An Automated Three-Dimensional Bone Pose Tracking Method Using Clinical Interleaved Biplane Fluoroscopy Systems: Application to the Knee
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
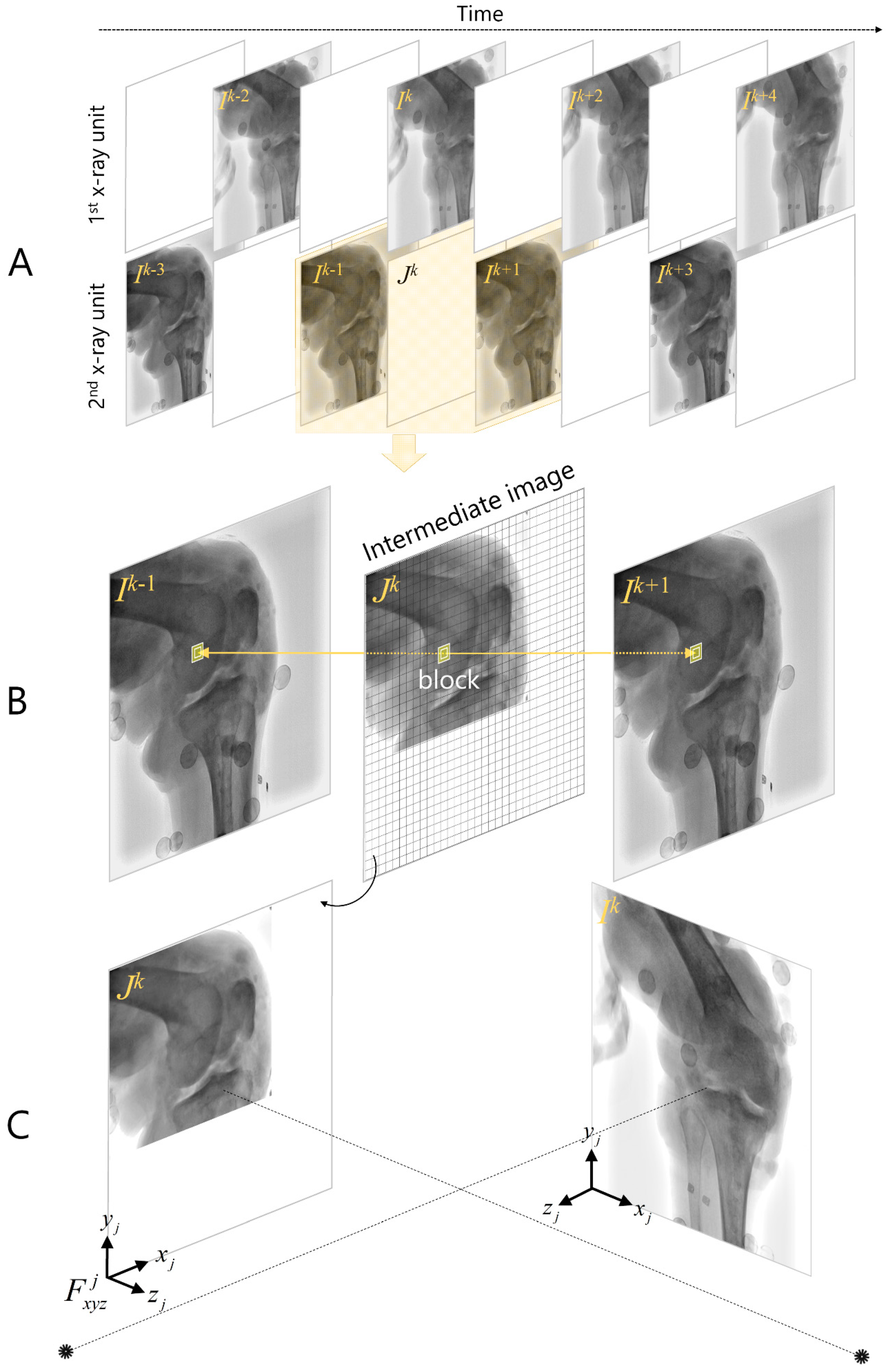
2.2. Asynchronous Biplane Fluoroscopy Imaging
2.3. CT-Based Bone Model
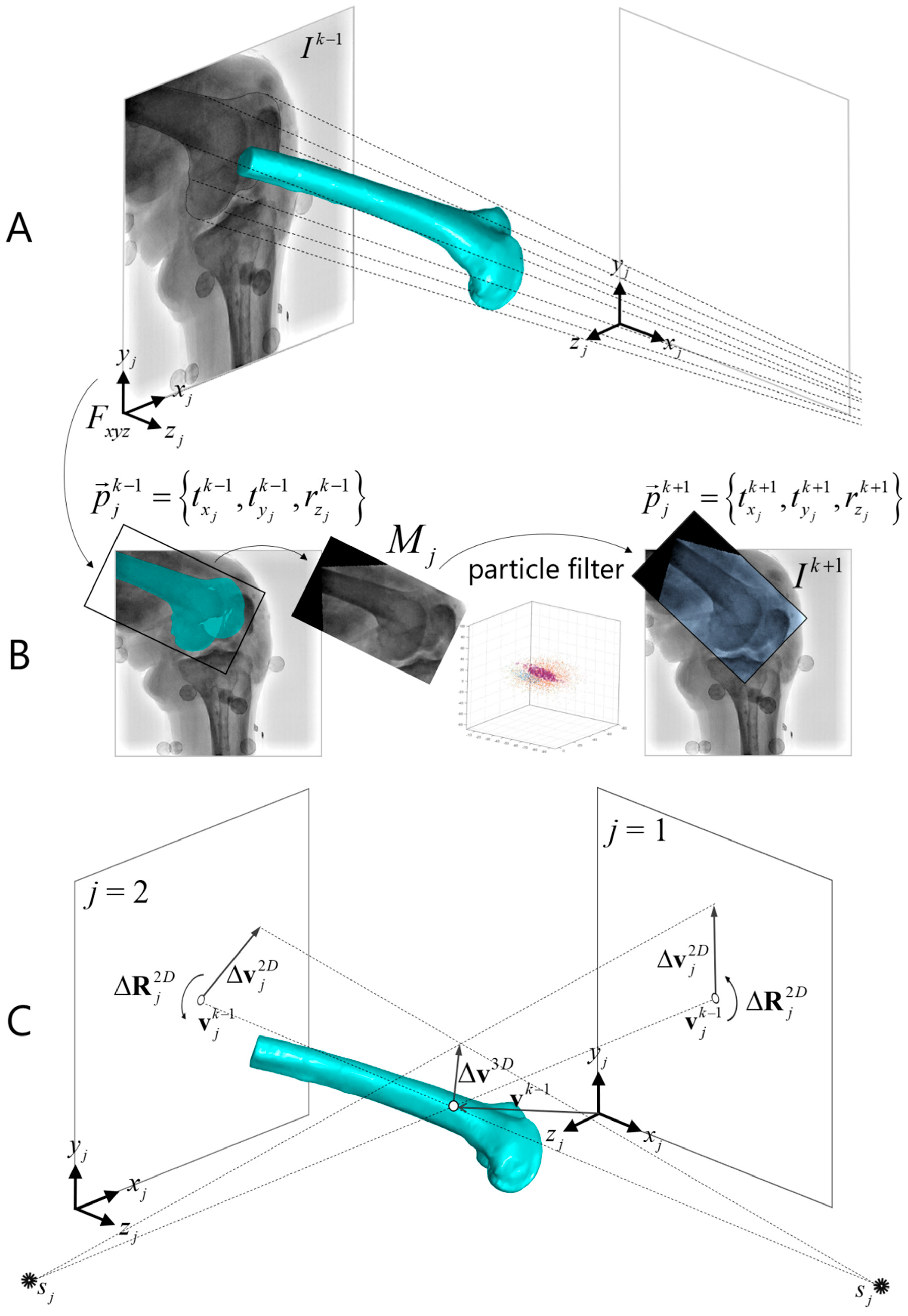
