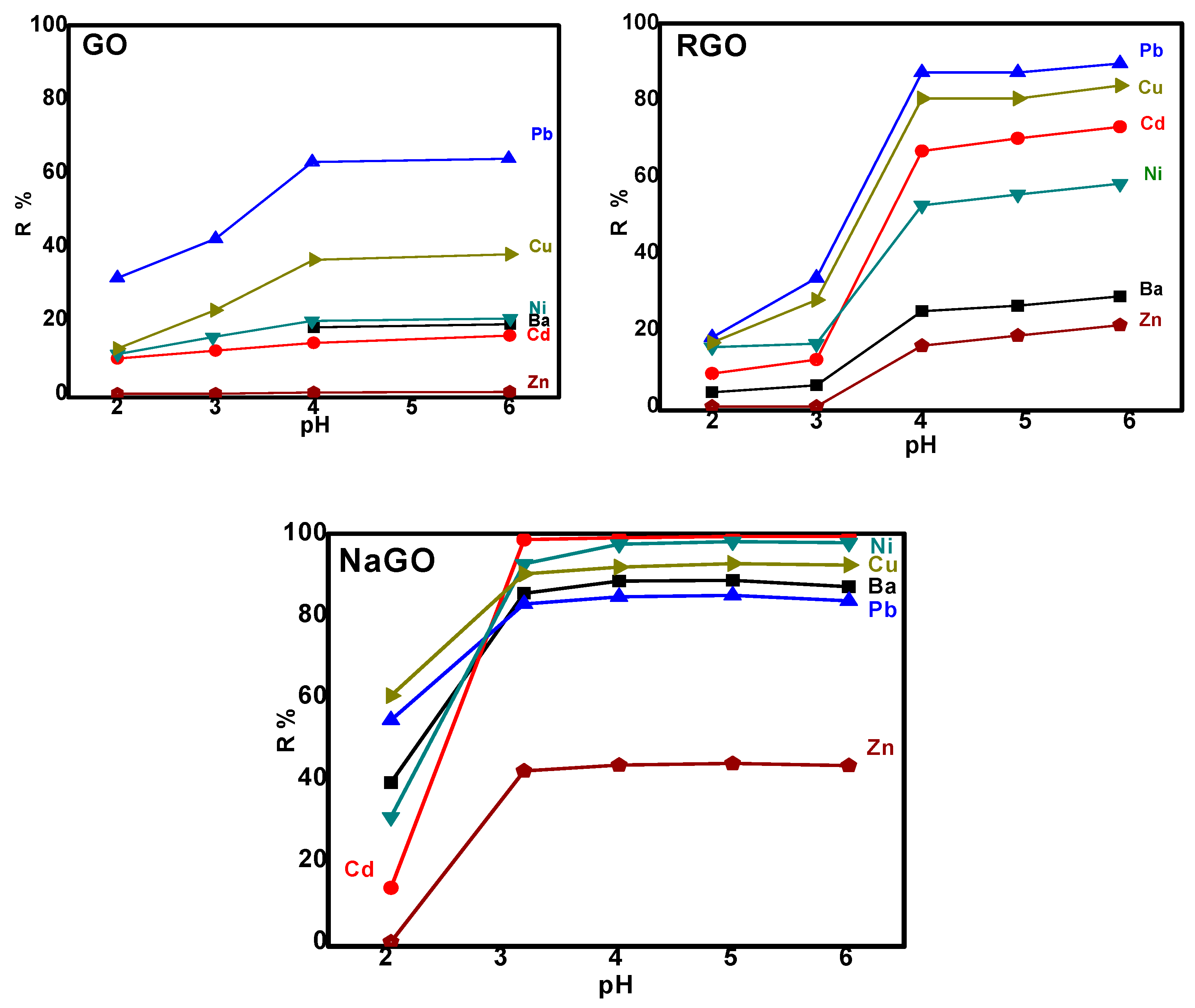
3.2.1. Selection of the Adsorbent
3.2.2. Selection of Emission Lines
3.2.3. Selection of Adsorption and Desorption Volume
3.2.4. Full Factorial Experimental Design for the Optimization of the d-SPE Method
3.3. Sorbent Capacity
3.4. Effect of Co-Existing Ions
3.5. Analytical Performance
3.6. Reusability of the Sorbent
3.7. Analysis of Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hidayah, N.M.S.; Liu, W.; Lai, C.; Noriman, N.Z.; Khe, C.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar]
- Du, J.; Cheng, H.M. The Fabrication, Properties, and Uses of Graphene/Polymer Composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Su, S.; Chen, B.; He, M.; Hu, B.; Xiao, Z. Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid-phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 2014, 119, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liang, Q.; Han, Q.; Zhang, X.; Ding, M. One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid-phase extraction of heavy metal ions from biological samples. Talanta 2015, 132, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liu, S.; Song, X.; Zhu, Y.; Jiang, S. Layer-by-layer self-assembled graphene oxide/silica microsphere composites as stationary phase for high performance liquid chromatography. Analyst 2012, 137, 5237–5244. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Yan, X.P. Fabrication of Graphene Oxide Nanosheets Incorporated Monolithic Column via One-Step Room Temperature Polymerization for Capillary Electrochromatography. Anal. Chem. 2011, 84, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Miao, S.; Yu, S.; Ma, L.P.; Sun, H.; Wang, S. Fabrication of Fe3O4/SiO2 core/shell nanoparticles attached to graphene oxide and its use as an adsorbent. J. Colloid Interf. Sci. 2010, 379, 20–26. [Google Scholar] [CrossRef]
- Kumara, A.S.K.; Jianga, S. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Yan, J.; Chen, G.; Cao, J.; Jang, W.; Xie, B.; Yang, M. Functionalized graphene oxide with ethylenediamine and 1,6-hexanediamine. New Carbon Mater. 2012, 27, 370–376. [Google Scholar] [CrossRef]
- Chandra, V.; Kim, K.S. Highly selective adsorption of Hg2+ by a polypyrrole–reduced graphene oxide composite. Chem. Commun. 2011, 47, 3942–3944. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption Behavior of EDTA-Graphene Oxide for Pb (II) Removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Yan, N.; Feng, J.; Ma, J.; Wen, Q.; Li, N.; Dong, Q. Adsorption mechanism of copper and lead ions onto graphene nanosheet/δ-MnO2. Mater. Chem. Phys. 2012, 136, 538–544. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Sun, X.; Su, Z.B.; Huang, Y.H.; Weng, W.; Hu, S.R.; Guo, H.X.; Wu, W.B.; He, Y.S. Highly effective removal of Cu(II) by triethylenetetramine -magnetic reduced graphene oxide composite. Appl. Surf. Sci. 2015, 356, 355–363. