Model-Based Roentgen Stereophotogrammetric Analysis Using Elementary Geometrical Shape Models: Reliability of Migration Measurements for an Anatomically Shaped Femoral Stem Component
Abstract
Featured Application
Abstract
1. Introduction
- Rater equivalence: the equivalence between raters was evaluated firstly, which was obtained when the inter-rater difference was so small that measurements from different raters were considered to be equivalent to the rater’s own repeated measurements.
- Intra-rater reliability: if the rater equivalence is acceptable, the investigating of rater reliability can be reduced to the evaluation of intra-rater reliability (as the definition of rater equivalence above).
- Intra-rater reliability of marker-based RSA and mbRSA-EGS
- Whether the intra-rater variability of mbRSA-EGS can be accepted compared with marker-based RSA and the upper limits of RSA accuracy.
2. Materials and Methods
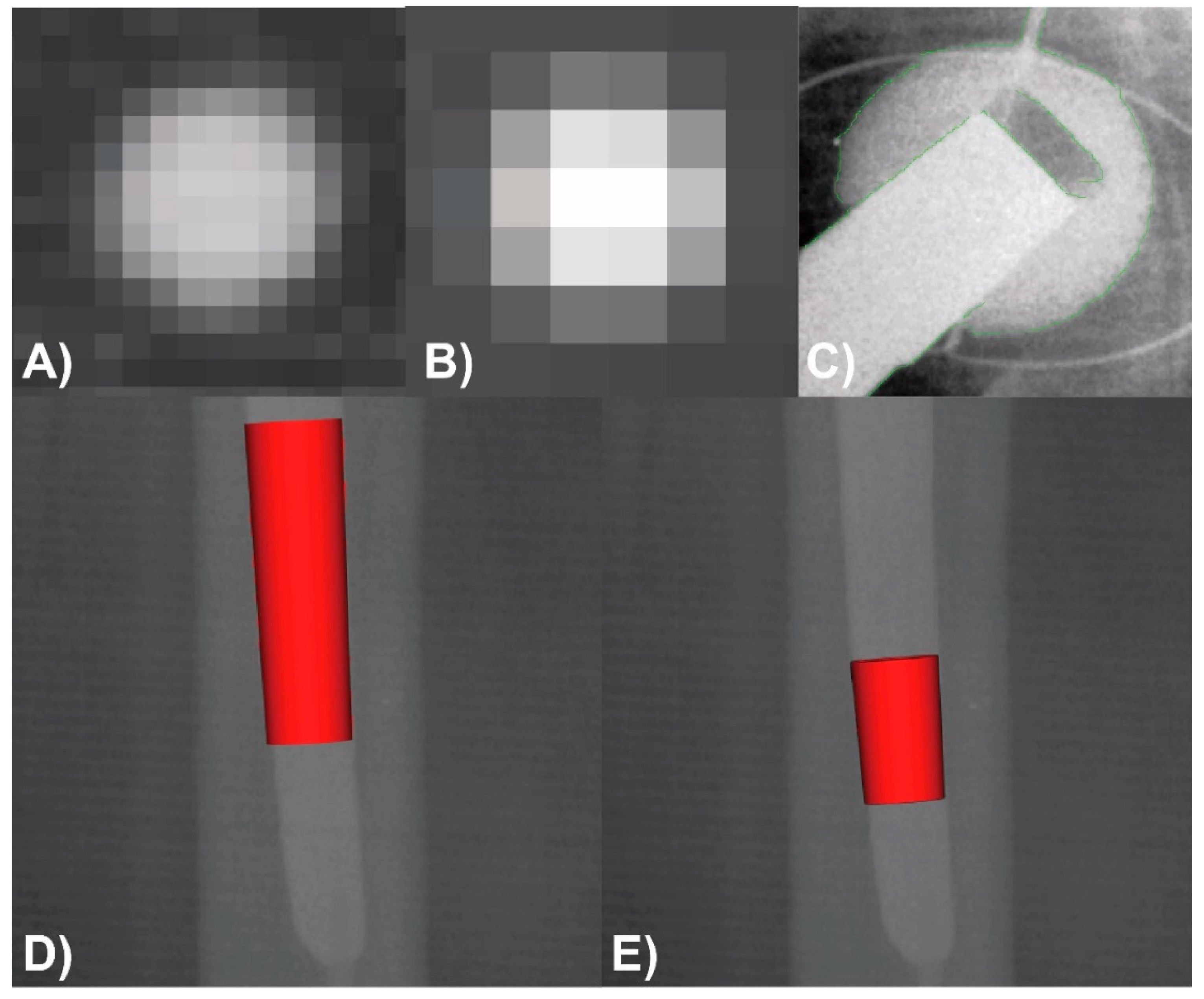
2.1. Image Acqusition and Analysis
2.2. Patient Cohort—Inclusion Criteria
2.3. Measurement and Analysis Protocol
2.4. Statistics
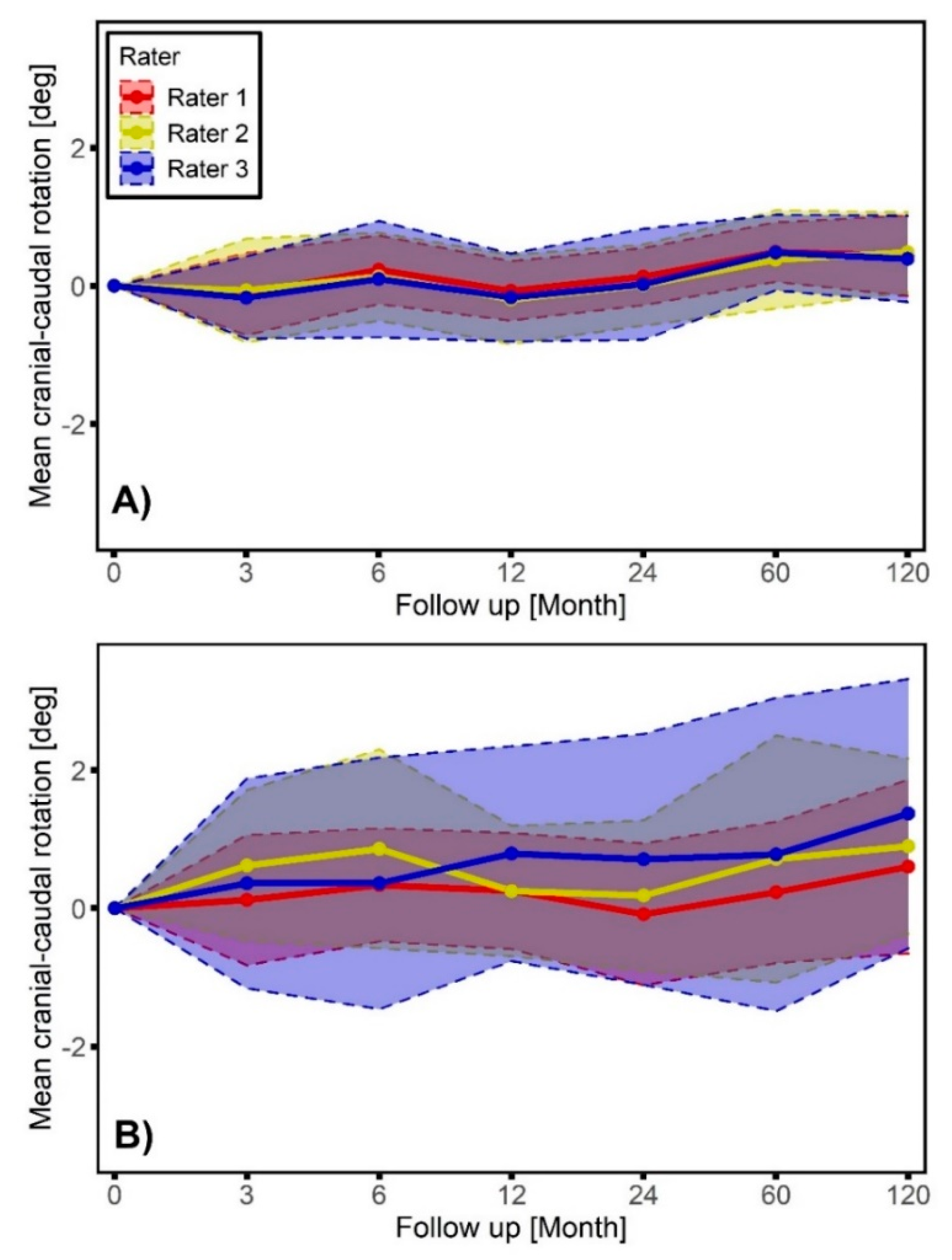
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef]
- Simurda, T.; Kubisz, P.; Dobrotova, M.; Necas, L.; Stasko, J. Perioperative Coagulation Management in a Patient with Congenital Afibrinogenemia during Revision Total Hip Arthroplasty. Semin. Thromb. Hemost. 2016, 42, 689–692. [Google Scholar] [CrossRef]
- Gautam, D.; Gupta, S.; Malhotra, R. Total hip arthroplasty in acetabular fractures. J. Clin. Orthop. Trauma 2020, 11, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.S.; Salib, C.G.; Rose, P.S.; Sim, F.H.; Lewallen, D.G.; Abdel, M.P. Reconstruction of the hip after resection of periacetabular oncological lesions: A systematic review. Bone Jt. J. 2018, 100, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Gallo, J. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J. Clin. Med. 2019, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Dobson, P.F.; Reed, M.R. Prevention of infection in primary THA and TKA. EFORT Open Rev. 2020, 5, 604–613. [Google Scholar] [CrossRef]
- Lenguerrand, E.; Whitehouse, M.R.; Beswick, A.D.; Kunutsor, S.K.; Foguet, P.; Porter, M.; Blom, A.W. Risk factors associated with revision for prosthetic joint infection following knee replacement: An observational cohort study from England and Wales. Lancet Infect. Dis. 2019, 19, 589–600. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; Mori, G.; Dibello, V.; Marrelli, M.; Mirgaldi, R.; De Vito, D.; Tatullo, M. Anti-plaque and antimicrobial efficiency of different oral rinses in a 3-day plaque accumulation model. J. Biol. Regul. Homeost. Agents 2016, 30, 1173–1178. [Google Scholar]
