Structure and Stability of Gas Adsorption Complexes in Periodic Porous Solids as Studied by VTIR Spectroscopy: An Overview
Abstract
1. Introduction
2. Background
2.1. Periodic Porous Solids
2.2. Outline of the VTIR Spectroscopic Method
2.3. Theoretical Calculations
3. Case Studies
3.1. Carbon Monoxide: Preliminary Considerations
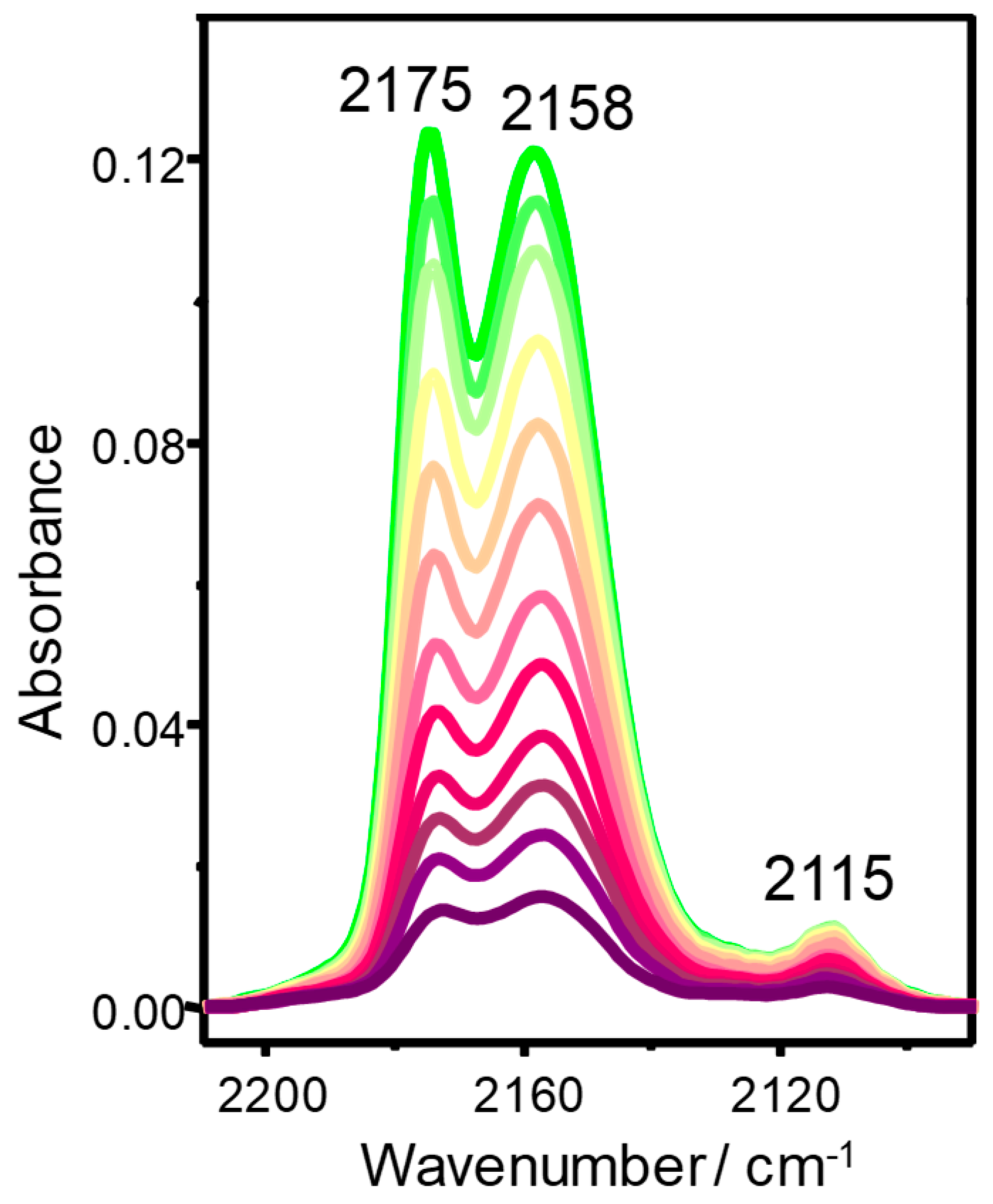
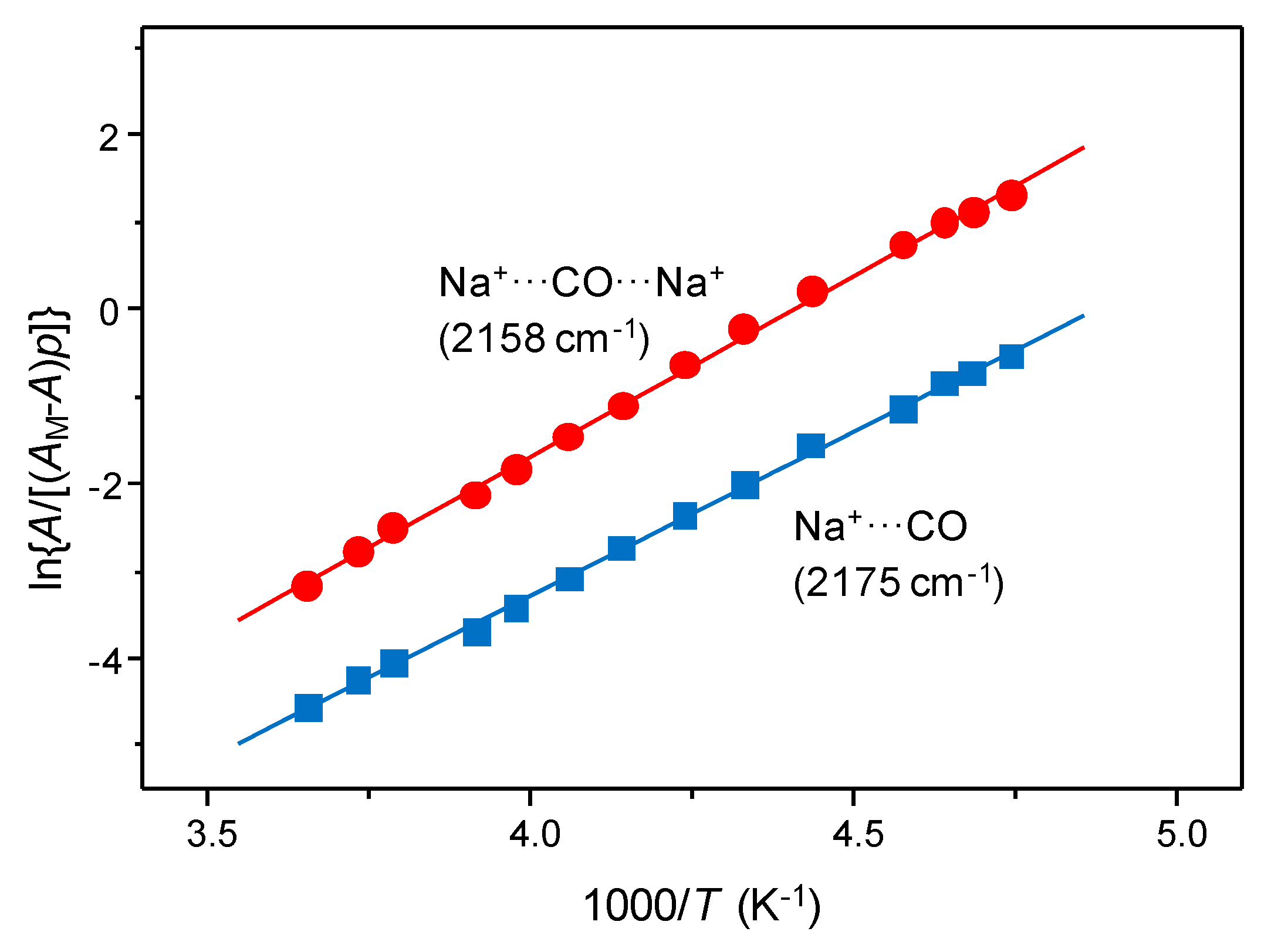
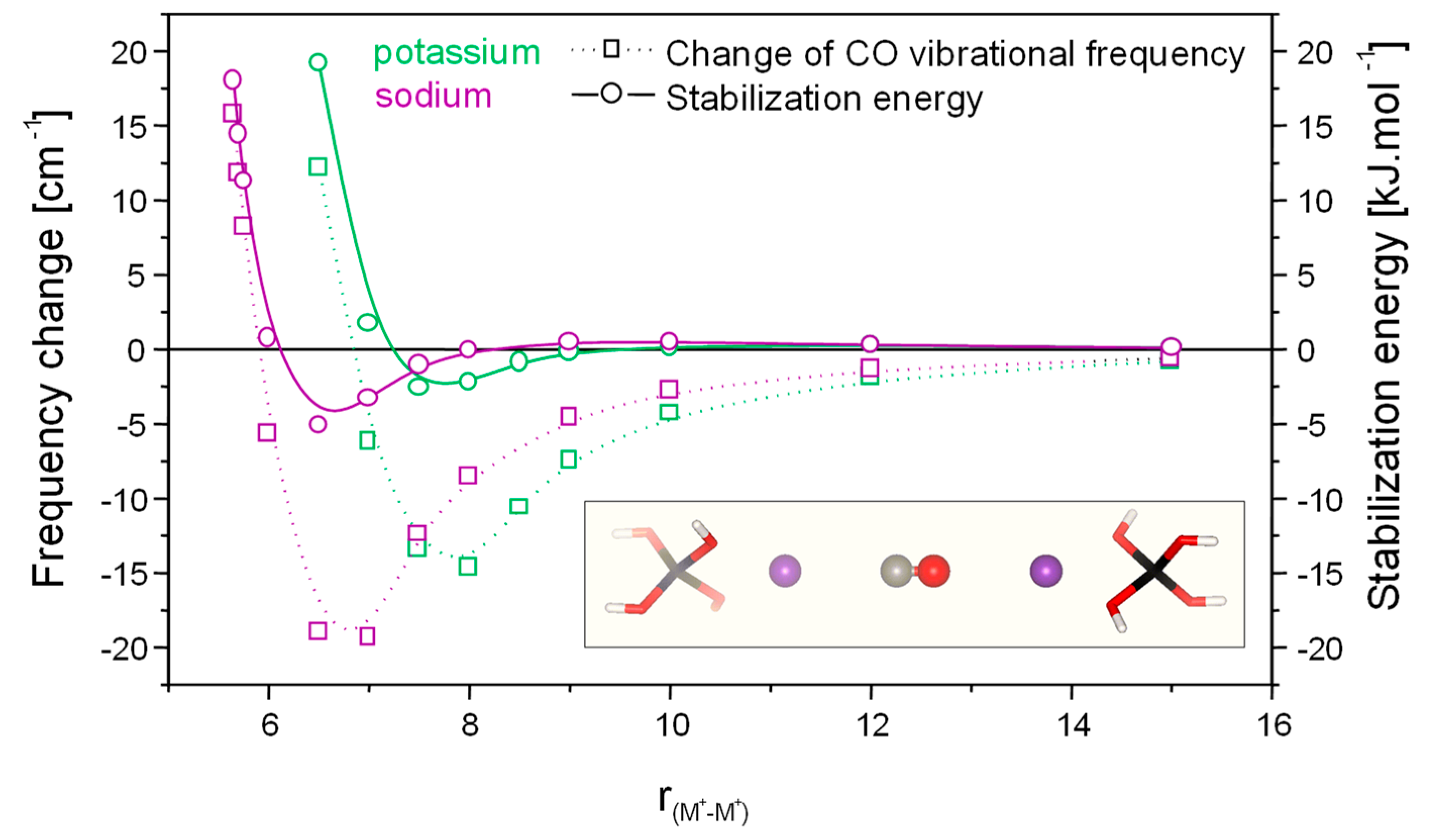
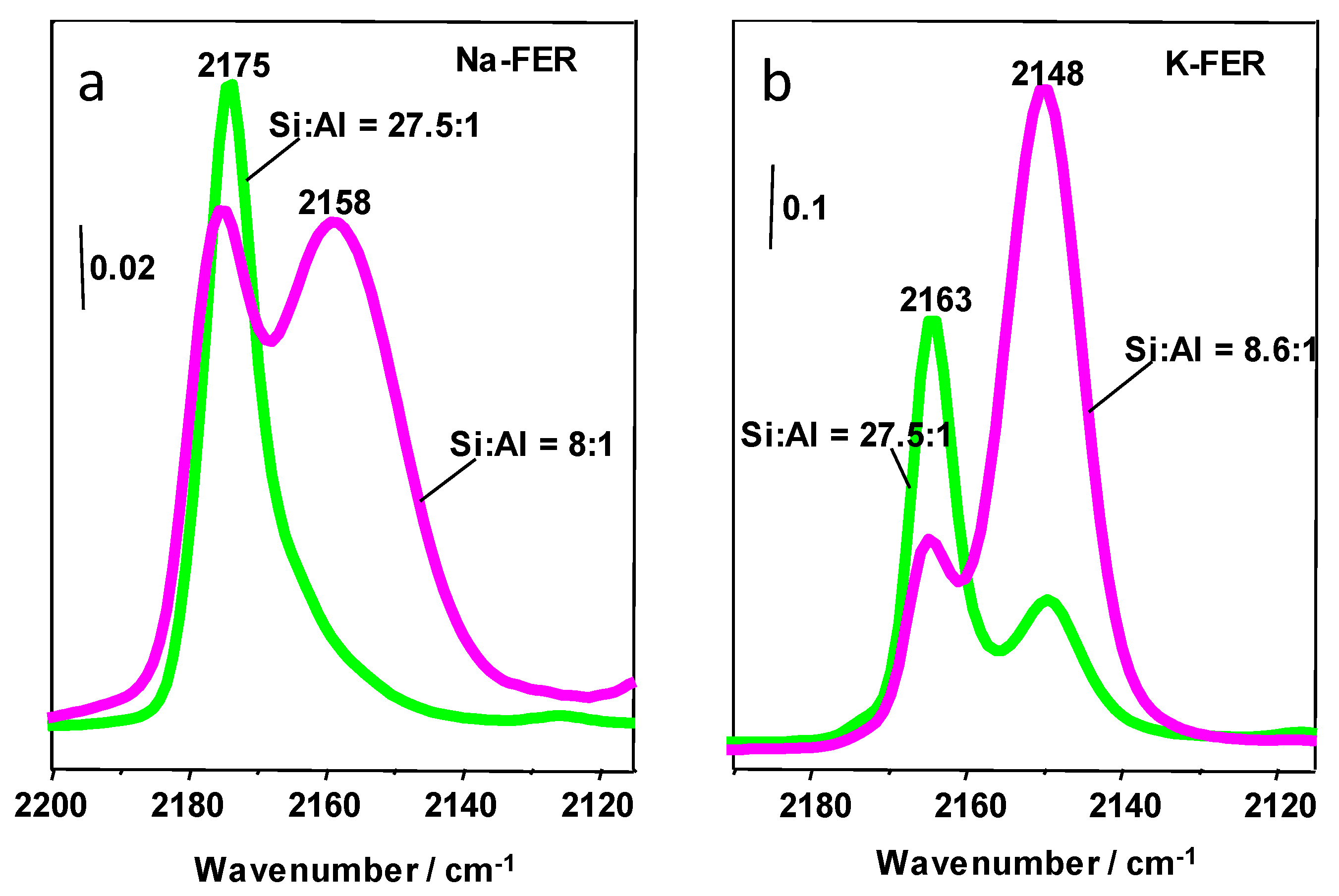
3.2. Carbon Monoxide Adsorption in Zeolites
3.3. Carbon Dioxide and Dinitrogen
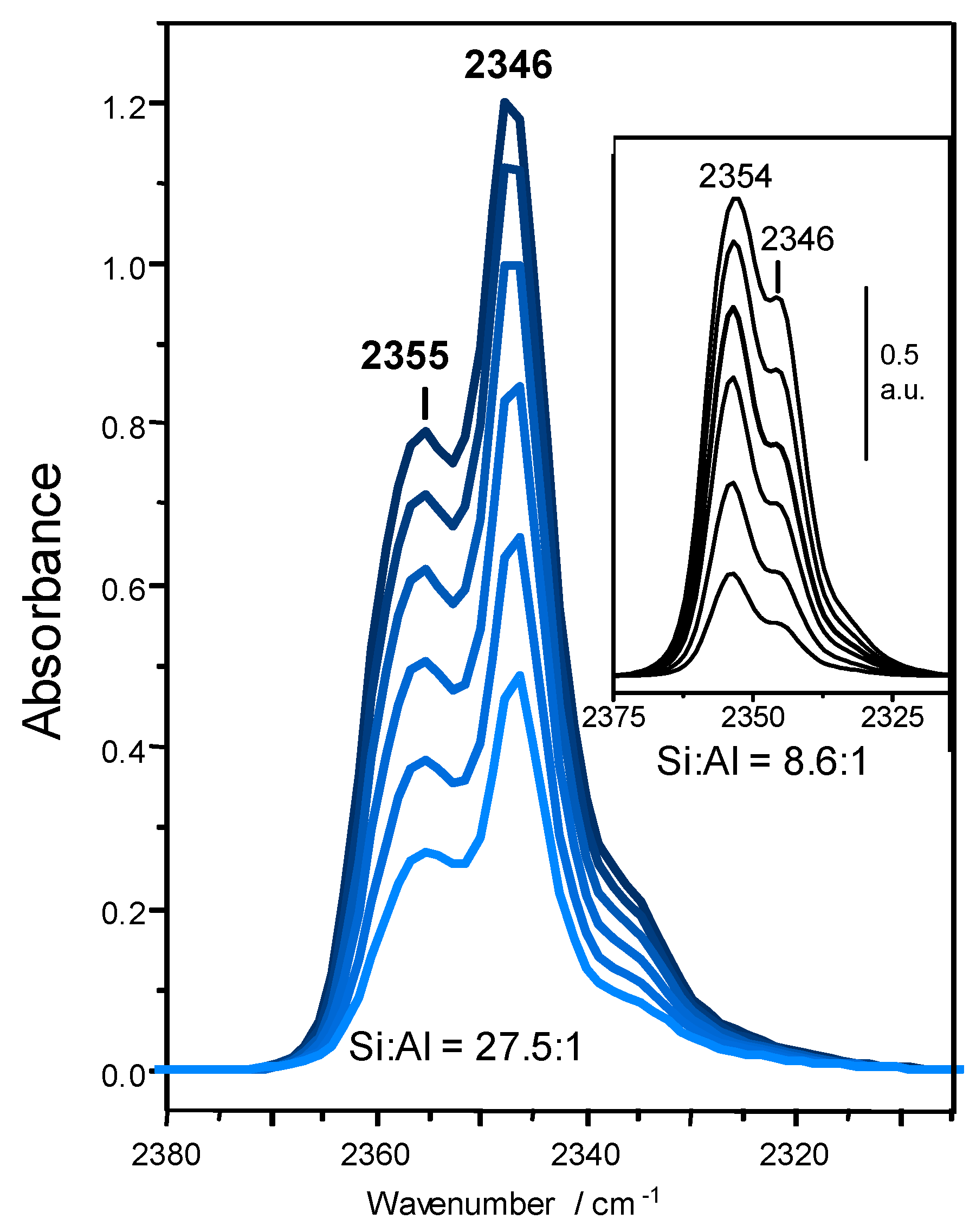
3.3.1. CO2 Adsorption in K-FER
3.3.2. Carbon Dioxide and Dinitrogen Adsorption in K-L
4. A Synopsis and Some Remarks
Funding
Conflicts of Interest
References
- Mendes, A.; Costa, C.A.; RodrIn Proceedings of theigues, A.E. Oxygen separation from air by PSA: Modelling and experimental results. Sep. Purif. Technol. 2001, 24, 173–188. [Google Scholar] [CrossRef]
- Mofarahi, M.; Towfighi, J.; Fathi, L. Oxygen Separation from Air by Four-Bed Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2009, 48, 5439–5444. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Hamed, H.H. Oxygen separation from air using zeolite type 5A. Int. J. Sci. Eng. Res. 2015, 6, 597. [Google Scholar]
- Zhao, X.X.; Sun, X.; Liu, X. Adsorption separation of carbon dioxide, methane and nitrogen on H and Na-exchanged-zeolite. J. Nat. Gas Chem. 2008, 17, 391. [Google Scholar]
- Montanari, T.; Finocchio, E.; Salvatore, E.; Garuti, G.; Giordano, A.; Pistarino, C.; Busca, G. CO2 separation and landfill biogas upgrading: A comparison of 4A and 13X zeolite adsorbents. Energy 2011, 36, 314–319. [Google Scholar] [CrossRef]
- Tagliabue, M.; Rizzo, C.; Onorati, N.B.; Gambarotta, E.F.; Carati, A.; Bazzano, F. Regenerability of zeolites as adsorbents for natural gas sweetening: A case-study. Fuel 2012, 93, 238–244. [Google Scholar] [CrossRef]
- Maghsoudi, H.; Soltanieh, M.; Bozorgzadeh, H.; Mohamadalizadeh, A. Adsorption isotherms and ideal selectivities of hydrogen sulfide and carbon dioxide over methane for the Si-CHA zeolite: Comparison of carbon dioxide and methane adsorption with the all-silica DD3R zeolite. Adsorption 2013, 19, 1045–1053. [Google Scholar] [CrossRef]
- Herm, Z.R.; Swisher, J.A.; Smit, B.; Krishna, R.; Long, J.R. Metal−Organic Frameworks as Adsorbents for Hydrogen Purification and Precombustion Carbon Dioxide Capture. J. Am. Chem. Soc. 2011, 133, 5664–5667. [Google Scholar] [CrossRef]
- Brea, P.; Delgado, J.; Águeda, V.I.; Gutiérrez, P.; Uguina, M.A. Multicomponent adsorption of H2, CH4, CO and CO2 in zeolites NaX, CaX and MgX. Evaluation of performance in PSA cycles for hydrogen purification. Microporous Mesoporous Mater. 2019, 286, 187–198. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, Y.; Chung, T.S. Poly-metal-benzimidazol nano-composite membrane for hydrogen purification. Energy Environ. Sci. 2011, 4, 4171. [Google Scholar] [CrossRef]
- D.’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225. [Google Scholar] [CrossRef]