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ramezani, M.; Jabbari, A. Preconcentration and determination of lead ions in fish and mollusk tissues by nanocomposite of Fe3O4@graphene oxide@polyimide as a solid-phase extraction sorbent. Food Chem. 2017, 237, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.; He, S.; Huang, F.; Peng, Z. Adsorption Behaviour of Reduced Graphene Oxide for Removal of Heavy Metal Ions. Asian J. Chem. 2014, 26, 4901–4906. [Google Scholar] [CrossRef]
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.A.; Zachariadis, G.A. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2020, 152, 104428. [Google Scholar] [CrossRef]
- Pastor, A.; Medina, J.; Del Ramo, J.; Torreblanca, A.; Diaz-Mayans, J.; Hernandez, F. Determination of lead in treated crayfish Procambarus clarkii: Accumulation in different tissues. Bull. Environ. Contam. Toxicol. 1988, 41, 412–418. [Google Scholar] [CrossRef]
- Demirezen, D.; Uruç, K. Comparative study of trace elements in certain fish, meat and meat products. Meat Sci. 2006, 74, 255–260. [Google Scholar] [CrossRef]
- Pelus, Ε.; Arnaud, J.; Faure, H.; Favier, A.; Roussel, A.M. Trace element (Cu, Zn, Fe, Mn, Se) intakes of a group of French men using the duplicate diet technique. Int. J. Food Sci. Nutr. 1994, 45, 63–70. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Zachariadis, G.A.; Stratis, J.A. On-line preconcentration and determination of nickel and zinc in natural water samples by flow injection—flame atomic absorption spectrometry using PTFE-turnings for column packing. Intern. J. Environ. Anal. Chem. 2010, 90, 127–136. [Google Scholar] [CrossRef]
- Seiler, G.; Sigel, A.; Sigel, A.H. Handbook on Metals in Clinical and Analytical Chemistry; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Momen, A.A.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Use of fractional factorial design for optimization of digestion procedures followed by multi-element determination of essential and non-essential elements in nuts using ICP-AES technique. Talanta 2007, 71, 443–451. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, M.; Miranda, M.; Castillo, C.; Hernández, J.; García-Vaquero, M.; Benedito, J.L. Toxic and essential metals in liver, kidney and muscle of pigs at slaughter in Galicia, north-west Spain. Food Addit. Contam. 2007, 24, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Castineira, E.; Gomez, R.; Carmona-Gonzalez, M.A.; Fernandez-Salgvero, J. Study of minerals in meat products. Alimentaria 1995, 262, 63–67. [Google Scholar]
- Demirbaş, A. Proximate and heavy metal composition in chicken meat and tissues. Food Chem. 1999, 67, 27–31. [Google Scholar] [CrossRef]