- Czuban, M.; Kulka, M.W.; Wang, L.; Koliszak, A.; Achazi, K.; Schlaich, C.; Donskyi, I.S.; Di Luca, M.; Mejia Oneto, J.M.; Royzen, M.; et al. Titanium coating with mussel inspired polymer and bio-orthogonal chemistry enhances antimicrobial activity against Staphylococcus aureus. Mater. Sci. Eng. C 2020, 116, 111109. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: Annual Report; Australian Orthopaedic Association National Joint Replacement Registry: Adelaide, Australia, 2019. [Google Scholar]
- Valstar, E.R.; Gill, R.; Ryd, L.; Flivik, G.; Borlin, N.; Karrholm, J. Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop. 2005, 76, 563–572. [Google Scholar] [CrossRef]
- Valstar, E.R. Digital Roentgen Stereophotogrammetry: Development, Validation, and Clinical Application; Leiden University: Leiden, The Netherlands, 2002. [Google Scholar]
- Kaptein, B.L.; Valstar, E.R.; Spoor, C.W.; Stoel, B.C.; Rozing, P.M. Model-based RSA of a femoral hip stem using surface and geometrical shape models. Clin. Orthop. Relat. Res. 2006, 448, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H.; Hurschler, C. Experimental analysis of Model-Based Roentgen Stereophotogrammetric Analysis (MBRSA) on four typical prosthesis components. J. Biomech. Eng. 2009, 131, 041004. [Google Scholar] [CrossRef] [PubMed]
- Hurschler, C.; Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H. Comparison of the model-based and marker-based roentgen stereophotogrammetry methods in a typical clinical setting. J. Arthroplast. 2009, 24, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rohrl, S.M.; Boe, B.; Nordsletten, L. Comparison of two different Radiostereometric analysis (RSA) systems with markerless elementary geometrical shape modeling for the measurement of stem migration. Clin. Biomech. 2014, 29, 950–955. [Google Scholar] [CrossRef]
- Xu, J.; Sonntag, R.; Kretzer, J.P.; Taylor, D.; Forst, R.; Seehaus, F. Model-Based Roentgen Stereophotogrammetric Analysis to Monitor the Head-Taper Junction in Total Hip Arthroplasty in Vivo-And They Do Move. Materials 2020, 13, 1543. [Google Scholar] [CrossRef]
- Seehaus, F.; Schwarze, M.; Flörkemeier, T.; von Lewinski, G.; Kaptein, B.L.; Jakubowitz, E.; Hurschler, C. Use of single-representative reverse-engineered surface-models for RSA does not affect measurement accuracy and precision. J. Orthop. Res. 2016, 34, 903–910. [Google Scholar] [CrossRef][Green Version]