- Pulido, A.; Delgado, M.R.; Bludsky, O.; Rubes, M.; Nachtigall, P.; Arean, C.O. Combined DFT/CC and IR spectroscopic studies on carbon dioxide adsorption in the zeolite H-FER. Energy Environ. Sci. 2009, 2, 1187. [Google Scholar] [CrossRef]
- Thang, H.V.; Grajciar, L.; Nachtigall, P.; Bludský, O.; Areán, C.O.; Frýdová, E.; Bulánek, R. Adsorption of CO2 in FAU zeolites: Effect of zeolite composition. Catal. Today 2014, 227, 50–56. [Google Scholar] [CrossRef]
- Kwong, P.; Chao, C.Y.; Hui, O.K.S.; Wan, M.; Chao, C.Y.-H. Removal of VOCs from indoor environment by ozonation over different porous materials. Atmos. Environ. 2008, 42, 2300–2311. [Google Scholar] [CrossRef]
- Liu, M.; Yang, D.; Pang, L.; Yu, Q.; Huang, Y. Experimental and computational investigation of adsorption performance of TC-5A and PSA-5A for manned spacecraft. Chin. J. Aeronaut. 2015, 28, 1583–1592. [Google Scholar] [CrossRef]
- Li, G.; Pang, L.; Liu, M.; Yang, D.; Yu, Q.; Rong, A. Multiobjective optical method for carbon dioxide removal assembly in manned spacecraft. J. Aerosp. Eng. 2016, 29, 040016052. [Google Scholar]
- Sidheswaran, M.A.; Destaillats, H.; Sullivan, D.P.; Cohn, S.; Fisk, W.J. Energy efficient indoor VOC air cleaning with activated carbon fiber (ACF) filters. Build. Environ. 2012, 47, 357–367. [Google Scholar] [CrossRef]
- Menon, V.C.; Komarneni, S. Porous Adsorbents for Vehicular Natural Gas Storage: A Review. J. Porous Mater. 1998, 5, 43–58. [Google Scholar] [CrossRef]
- Makal, T.A.; Li, J.-R.; Lu, W.; Zhou, H.-C. Methane storage in advanced porous materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef] [PubMed]
- Armandi, M.; Bonelli, B.; Areán, C.O.; Garrone, E. Role of microporosity in hydrogen adsorption on templated nanoporous carbons. Microporous Mesoporous Mater. 2008, 112, 411–418. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal-organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]
- Lin, K.; Adhikari, A.K.; Ku, C.-N.; Chiang, C.-L.; Kuo, H. Synthesis and characterization of porous HKUST-1 metal organic frameworks for hydrogen storage. Int. J. Hydrogen Energy 2012, 37, 13865–13871. [Google Scholar] [CrossRef]
- Lin, K.-S.; Adhikari, A.K.; Tu, M.-T.; Wang, C.-H.; Chiang, C.-L. Preparation, characterization, and hydrogen storage capacity of MIL-53 metal-organic frameworks. J. Nanosci. Nanotechnol. 2013, 13, 2549–2556. [Google Scholar] [CrossRef]
- Wijiyanti, R.; Gunawan, T.; Nasri, N.S.; Karim, Z.A.; Ismail, A.F.; Widiastuti, N. Hydrogen Adsorption Characteristics for Zeolite-Y Templated Carbon. Indones. J. Chem. 2019, 20, 29. [Google Scholar] [CrossRef]
- Berg, A.W.C.V.D.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 6, 668. [Google Scholar] [CrossRef]
- Dincă, M.; Long, J.R. Hydrogen Storage in Microporous Metal-Organic Frameworks with Exposed Metal Sites. Angew. Chem. Int. Ed. 2008, 47, 6766–6779. [Google Scholar] [CrossRef]
- Verstraete, D.; Hendrick, P.; Pilidis, P.; Ramsden, K. Hydrogen fuel tanks for subsonic transport aircraft. Int. J. Hydrogen Energy 2010, 35, 11085–11098. [Google Scholar] [CrossRef]