2.4. Model-Based Interleaved Biplane Fluoroscopy Image Tracking
2.4.1. Two-Dimensional/2D Template Registration
2.4.2. Frame Interpolation
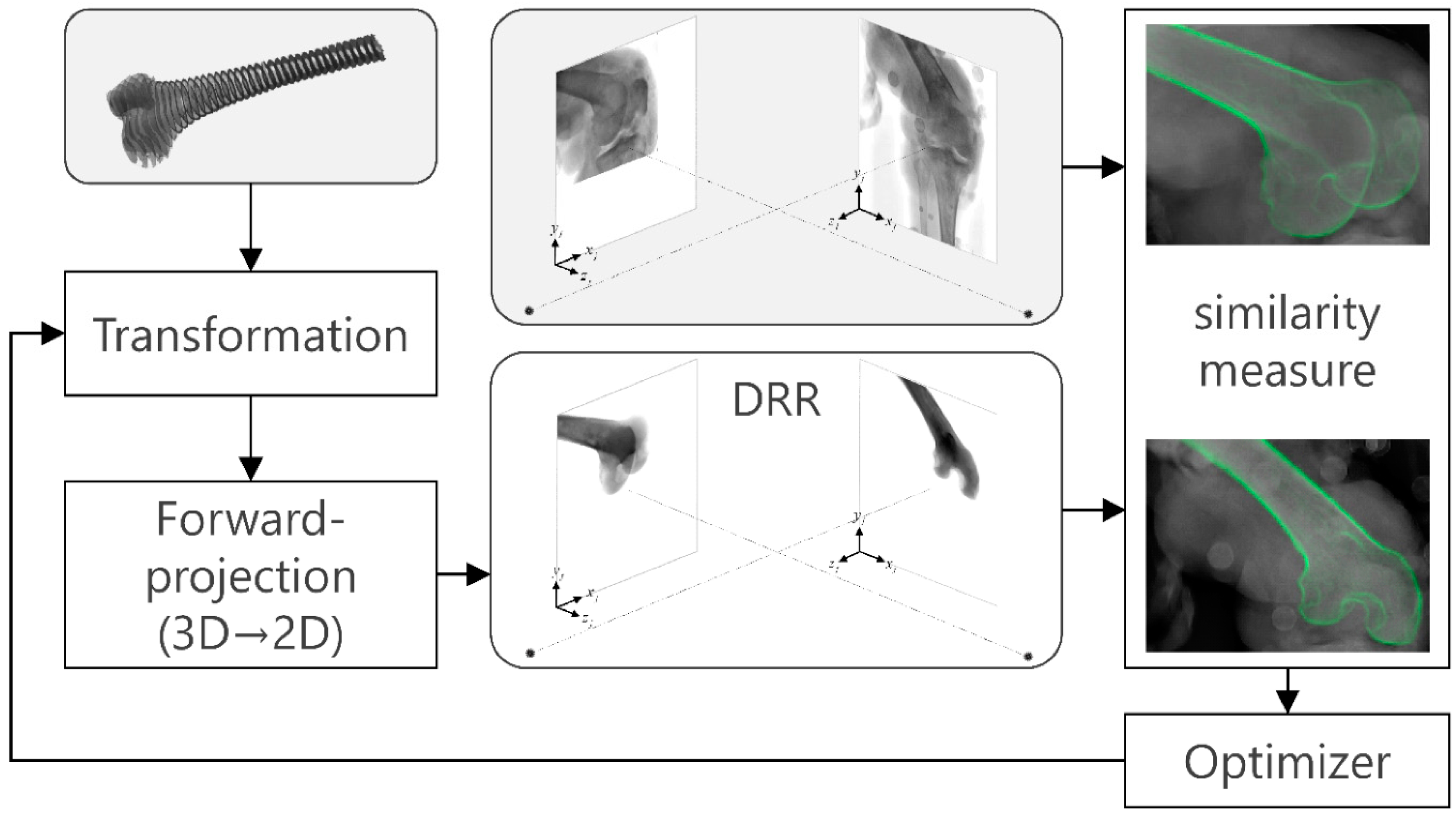
2.4.3. Biplane 3D/2D Image Registration
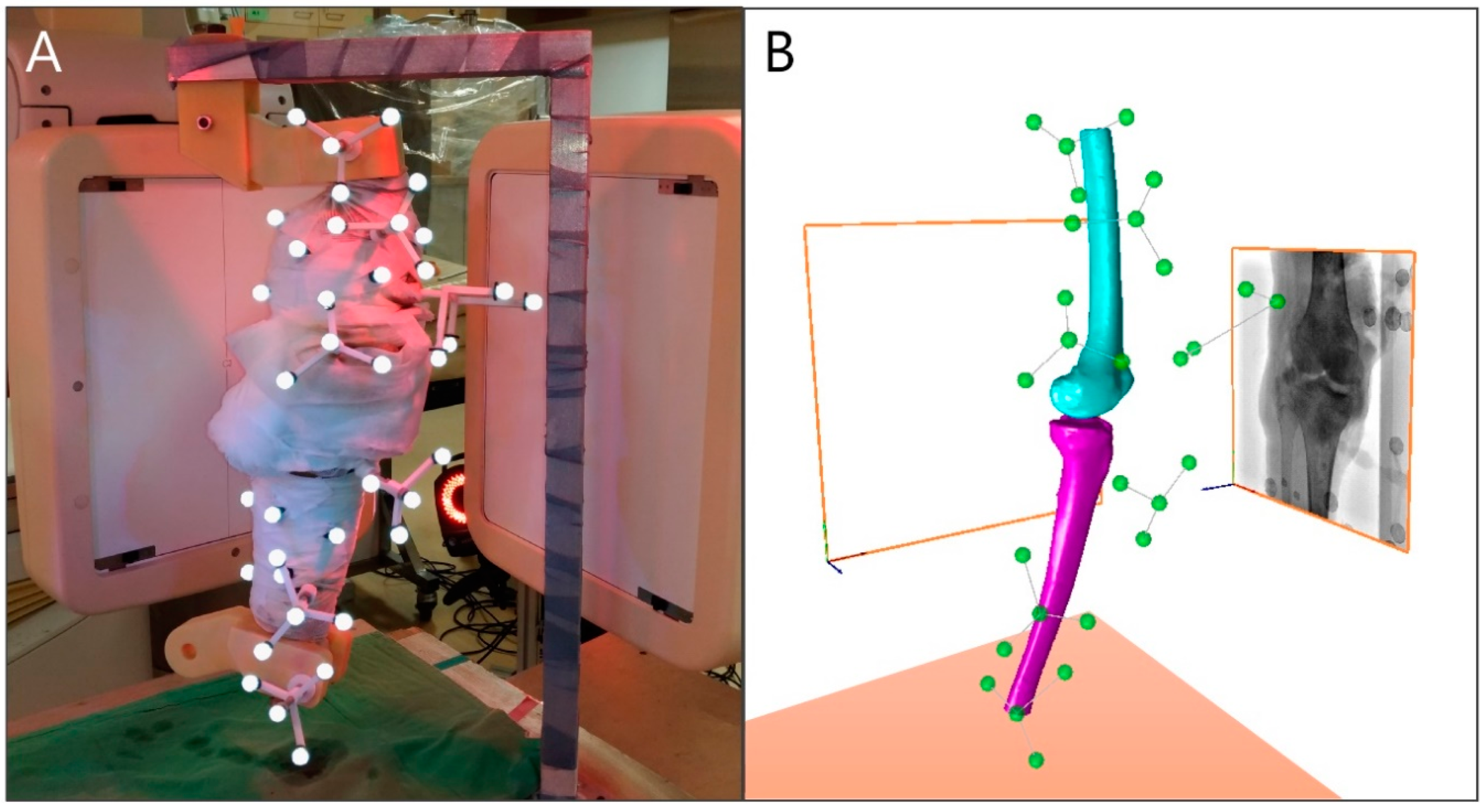
2.5. In Vitro Motion Experiment
2.6. Evaluation of the MIBFT