- Hurschler, C.; Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H. Accuracy of model-based RSA contour reduction in a typical clinical application. Clin. Orthop. Relat. Res. 2008, 466, 1978–1986. [Google Scholar] [CrossRef]
- Kaptein, B.; Valstar, E.; Stoel, B.; Rozing, P.; Reiber, J. A new model-based RSA method validated using CAD models and models from reversed engineering. J. Biomech. 2003, 36, 873–882. [Google Scholar] [CrossRef]
- Hu, C.Y.; Yoon, T.R. Recent updates for biomaterials used in total hip arthroplasty. Biomater. Res. 2018, 22, 33. [Google Scholar] [CrossRef]
- Vrooman, H.A.; Valstar, E.R.; Brand, G.J.; Admiraal, D.R.; Rozing, P.M.; Reiber, J.H. Fast and accurate automated measurements in digitized stereophotogrammetric radiographs. J. Biomech. 1998, 31, 491–498. [Google Scholar] [CrossRef]
- Sesselmann, S.; Hong, Y.; Schlemmer, F.; Wiendieck, K.; Söder, S.; Hussnaetter, I.; Müller, L.A.; Forst, R.; Wierer, T. Migration measurement of the cemented Lubinus SP II hip stem–a 10-year follow-up using radiostereometric analysis. Biomed. Tech. 2017, 62, 271–278. [Google Scholar] [CrossRef] [PubMed]
- RSAcore. Model-Based RSA 4.1: User Manual; Leiden University Medical Center: Leiden, The Netherlands, 2015. [Google Scholar]
- Barnhart, H.X.; Kosinski, A.S.; Haber, M.J. Assessing individual agreement. J. Biopharm. Stat. 2007, 17, 697–719. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Statistical Ppproaches to Establishing Bioequivalence, Food and Drug Administration; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2001.
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assoc. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Norman, G.R.; Streiner, D.L. Analysis of Variance. In Biostatistics: The Bare Essentials, 3rd ed.; B.C. Decker: Hamilton, ON, Canada, 2008; pp. 77–80. [Google Scholar]
- Karrholm, J.; Borssen, B.; Lowenhielm, G.; Snorrason, F. Does early micromotion of femoral stem prostheses matter? 4–7-year stereoradiographic follow-up of 84 cemented prostheses. J. Bone Jt. Surg. Br. 1994, 76, 912–917. [Google Scholar] [CrossRef]
- Ryd, L.; Albrektsson, B.; Carlsson, L.; Dansgard, F.; Herberts, P.; Lindstrand, A.; Regner, L.; Toksvig-Larsen, S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J. Bone Joint Surg. Br. 1995, 77, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, P.; Pijls, B.G.; Nieuwenhuijse, M.J.; Jasper, J.; Fiocco, M.; Plevier, J.W.; Middeldorp, S.; Valstar, E.R.; Nelissen, R.G. Early subsidence of shape-closed hip arthroplasty stems is associated with late revision. A systematic review and meta-analysis of 24 RSA studies and 56 survival studies. Acta Orthop. 