- Nojoumi, H.; Dincer, I.; Naterer, G. Greenhouse gas emissions assessment of hydrogen and kerosene-fueled aircraft propulsion. Int. J. Hydrogen Energy 2009, 34, 1363–1369. [Google Scholar] [CrossRef]
- Hames, Y.; Kaya, K.; Baltacioglu, E.; Turksoy, A. Analysis of the control strategies for fuel saving in the hydrogen fuel cell vehicles. Int. J. Hydrogen Energy 2018, 43, 10810–10821. [Google Scholar] [CrossRef]
- Dutczak, J. Issues related to fuel cells application to small drones propulsion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 421, 042014. [Google Scholar] [CrossRef]
- Verstraete, D. Long range transport aircraft using hydrogen fuel. Int. J. Hydrogen Energy 2013, 38, 14824–14831. [Google Scholar] [CrossRef]
- Khandelwal, B.; Karakurt, A.; Sekaran, P.R.; Sethi, V.; Singh, R. Hydrogen powered aircraft: The future of air transport. Prog. Aerosp. Sci. 2013, 60, 45–59. [Google Scholar] [CrossRef]
- Sharpe, J.E.; Bimbo, N.; Ting, V.P.; Rechain, B.; Joubert, E.; Mays, T.J. Modelling the potential of adsorbed hydrogen for use in aviation. Microporous Mesoporous Mater. 2015, 209, 135–140. [Google Scholar] [CrossRef]
- Lapeña-Rey, N.; Blanco, J.; Ferreyra, E.; Lemus, J.; Pereira, S.; Serrot, E. A fuel cell powered unmanned aerial vehicle for low altitude surveillance missions. Int. J. Hydrogen Energy 2017, 42, 6926–6940. [Google Scholar] [CrossRef]
- Arean, C.O.; Manoilova, O.V.; Palomino, G.T.; Delgado, M.R.; Tsyganenko, A.A.; Bonelli, B.; Garrone, E. Variable-temperature infrared spectroscopy and access to adsorption thermodynamics of weakly interacting systems. Phys. Chem. Chem. Phys. 2002, 4, 5713. [Google Scholar] [CrossRef]
- Garrone, E.; Areán, C.O. Variable temperature infrared spectroscopy: A convenient tool for studying the thermodynamics of weak solid-gas interactions. Chem. Soc. Rev. 2005, 34, 846–857. [Google Scholar] [CrossRef]
- Areán, C.O. Probing Bronsted acidity of protonic zeolites with variable-temperature infrared spectroscopy. Ukr. J. Phys. 2018, 63, 538. [Google Scholar] [CrossRef]
- Bhagiyalakshmi, M.; Yun, L.J.; Anuradha, R.; Jang, H.T. Synthesis of chloropropylamine grafted mesoporous MCM-41, MCM-48 and SBA-15 from rice husk ash: Their application to CO2 chemisorption. J. Porous Mater. 2009, 17, 475–484. [Google Scholar] [CrossRef]
- Rao, N.; Wang, M.; Shang, Z.; Hou, Y.; Fan, G.; Li, J. CO2 Adsorption by Amine-Functionalized MCM-41: A Comparison between Impregnation and Grafting Modification Methods. Energy Fuels 2018, 32, 670–677. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.-S.; Snurr, R.Q. Development and Evaluation of Porous Materials for Carbon Dioxide Separation and Capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191. [Google Scholar] [CrossRef]
- Pettinari, C.; Tombesi, A. Metal-organic frameworks for carbon dioxide capture. In MRS Energy & Sustainability; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Armandi, M.; Bonelli, B.; Bottero, I.; Areán, C.O.; Garrone, E. Synthesis and characterization of ordered porous carbons with potential applications as hydrogen storage media. Microporous Mesoporous Mater. 2007, 103, 150–157. [Google Scholar] [CrossRef]