2.6.1. Standard Reference Determination
2.6.2. Error Metrics
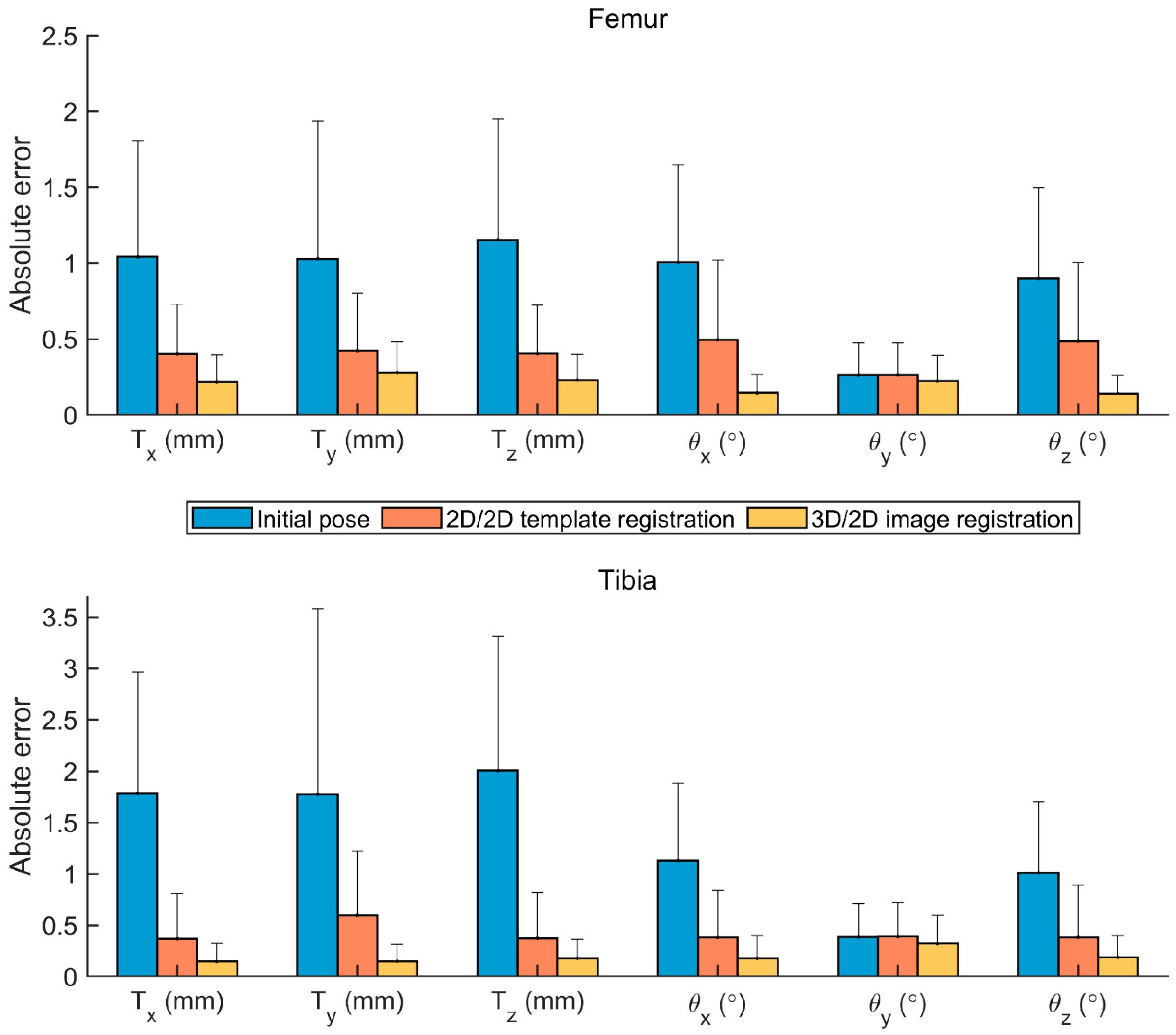
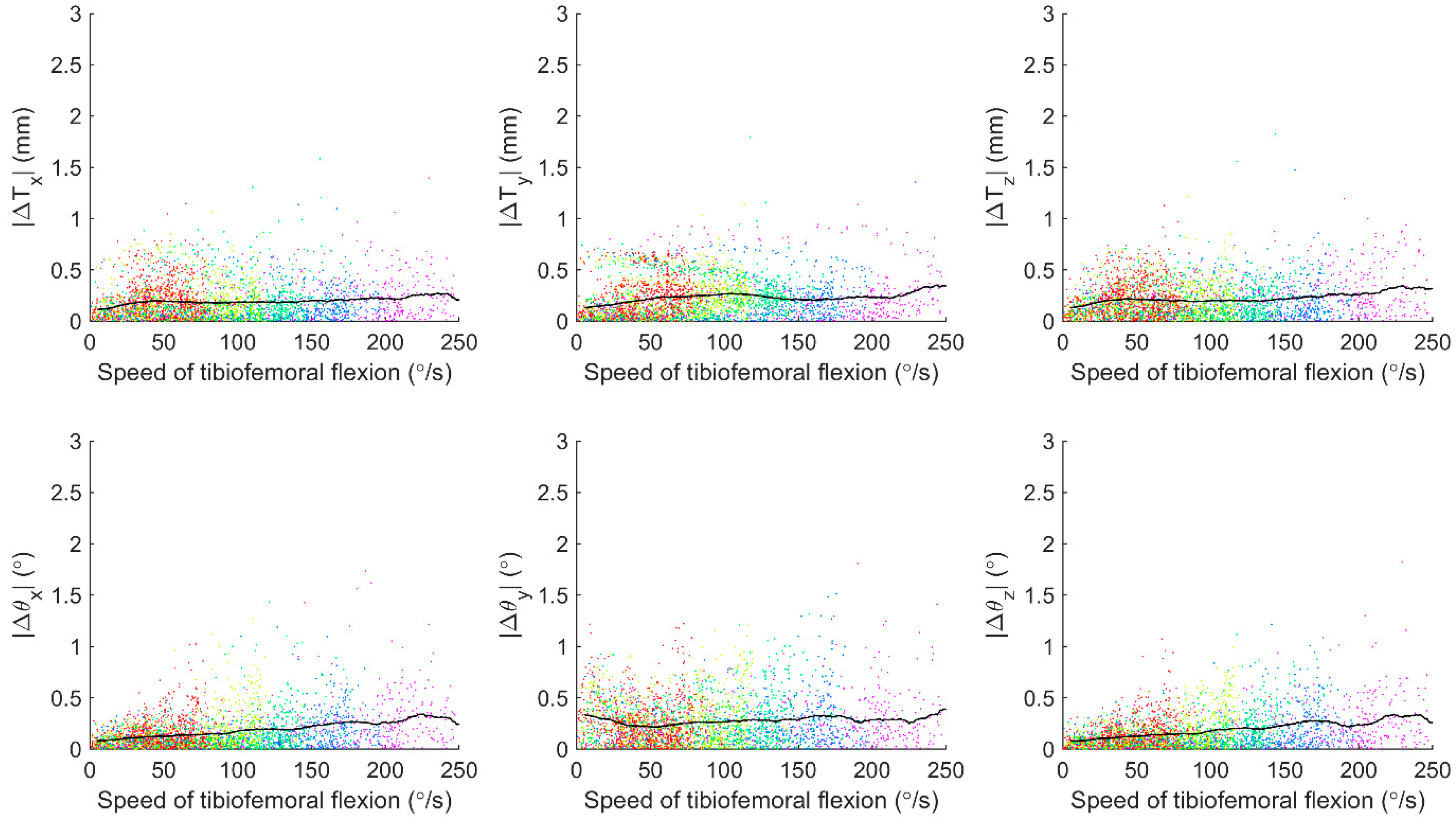
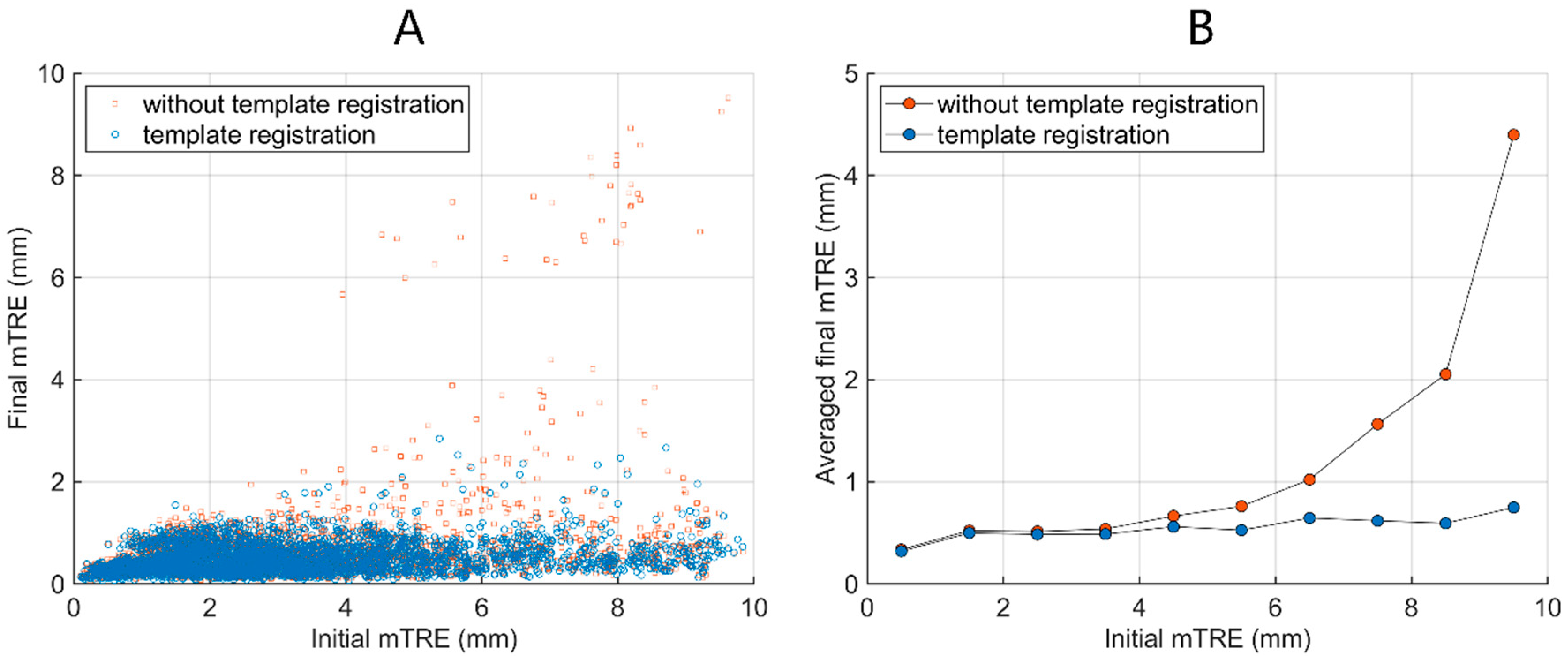
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, D.R.; Feikes, J.D.; O’Connor, J.J. Ligaments and articular contact guide passive knee flexion. J. Biomech. 