2015, 86, 575–585. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Olofsson, K.; Digas, G.; Karrholm, J. Influence of design variations on early migration of a cemented stem in THA. Clin. Orthop. Relat. Res. 2006, 448, 67–72. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Ding, M.; Jensen, C.; Bragdon, C.; Malchau, H.; Andreasen, C.M.; Ovesen, O.; Hofbauer, C.; Overgaard, S. Vitamin E-doped total hip arthroplasty liners show similar head penetration to highly cross-linked polyethylene at five years: A multi-arm randomized controlled trial. Bone Jt. J. 2020, 102, 1303–1310. [Google Scholar] [CrossRef]
- Nieuwenhuijse, M.J.; Vehmeijer, S.B.W.; Mathijsen, N.M.C.; Keizer, S.B. Fixation of the short global tissue-sparing hip stem. Bone Jt. J. 2020, 102, 699–708. [Google Scholar] [CrossRef]
- Tabori-Jensen, S.; Mosegaard, S.B.; Hansen, T.B.; Stilling, M. Inferior stabilization of cementless compared with cemented dual-mobility cups in elderly osteoarthrosis patients: A randomized controlled radiostereometry study on 60 patients with 2 years’ follow-up. Acta Orthop. 2020, 91, 246–253. [Google Scholar] [CrossRef]
- Hasan, S.; van Hamersveld, K.T.; Marang-van de Mheen, P.J.; Kaptein, B.L.; Nelissen, R.; Toksvig-Larsen, S. Migration of a novel 3D-printed cementless versus a cemented total knee arthroplasty: Two-year results of a randomized controlled trial using radiostereometric analysis. Bone Jt. J. 2020, 102, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Marang-Van De Mheen, P.J.; Kaptein, B.L.; Nelissen, R.G.H.H.; Toksvig-Larsen, S. All-polyethylene versus metal-backed posterior stabilized total knee arthroplasty: Similar 2-year results of a randomized radiostereometric analysis study. Acta Orthop. 2019, 90, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Maletta, C.; Inchingolo, F.; Alfano, M.; Tatullo, M. Three-point bending tests of zirconia core/veneer ceramics for dental restorations. Int. J. Dent. 2013, 2013, 831976. [Google Scholar] [CrossRef] [PubMed]
- Seehaus, F.; Sonntag, R.; Schwarze, M.; Jakubowitz, E.; Sesselmann, S.; Kretzer, J.P.; Hurschler, C. Früherkennung des Risikos der späteren Implantatlockerung mittels der Röntgen Stereophotogrammetrischen Analyse (RSA). Der Orthopäde 2020, 49, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, R.G.; Pijls, B.G.; Karrholm, J.; Malchau, H.; Nieuwenhuijse, M.J.; Valstar, E.R. RSA and registries: The quest for phased introduction of new implants. J. Bone Jt. Surg. Am. 2011, 93 (Suppl. 3), 62–65. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC; European Union: Brussels, Belgium, 2017. [Google Scholar]