- Nishihara, H.; Hou, P.-X.; Li, L.-X.; Ito, M.; Uchiyama, M.; Kaburagi, T.; Ikura, A.; Katamura, J.; Kawarada, T.; Mizuuchi, K.; et al. High-Pressure Hydrogen Storage in Zeolite-Templated Carbon. J. Phys. Chem. C 2009, 113, 3189–3196. [Google Scholar] [CrossRef]
- Konwar, R.J.; De, M. Effects of synthesis parameters on zeolite templated carbon for hydrogen storage application. Microporous Mesoporous Mater. 2013, 175, 16–24. [Google Scholar] [CrossRef]
- Youn, H.-K.; Kim, J.; Chandrasekar, G.; Jin, H.; Ahn, W.-S. High pressure carbon dioxide adsorption on nanoporous carbons prepared by Zeolite Y templating. Mater. Lett. 2011, 65, 1772–1774. [Google Scholar] [CrossRef]
- Armandi, M.; Bonelli, B.; Karaindrou, E.; Areán, C.O.; Garrone, E. Post-synthesis modifications of SBA-15 carbon replicas: Improving hydrogen storage by increasing microporous volume. Catal. Today 2008, 138, 244–248. [Google Scholar] [CrossRef]
- Areán, C.O.; Tsyganenko, A.A.; Platero, E.E.; Garrone, E.; Zecchina, A. Two Coordination Modes of CO in Zeolites: A Temperature-Dependent Equilibrium. Angew. Chem. Int. Ed. 1998, 37, 3161–3163. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Platero, E.E.; Arean, C.O.; Garrone, E.; Zecchina, A. Variable-temperature IR spectroscopic studies of CO adsorbed on Na-ZSM-5 and Na-Y zeolites. Catal. Lett. 1999, 61, 187. [Google Scholar] [CrossRef]
- Ugliengo, P.; Garrone, E.; Ferrari, A.M.; Zecchina, A.; Arean, C.O. Quantum Chemical Calculations and Experimental Evidence for O-Bonding of Carbon Monoxide to Alkali Metal Cations in Zeolites. J. Phys. Chem. B 1999, 103, 4839–4846. [Google Scholar] [CrossRef]
- Arean, C.O.; Manoilova, O.; Delgado, M.R.; Tsyganenko, A.; Garrone, E. Formation of several types of coordination complexes upon CO adsorption on the zeolite Li-ZSM-5. Phys. Chem. Chem. Phys. 2001, 3, 4187–4188. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK, 1978. [Google Scholar]
- Szostak, R. Molecular Sieves: Principles of Synthesis and Identification; Van Nostrand Reinhold: New York, NY, USA, 1989. [Google Scholar]
- Meier, W.; Olson, D.H. Altas of Zeolite Structure Types; Butterworth-Heinemann: London, UK, 1992. [Google Scholar]
- Areán, C.O. Zeolites and Intrazeolite Chemistry: Insights from Infrared Spectroscopy. Comments Inorg. Chem. 2000, 22, 241–273. [Google Scholar] [CrossRef]
- Garrone, E.; Areán, C.O.; Delgado, M.R.; Bonelli, B. Molar Entropy and Enthalpy of CO Adsorbed in Zeolites as Derived from VTIR Data: Role of Intermolecular Modes. ChemistryOpen 2020, 9, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Bond, G.C.; Keane, M.A.; Kral, H.; Lercher, J.A. Compensation Phenomena in Heterogeneous Catalysis: General Principles and a Possible Explanation. Catal. Rev. 2000, 42, 323–383. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Storozhev, P.Y.; Arean, C.O. Infrared spectroscopic studies on the binding isomerism of adsorbed molecules. Kinet. Catal. 2004, 45, 530. [Google Scholar] [CrossRef]
- Bolis, V.; Fubini, B.; Garrone, E.; Morterra, C. Thermodynamic and vibrational characterization of CO adsorption on variously pretreated anatase. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 1383–1395. [Google Scholar] [CrossRef]