- Papadomichelakis, G.; Zoidis, E.; Pappas, A.C.; Danezis, G.; Georgiou, C.A.; Fegeros, K. Dietary organic selenium addition and accumulation of toxic and essential trace elements in liver and meat of growing rabbits. Meat Sci. 2018, 145, 383–388. [Google Scholar] [CrossRef]
- Falandysz, J. Manganese, copper, zinc, iron, cadmium, mercury and lead in muscle meat, liver and kidneys of poultry, rabbit and sheep slaughtered in the northern part of Poland, 1987. Food Addit. Contam. 1991, 8, 71–83. [Google Scholar] [CrossRef]
- Little, T.A. Design of experiment is a powerful development tool for method characterization and method validation. BioPharm Int. 2014, 27, 40–45. [Google Scholar]
- Zacharis, C.K.; Vastardi, E. Application of analytical quality by design principles for the determination of alkyl p-toluenesulfonates impurities in Aprepitant by HPLC. Validation using total-error concept. J. Pharm. Biomed. Anal. 2017, 150, 152–161. [Google Scholar] [CrossRef]
- Escudero, L.A.; Cerutti, S.; Olsina, R.A.; Salonia, J.A.; Gasquez, J.A. Factorial design optimization of experimental variables in the on-line preparation/preconcentration of copper in water samples using solid-phase extraction and ICP-AES determination. J. Hazard. Mater. 2010, 183, 218–223. [Google Scholar] [CrossRef]
- Zachariadis, G.A.; Archontas, K.N. The Potential of Desirability Function Strategy in Chemometric Optimization of ICP-AES for Platinum Group Elements and Gold. Curr. Anal. Chem. 2016, 12, 147–158. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Karageorgou, E.; Samanidou, V. Youden test application in robustness assays during method validation. J. Chromatogr. A 2014, 1351, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.R.; Shemirani, F.; Jamali, M.R. Continuous pressurized solvent extraction of polycyclic aromatic hydrocarbons from biosolids. Assessment of their lability in soils amended with biosolids. Anal. Lett. 2010, 16, 2465–2476. [Google Scholar]
- Jeong, H.K.; Noh, H.J.; Kim, J.Y.; Jin, M.H.; Park, C.Y.; Lee, Y.H. X-ray absorption spectroscopy of graphite oxide. EPL 2008, 82, 1–5. [Google Scholar] [CrossRef]
- Seredych, M.; Tamashausky, A.V.; Bandosz, T.J. Graphite oxides obtained from porous graphite: The role of surface chemistry and texture in ammonia retention at ambient conditions. Adv. Funct. Mater. 2010, 20, 1670–1679. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation of activated carbon from date stones by microwave induced chemical activation: Application for methylene blue adsorption. Chem. Eng. J. 2011, 170, 338–341. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Anirudhan, T.S. Kinetic and equilibrium modeling of cobalt(ii) adsorption onto bagasse pith based sulphurised activated carbon. Chem. Eng. J. 2008, 137, 257–264. [Google Scholar] [CrossRef]