- Pijls, B.G.; Plevier, J.W.M.; Nelissen, R. RSA migration of total knee replacements. Acta Orthop. 2018, 89, 320–328. [Google Scholar] [CrossRef]
- Laende, E.K.; Richardson, C.G.; Dunbar, M.J. Predictive value of short-term migration in determining long-term stable fixation in cemented and cementless total knee arthroplasties. Bone Jt. J. 2019, 101, 55–60. [Google Scholar] [CrossRef]
- Galea, V.P.; Connelly, J.W.; Shareghi, B.; Karrholm, J.; Skoldenberg, O.; Salemyr, M.; Laursen, M.B.; Muratoglu, O.; Bragdon, C.; Malchau, H. Evaluation of in vivo wear of vitamin E-diffused highly crosslinked polyethylene at five years: A multicentre radiostereometric analysis study. Bone Jt. J. 2018, 100, 1592–1599. [Google Scholar] [CrossRef]
- Johanson, P.-E.; Shareghi, B.; Eriksson, M.; Kärrholm, J. Wear measurements with use of radiostereometric analysis in total hip arthroplasty with obscured femoral head. J. Orthop. Res. 2020. [Google Scholar] [CrossRef]
- Gascoyne, T.; Parashin, S.; Teeter, M.; Bohm, E.; Laende, E.; Dunbar, M.; Turgeon, T. In vivo wear measurement in a modern total knee arthroplasty with model-based radiostereometric analysis. Bone Jt. J. 2019, 101, 1348–1355. [Google Scholar] [CrossRef]
- Hansen, L.; De Raedt, S.; Jørgensen, P.B.; Mygind-Klavsen, B.; Kaptein, B.; Stilling, M. Marker free model-based radiostereometric analysis for evaluation of hip joint kinematics. Bone Jt. Res. 2018, 7, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; de Raedt, S.; Jørgensen, P.B.; Mygind-Klavsen, B.; Kaptein, B.; Stilling, M. Dynamic radiostereometric analysis for evaluation of hip joint pathomechanics. J. Exp. Orthop. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Humadi, A.; Dawood, S.; Halldin, K.; Freeman, B. RSA in Spine: A Review. Glob. Spine J. 2017, 7, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.N.; Tsukanaka, M.; Fjalestad, T.; Madsen, J.E.; Röhrl, S.M. Model-based RSA is suitable for clinical trials on the glenoid component of reverse total shoulder arthroplasty. J. Orthop. Res. 2018, 36, 3299–3307. [Google Scholar] [CrossRef]


| Translation | Rotation | |||||
|---|---|---|---|---|---|---|
| m.l.1 | c.c.2 | a.p.3 | m.l.1 | c.c.2 | a.p.3 | |
| RSA-marker 4 | 0.42 (> 0.27) | 0.58 (> 0.44) | 0.81 (> 0.72) | 0.80 (> 0.70) | 0.83 (> 0.75) | 0.37 (> 0.22) |