- Jeremiah, D.W.; Gassensmith, J.; Gouveâ, D.; Ushakov, S.; Stoddart, J.F.; Navrotsky, A. Direct Calorimetric Measurement of Enthalpy of Adsorption of Carbon Dioxide on CD-MOF-2, a Green Metal−Organic Framework. J. Am. Chem. Soc. 2013, 135, 6790. [Google Scholar]
- Nachtigall, P.; Delgado, M.R.; Nachtigallova, D.; Arean, C.O. The nature of cationic adsorption sites in alkaline zeolites- single, dual and multiple cation sites. Phys. Chem. Chem. Phys. 2012, 14, 1552. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab-initio molecular dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Arean, C.O.; Delgado, M.R.; Bauçà, C.L.; Vrbka, L.; Nachtigall, P. Carbon monoxide adsorption on low-silica zeolites—from single to dual and to multiple cation sites. Phys. Chem. Chem. Phys. 2007, 9, 4657. [Google Scholar] [CrossRef] [PubMed]
- Areán, C.O.; Nachtigallová, D.; Nachtigall, P.; Garrone, E.; Delgado, M.R. Thermodynamics of reversible gas adsorption on alkali-metal exchanged zeolites—the interplay of infrared spectroscopy and theoretical calculations. Phys. Chem. Chem. Phys. 2007, 9, 1421–1437. [Google Scholar] [CrossRef]
- Kao, L.W.; Nanagas, K.A. Carbon monoxide poisoning. Emerg. Med. Clin. N. Am. 2004, 22, 985. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K. Carbon monoxide poisoning. N. Eng. J. Med. 2009, 360, 1217. [Google Scholar] [CrossRef]
- Camara, G.A.; Ticianelli, E.A.; Mukerjee, S.; Lee, S.J.; McBreen, J. The CO Poisoning Mechanism of the Hydrogen Oxidation Reaction in Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2002, 149, A748–A753. [Google Scholar] [CrossRef]
- Ju, J.; Lee, K.S.; Um, S. Multi-dimentional modeling of copoisoning effects on proton Exchange membrane fuel cells (PEMFCs). J. Mech. Sci. Technol. 2008, 22, 991. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Gao, J.-A.; Jensen, J.O.; Bjerrum, N.J. The CO Poisoning Effect in PEMFCs Operational at Temperatures up to 200 °C. J. Electrochem. Soc. 2003, 150, A1599–A1605. [Google Scholar] [CrossRef]
- Zecchina, A.; Areán, C.O. Diatomic molecular probes for mid-IR studies of zeolites. Chem. Soc. Rev. 1996, 25, 187–197. [Google Scholar] [CrossRef]
- Knözinger, H.; Huber, S. IR spectroscopy of small and weakly interacting molecular probes for acidic and basic zeolites. J. Chem. Soc. Faraday Trans. 1998, 94, 2047–2059. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Lamberti, C.; Zecchina, A.; Groppo, E.; Bordiga, S. Probing the surfaces of heterogeneous catalysts by in situ IR spectroscopy. Chem. Soc. Rev. 2010, 39, 4951–5001. [Google Scholar] [CrossRef]
- Delgado, M.R.; De Yuso, A.M.; Bulánek, R.; Areán, C.O. Infrared spectroscopic and thermodynamic assessment of extraframework cationic adsorption sites in the zeolite K-L by using CO as probe molecule. Chem. Phys. Lett. 2015, 639, 195–198. [Google Scholar] [CrossRef]
- Delgado, M.R.; Bulanek, R.; Chlubna, P.; Arean, C.O. Brønsted acidity of H-MCM-22 as probed by variable-temperature infrared spectroscopy of adosrbed CO and N2. Catal. Today 2014, 227, 45. [Google Scholar] [CrossRef]