- Vukovic, G.D.; Marinkovic, A.D.; Skapin, S.D.; Ristic, M.D.; Aleksic, R.; Peric-Grujic, A.A.; Uskokovic, P.S. Removal of lead from water by amino modified multi-walled carbon nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M. Adsorption of Cr(III) on ozonised activated carbon. Importance of Cpi-cation interactions. Water Res. 2003, 37, 3335–3340. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Mitchell, J.K.; Bandosz, T.J. Reactive adsorption of mustard gas surrogate on zirconium (hydr)oxide/graphite oxide composites: The role of surface and chemical features. J. Mat. Chem. A 2016, 4, 1008–1019. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Giannakoudakis, D.A.; Bandosz, T.J. Zinc (hydr)oxide/graphite oxide/AuNPs composites: Role of surface features in H2S reactive adsorption. J. Colloid Interface Sci. 2014, 436, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Deliyanni, E.; Arabatzidou, A.; Tzoupanos, N.; Matis, K.A. Adsorption of Pb2+ Using Mesoporous Activated Carbon and Its Effects on Surface Modifications. Adsorpt. Sci. Technol. 2012, 30, 627–645. [Google Scholar] [CrossRef]
- Myglovets, M.; Poddubnaya, O.I.; Sevastyanova, O.; Lindström, M.E.; Gawdzik, B.; Sobiesiak, M.; Tsyba, M.M.; Sapsay, V.I.; Klymchuk, D.O.; Puziy, A.M. Preparation of carbon adsorbents from lignosulfonate by phosphoric acid activation for the adsorption of metal ions. Carbon 2014, 80, 771–783. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Matis, K.A. Activated carbons produced by pyrolysis of waste potato peels: Cobalt ions removal by adsorption. Colloids. Surf. A 2016, 490, 74–83. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Mitropoulos, A.C.; Matis, K.A. Hydrothermally produced activated carbons from zero-cost green sources for cobalt ions removal. Desalin. Water Treat. 2018, 123, 288–299. [Google Scholar] [CrossRef]
- Bibaj, E.; Lysigaki, K.; Nolan, J.W.; Seyedsalehi, M.; Deliyanni, E.A.; Mitropoulos, A.C.; Kyzas, G.Z. Activated carbons from banana peels for the removal of nickel ions. Int. J. Environ. Sci. Technol. 2019, 16, 667–680. [Google Scholar] [CrossRef]
- Angel Maqulelra, H.A.M.E.; Puchades, R. Use of Saccharomyces cerevisiae in Flow Injection Atomic Absorption Spectrometry for Trace Metal Preconcentration. Anal. Chem. 1994, 66, 3632–3638. [Google Scholar]