| mbRSA-EGS | 0.71 (> 0.55) | 0.64 (> 0.48) | 0.77 (> 0.69) | 0.76 (> 0.69) | 0.81 (> 0.74) | 0.65 (> 0.52) |
| Translation | Rotation | ||||||
|---|---|---|---|---|---|---|---|
| m.l.1 | c.c2 | a.p.3 | m.l.1 | c.c.2 | a.p.3 | ||
| RSA-marker 4 | Rater 1 | 0.93 | 0.90 | 0.75 | 0.79 | 0.63 | 0.92 |
| Rater 2 | 0.91 | 0.83 | 0.76 | 0.86 | 0.50 | 0.90 | |
| Rater 3 | 0.90 | 0.88 | 0.68 | 0.83 | 0.47 | 0.87 | |
| mbRSA-EGS | Rater 1 | 0.83 | 0.84 | 0.62 | 0.64 | 0.30 | 0.82 |
| Rater 2 | 0.77 | 0.76 | 0.53 | 0.59 | 0.21 | 0.76 | |
| Rater 3 | 0.65 | 0.70 | 0.32 | 0.51 | 0.11 | 0.64 | |
| Translation (mm) | Rotation (deg) | ||||||
|---|---|---|---|---|---|---|---|
| m.l.1 | c.c.2 | a.p.3 | m.l.1 | c.c.2 | a.p.3 | ||
| RSA-marker 4 | Rater 1 | 0.07 *† | 0.07 *† | 0.15 * | 0.19 * | 0.50 * | 0.07 * |
| Rater 2 | 0.08 *† | 0.09 *† | 0.15 * | 0.15 * | 0.65 * | 0.08 * | |
| Rater 3 | 0.09 *† | 0.07 *† | 0.18 * | 0.17 * | 0.69 * | 0.09 * | |
| mbRSA-EGS | Rater 1 | 0.09 *† | 0.09 *† | 0.17 * | 0.28 * | 0.99 * | 0.11 * |
| Rater 2 | 0.11 *† | 0.11 *† | 0.21 * | 0.31 * | 1.27 | 0.14 * | |
| Rater 3 | 0.15 * | 0.13 *† | 0.32 * | 0.37 * | 1.81 | 0.18 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Cao, H.; Sesselmann, S.; Taylor, D.; Forst, R.; Seehaus, F. Model-Based Roentgen Stereophotogrammetric Analysis Using Elementary Geometrical Shape Models: Reliability of Migration Measurements for an Anatomically Shaped Femoral Stem Component. Appl. Sci. 2020, 10, 8507. https://doi.org/10.3390/app10238507
Xu J, Cao H, Sesselmann S, Taylor D, Forst R, Seehaus F. Model-Based Roentgen Stereophotogrammetric Analysis Using Elementary Geometrical Shape Models: Reliability of Migration Measurements for an Anatomically Shaped Femoral Stem Component. Applied Sciences. 2020; 10(23):8507. https://doi.org/10.3390/app10238507
Chicago/Turabian StyleXu, Jing, Han Cao, Stefan Sesselmann, Dominic Taylor, Raimund Forst, and Frank Seehaus. 2020. "Model-Based Roentgen Stereophotogrammetric Analysis Using Elementary Geometrical Shape Models: Reliability of Migration Measurements for an Anatomically Shaped Femoral Stem Component" Applied Sciences 10, no. 23: 8507. https://doi.org/10.3390/app10238507
APA StyleXu, J., Cao, H., Sesselmann, S., Taylor, D., Forst, R., & Seehaus, F. (2020). Model-Based Roentgen Stereophotogrammetric Analysis Using Elementary Geometrical Shape Models: Reliability of Migration Measurements for an Anatomically Shaped Femoral Stem Component. Applied Sciences, 10(23), 8507. https://doi.org/10.3390/app10238507