- Arean, C.O.; Delgado, M.R.; Nachtigall, P.; Thang, H.V.; Rubes, M.; Bulanek, R.; Chlubna-Eliasova, P. Measuring the Brønsted acid strength of zeolites—Does it correlate with the O-H frequency shift probed by a weak base? Phys. Chem. Chem. Phys. 2014, 15, 10129. [Google Scholar] [CrossRef]
- Areán, C.O.; Delgado, M.R.; Frolich, K.; Bulánek, R.; Pulido, A.; Bibiloni, G.F.; Nachtigall, P. Computational and Fourier Transform Infrared Spectroscopic Studies on Carbon Monoxide Adsorption on the Zeolites Na-ZSM-5 and K-ZSM-5: Evidence of Dual-Cation Sites. J. Phys. Chem. C 2008, 112, 4658–4666. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Eaton, D.F.; Caspar, J.V. Photochemical and photophysical studies of organic molecules included within zeolites. Accounts Chem. Res. 1992, 25, 299–307. [Google Scholar] [CrossRef]
- Derouane, E.G. Zeolites as solid solvents. J. Mol. Catal. A 1998, 134, 29. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. The catalytic activity of zeolites: Confinement effect within voids of molecular dimensions. Chem. Commun. 2013, 49, 3491. [Google Scholar] [CrossRef]
- Polarz, S.; Kuschel, A. Chemistry in Confining Reaction Fields with Special Emphasis on Nanoporous Materials. Chem. A Eur. J. 2008, 14, 9816–9829. [Google Scholar] [CrossRef]
- Fischer, M.; Delgado, M.R.; Areán, C.O.; Duran, C.O. CO adsorption complexes in zeolites: How does the inclusion of dispersion interactions affect predictions made from DFT calculations? The case of Na-CHA. Theor. Chem. Accounts 2015, 134, 91. [Google Scholar] [CrossRef]
- Nachtigallová, D.; Bludský, O.; Areán, C.O.; Bulánek, R.; Nachtigall, P. The vibrational dynamics of carbon monoxide in a confined space—CO in zeolites. Phys. Chem. Chem. Phys. 2006, 8, 4849–4852. [Google Scholar] [CrossRef]
- Nachtigall, P.; Delgado, M.R.; Frolich, K.; Bulánek, R.; Palomino, G.T.; Bauçà, C.L.; Arean, C.O. Periodic density functional and FTIR spectroscopic studies on CO adsorption on the zeolite Na-FER. Microporous Mesoporous Mater. 2007, 106, 106–162. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Massiani, P.; Knözinger, H. Low-temperature CO and 15N2 adsorption and co-adsorption on alkali cation exchanged EMT zeolites: An FTIR study. Phys. Chem. Chem. Phys. 1999, 1, 3831–3838. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Knözinger, H. FTIR study of the low-temperature adsorption and co-adsorption of CO and N2 on NaY zeolite: Evidence of simultaneous coordination of two molecules to one Na+ site. Chem. Phys. Lett. 1999, 303, 513–520. [Google Scholar] [CrossRef]
- Hush, N.S.; Williams, M.L. Carbon monoxide bond-length, force constant and infrared intensity variations in strong electric fields: Valence shell calculations, with applications to properties of adsorbed and complexed CO. J. Mol. Spectrosc. 1974, 50, 349. [Google Scholar] [CrossRef]
- Lamberti, C.; Bordiga, S.; Geobaldo, F.; Zecchina, A.; Areán, C.O. Stretching frequencies of cation-CO adducts in alkali-metal exchanged zeolites: An elementary electrostatic approach. J. Chem. Phys. 1995, 103, 3158–3165. [Google Scholar] [CrossRef]