- Su, S.; Chen, B.; He, M.; Hu, B. Graphene oxide–silica composite coating hollow fiber solid-phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 2014, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Hu, B.; Jiang, Z.C. On-line preconcentration and separation of Co, Ni and Cd via capillary microextraction on ordered mesoporous alumina coating and determination by inductively plasma mass spectrometry (ICP-MS). Anal. Chim. Acta 2006, 572, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Hu, B. MPTS-silica coated capillary microextraction on line hyphenated with inductively coupled plasma atomic emission spectrometry for the determination of Cu, Hg and Pb in biological samples. Talanta 2007, 73, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Salarian, M.; Bagheri, A.; Tabani, H.; Omidi, F.; Fakhari, A. Synthesis, characterization and analytical application of Zn(II)-imprinted polymer as an efficient solid-phase extraction technique for trace determination of zinc ions in food samples. J. Food. Compos. Anal. 2014, 34, 81–89. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Talik, E.; Janik, P.; Osoba, G.; Feist, B.; Malicka, E. Spherical silica particles decorated with graphene oxide nanosheets as a new sorbent in inorganic trace analysis. Anal. Chim. Acta 2014, 834, 22–29. [Google Scholar] [CrossRef]
- Pourjavid, M.; Arabieh, M.; Yousefi, S.; Jamali, M.; Rezaee, M.; Hosseini, M.; Sehat, A. Study on column SPE with synthesized graphene oxide and FAAS for determination of trace amount of Co(II) and Ni(II) ions in real samples. Mat. Sci. Eng. C 2015, 47, 114–122. [Google Scholar] [CrossRef]
- Yavuz, E.; Tokalıoğlu, S.; Sxahan, H.; Patat, S.A. A graphene/Co3O4 nanocomposite as a new adsorbent for solid phase extraction of Pb(II), Cu(II) and Fe(III) ions in various samples. RSC Adv. 2013, 3, 24650–24657. [Google Scholar] [CrossRef]
- Sayar, O.; Mehrani, K.; Hoseinzadeh, F.; Mehrani, A.; Sadeghi, O. Comparison of the performance of different modified graphene oxide nanosheets for the extraction of Pb(II) and Cd(II) from natural samples. Microchim. Acta 2014, 181, 313–320. [Google Scholar] [CrossRef]
- Zheng, H.; Jia, B.; Zhu, Z.; Tang, Z.; Hu, S. Determination of trace amounts of Pb, Cd, Ni and Co by wavelength-dispersive Xray fluorescence spectrometry after preconcentration with dithizone functionalized graphene. Anal. Methods 2014, 6, 8569–8576. [Google Scholar] [CrossRef]
- Kocot, K.; Sitko, R. Trace and ultratrace determination of heavy metal ions by energy-dispersive X-ray fluorescence spectrometry using graphene as solid sorbent in dispersive micro solid-phase extraction. Spectrochim. Acta B 2014, 94–95, 7–13. [Google Scholar] [CrossRef]
| Analyte | Adsorption Capacity (mg g−1) | ||||||
|---|---|---|---|---|---|---|---|
| NaGO (This Work) | GO-Silica [53] | Al2O3 [54] | Sol Gel 3-Mercaptopropyltrimethoxysilane Silica [55] | Zn-Molecularly Imprinted Polymer [56] | GO/SiO2 [57] | GO [58] | |
| Cu | 40.3 | 5.5 | - | - | - | 6.0 | - |
| Zn | 228.1 | - | - | - | 68.6 | - | - |
| Ba | 4.8 | - | - | - | - | - | - |
| Cd | 4.3 | 16 | 1.90 | - | - | - | - |
| Ni | 3.9 | 7.4 | 1.19 | - | - | - | 7.0 |