- Storozhev, P.; Yanko, V.; Tsyganenko, A.; Palomino, G.T.; Delgado, M.R.; Arean, C.O. Isomeric states of polar molecules on ionic surfaces: Electrostatic model and FTIR studies. Appl. Surf. Sci. 2004, 238, 390–394. [Google Scholar] [CrossRef]
- Dunne, J.A.; Rao, M.; Sircar, S.; Gorte, R.J.; Myers, A.L. Calorimetric heats of adsorption and adsorption isotherms. 2. O2, N2, Ar, CO2, CH4, C2H6 and SF6 on NaX, H-ZSM-5, and Na-ZSM-5 zeolites. Langmuir 1996, 12, 5896. [Google Scholar] [CrossRef]
- Arean, C.O.; Delgado, M.R.; Bibiloni, G.F.; Bludsky, O.; Nachtigall, P. Variable-Temperature IR Spectroscopic and Theoretical Studies on CO2 Adsorbed in Zeolite K-FER. ChemPhysChem 2011, 12, 1435–1443. [Google Scholar] [CrossRef]
- Bonelli, B.; Civalleri, B.; Fubini, B.; Ugliengo, P.; Arean, C.O.; Garrone, E. Experimental and quantum-chemical studies on the adsorption of carbon dioxide in alkali-metal-exchanged ZSM-5 zeolites. J. Phys. Chem. B 2000, 104, 10978. [Google Scholar] [CrossRef]
- Meir, W.M.; Olson, D.H. Atlas of Zeolite Structure Types; Butterworth: London, UK, 1987. [Google Scholar]
- Arean, C.O.; Bibiloni, G.; Delgado, M.R. FT-IR spectroscopic and thermodynamic study on the adsorption of carbon dioxide and dinitrogen in the alkaline zeolite K-L. Appl. Surf. Sci. 2012, 259, 367–370. [Google Scholar] [CrossRef]
- Förster, H.; Schuldt, M. Infrared active fundamentals of deuterium, nitrogen, and oxygen in zeolitic matrices. J. Chem. Phys. 1977, 66, 5237. [Google Scholar] [CrossRef]
- Bulanin, K.M.; Lobo, R.F.; Bulanin, M.O. Low temperature adsorption of N2, O2 and D2 on LiX, NaX and NaLiX zeolites studied by FT-IR spectroscopy. J. Chem. Phys. B 2000, 104, 1269. [Google Scholar] [CrossRef]
- Delgado, M.R.; Areán, C.O. Non-Linear Enthalpy-Entropy Correlation for Nitrogen Adsorption in Zeolites. Molecules 2018, 23, 2978. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Scarano, D.; Petrini, G.; Leofanti, G.; Padovan, M.; Areán, C.O. Low-temperature Fourier-transform infrared investigation of the interaction of CO with nanosized ZSM5 and silicalite. J. Chem. Soc. Faraday Trans. 1992, 88, 2959–2969. [Google Scholar] [CrossRef]
- Delgado, M.R.; Bulanek, R.; Arean, C.O. Answer to the comment by O. Cairon on “Brønsted acidity of H-MCM-22 as probed by variable-temperature infrared spectroscopy of adosrbed CO and N2”. Catal. Today 2015, 252, 214. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Palomino, G.T.; Arean, C.O.; Bordiga, S. Computational and Experimental Studies on the Adsorption of CO, N2, and CO2 on Mg-MOF-74. J. Phys. Chem. C 2010, 114, 11185–11191. [Google Scholar] [CrossRef]
- Gropp, C.; Canossa, S.; Wuttke, S.; Gándara, F.; Li, Q.; Gagliardi, L.; Yaghi, O.M. Standard Practices of Reticular Chemistry. ACS Central Sci. 2020, 6, 1255–1273. [Google Scholar] [CrossRef]
- Civalleri, B.; Maurin, G.; Van Speybroeck, V. Frontiers in modeling metal-organic frameworks. Adv. Theory Simul. 2019, 2, 1900196. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.-S.; Wilmer, C.E.; Snurr, R.Q. Review and Analysis of Molecular Simulations of Methane, Hydrogen, and Acetylene Storage in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef]
- Rzepka, P.; Bacsik, Z.; Smeets, S.; Hansen, T.C.; Hedin, N.; Wardecki, D. Site-Specific Adsorption of CO2 in Zeolite NaK-A. J. Phys. Chem. C 2018, 122, 27005–27015. [Google Scholar] [CrossRef]
- Conterosito, E.; Palin, L.; Caliandro, R.; Van Beek, W.; Chernyshov, D.; Milanesio, M. CO2 adsorption in Y zeolite: A structural and dynamic view by a novel principal-component-analysis-assisted in situ single-crystal X-ray diffraction experiment. Acta Crystallogr. Sect. A Found. Adv. 2019, 75, 214–222. [Google Scholar] [CrossRef]
- Rzepka, P.; Wardecki, D.; Smeets, S.; Müller, M.; Gies, H.; Zou, X.; Hedin, N. CO2-Induced Displacement of Na+ and K+ in Zeolite |NaK|-A. J. Phys. Chem. C 2018, 122, 17211–17220. [Google Scholar] [CrossRef]
- Griaznova, T.P.; Katsyuba, S.A.; Shakirova, O.G.; Lavrenova, L.G. Variable temperature IR spectroscopy and quantum chemistry as the tool for diagnostics of metal spin state. Chem. Phys. Lett. 2010, 495, 50. [Google Scholar] [CrossRef]
- Herber, R.; Casson, L.M. ChemInform Abstract: Light-Induced Excited-Spin-State Trapping: Evidence from VTFTIR Measurements. Chem. Inf. 1986, 17, 847. [Google Scholar] [CrossRef]
- Neya, S.; Takahashi, A.; Ode, H.; Hoshino, T.; Hata, M.; Ikezaki, A.; Ohgo, Y.; Takahashi, M.; Hiramatsu, H.; Kitagawa, T.; et al. Magnetic and Infrared Properties of the Azide Complex of (2,7,12,17-Tetrapropylporphycenato)iron(III): A Novel Admixing Mechanism of the S = 5/2 and S = 3/2 States. Eur. J. Inorg. Chem. 2007, 2007, 3188–3194. [Google Scholar] [CrossRef]

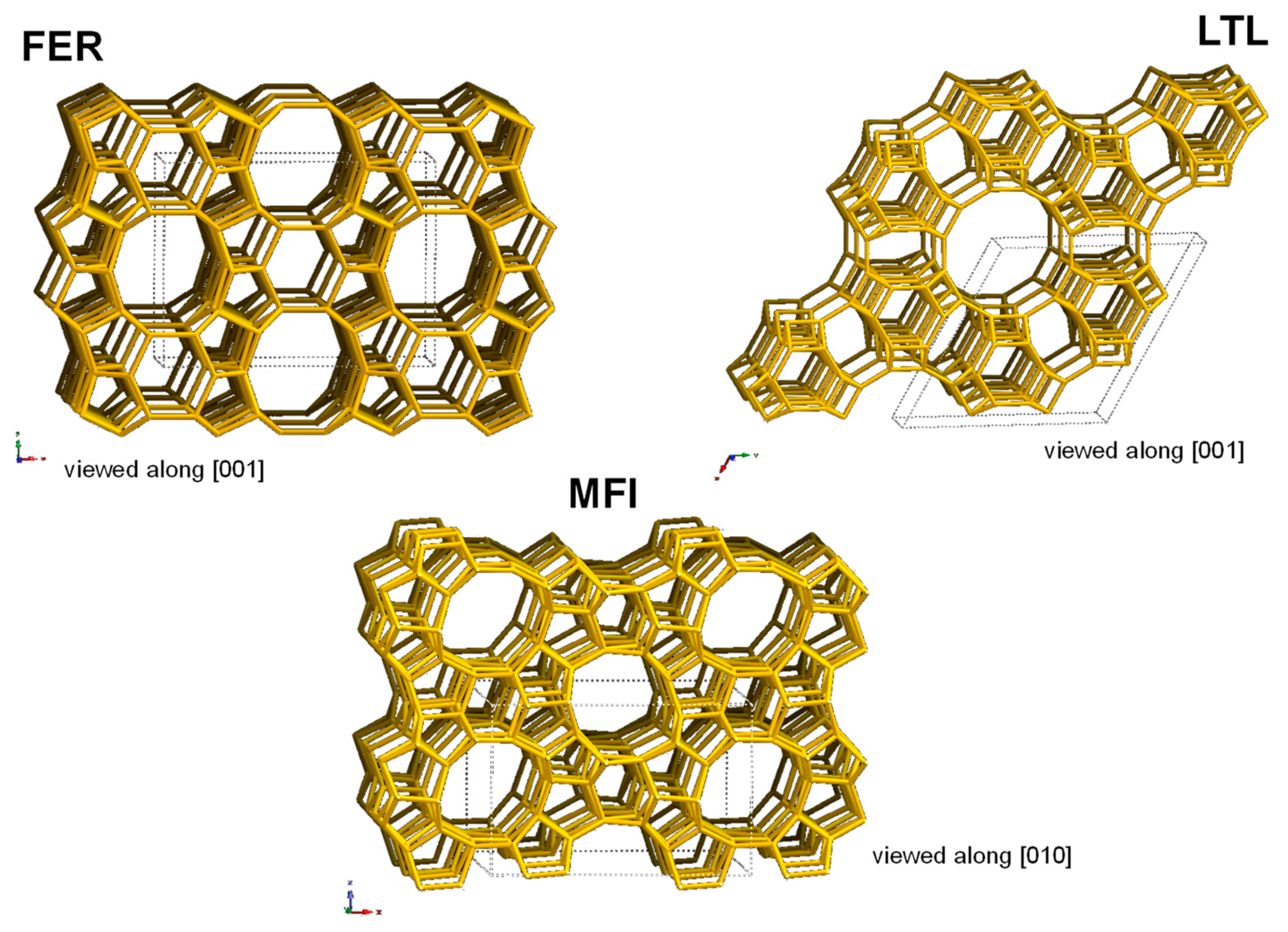





| Structure Type-Code | Selected Isotypes | Dimensionality | Pore Size (Å) |
|---|---|---|---|
| Small pore | |||
| STI | Stilbite, Stellerite | 1D | 2.7 × 5.6 |
| ERI | Erionite | 3D | 3.6 × 5.1 |
| CHA | Chabazite | 3D | 3.8 |
| LTA | Linde A | 3D | 4.1 |
| Medium pore | |||
| FER | Ferrierite, ZSM-35 | 2D | 4.2 × 5.4 |
| MTT | ZSM-23, EU-13 | 1D | 4.5 × 5.2 |
| MFI | Silicalite, ZSM-5 | 3D | 5.5 |
| Large pore | |||
| MOR | Mordenite, LZ-211 | 2D | 6.5 × 7.0 |
| LTL | Perlialite, L | 1D | 7.1 |
| FAU | Faujasite, X, Y | 3D | 7.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. Delgado, M. Structure and Stability of Gas Adsorption Complexes in Periodic Porous Solids as Studied by VTIR Spectroscopy: An Overview. Appl. Sci. 2020, 10, 8589. https://doi.org/10.3390/app10238589
R. Delgado M. Structure and Stability of Gas Adsorption Complexes in Periodic Porous Solids as Studied by VTIR Spectroscopy: An Overview. Applied Sciences. 2020; 10(23):8589. https://doi.org/10.3390/app10238589
Chicago/Turabian StyleR. Delgado, Montserrat. 2020. "Structure and Stability of Gas Adsorption Complexes in Periodic Porous Solids as Studied by VTIR Spectroscopy: An Overview" Applied Sciences 10, no. 23: 8589. https://doi.org/10.3390/app10238589
APA StyleR. Delgado, M. (2020). Structure and Stability of Gas Adsorption Complexes in Periodic Porous Solids as Studied by VTIR Spectroscopy: An Overview. Applied Sciences, 10(23), 8589. https://doi.org/10.3390/app10238589