| Pb | 24.9 | 25 | - | 1.19 | - | 13.6 | - |
| Element | Calibration Curves | R | Linear Range (μg g−1) | LOD (μg g−1) | LOQ (μg g−1) | ER 1% | EF 2 |
|---|---|---|---|---|---|---|---|
| Ba | y = (80.8 ± 2.0)x − (7.6 ± 1.2) | 0.9990 | 5–100 | 0.01 | 0.03 | 73.8 | 15 |
| Cd | y = (68.2 ± 1.3)x − (321.8 ± 31.9) | 0.9990 | 5–500 | 0.03 | 0.09 | 91.2 | 18 |
| Pb | y = (3.7 ± 0.3)x − (180.2 ± 10.1) | 0.9990 | 25–500 | 0.21 | 0.63 | 69.3 | 14 |
| Ni | y = (24.5 ± 0.3)x − (66.9 ± 5.8) | 0.9996 | 5–500 | 0.08 | 0.24 | 79.7 | 16 |
| Cu | y = (2551 ± 77.9)x + (71826 ± 363.9) | 0.9997 | 20–1000 | 0.10 | 0.30 | 58.7 | 12 |
| Zn | y = (60.6 ± 1.5)x + (544 ± 37.5) | 0.9997 | 20–5000 | 0.10 | 0.32 | 95.1 | 19 |
| Element | Added (μg) | Intra-Day (n = 5) | Inter-Day (n = 5 × 3) | Instrumental RSD (n = 7) (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Found (μg) | RSD% | Relative Recovery (%) | Found (μg) | RSD% | Relative Recovery (%) | |||
| Ba | 12.5 | 13.4 ± 0.2 | 1.1 | 107.4 | 14.5 ± 0.6 | 4.0 | 116.2 | 0.2 |
| Cd | 12.5 | 13.5 ± 0.1 | 0.8 | 107.8 | 12.4 ± 0.4 | 3.5 | 99.0 | 0.5 |
| Pb | 12.5 | 14.7 ± 0.1 | 0.6 | 117.8 | 12.6 ± 0.4 | 3.2 | 101.1 | 1.4 |
| Ni | 12.5 | 13.5 ± 0.1 | 1.0 | 108.0 | 13.1 ± 0.3 | 2.6 | 104.6 | 0.6 |
| Cu | 50 | 45.1 ± 0.6 | 1.3 | 90.1 | 45.7 ± 2.2 | 4.7 | 91.4 | 1.0 |
| Zn | 50 | 47.2 ± 0.9 | 1.9 | 94.3 | 46.6 ± 1.1 | 2.3 | 93.1 | 0.4 |
| Element | Chicken (μg g−1) | Bovine (μg g−1) | Pork (μg g−1) |
|---|---|---|---|
| Ba | <LOQ | <LOQ | <LOQ |
| Cd | <LOQ | <LOQ | <LOQ |
| Pb | <LOQ | <LOQ | <LOQ |
| Ni | 21.1 ± 0.3 | 25.2 ± 0.2 | 22.3 ± 0.4 |
| Cu | 4.8 ± 0.2 | <LOQ | <LOQ |
| Zn | 67.0 ± 3.2 | 205.1 ± 9.2 | 55.8 ± 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Deliyanni, E.; Zachariadis, G. Multi-Element Determination of Toxic and Nutrient Elements by ICP-AES after Dispersive Solid-Phase Extraction with Modified Graphene Oxide. Appl. Sci. 2020, 10, 8722. https://doi.org/10.3390/app10238722
Manousi N, Deliyanni E, Zachariadis G. Multi-Element Determination of Toxic and Nutrient Elements by ICP-AES after Dispersive Solid-Phase Extraction with Modified Graphene Oxide. Applied Sciences. 2020; 10(23):8722. https://doi.org/10.3390/app10238722
Chicago/Turabian StyleManousi, Natalia, Eleni Deliyanni, and George Zachariadis. 2020. "Multi-Element Determination of Toxic and Nutrient Elements by ICP-AES after Dispersive Solid-Phase Extraction with Modified Graphene Oxide" Applied Sciences 10, no. 23: 8722. https://doi.org/10.3390/app10238722
APA StyleManousi, N., Deliyanni, E., & Zachariadis, G. (2020). Multi-Element Determination of Toxic and Nutrient Elements by ICP-AES after Dispersive Solid-Phase Extraction with Modified Graphene Oxide. Applied Sciences, 10(23), 8722. https://doi.org/10.3390/app10238722