1998, 31, 1127–1136. [Google Scholar] [CrossRef]
- Navacchia, A.; Kefala, V.; Shelburne, K.B. Dependence of Muscle Moment Arms on In Vivo Three-Dimensional Kinematics of the Knee. Ann. Biomed. Eng. 2017, 45, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Hosseini Nasab, S.H.; Smith, C.R.; Schütz, P.; Postolka, B.; List, R.; Taylor, W.R. Elongation Patterns of the Collateral Ligaments After Total Knee Arthroplasty Are Dominated by the Knee Flexion Angle. Front. Bioeng. Biotechnol. 2019, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Tomita, T.; Futai, K.; Yamazaki, T.; Tanaka, S.; Yoshikawa, H.; Sugamoto, K. In vivo three-dimensional kinematics of normal knees during different high-flexion activities. Bone Jt. J. 2018, 100, 50–55. [Google Scholar] [CrossRef]
- Gray, H.A.; Guan, S.; Thomeer, L.T.; Schache, A.G.; de Steiger, R.; Pandy, M.G. Three-dimensional motion of the knee-joint complex during normal walking revealed by mobile biplane x-ray imaging. J. Orthop. Res. 2019, 37, 615–630. [Google Scholar] [CrossRef]
- Kefala, V.; Cyr, A.J.; Harris, M.D.; Hume, D.R.; Davidson, B.S.; Kim, R.H.; Shelburne, K.B. Assessment of Knee Kinematics in Older Adults Using High-Speed Stereo Radiography. Med. Sci. Sports Exerc. 2017, 49, 2260–2267. [Google Scholar] [CrossRef]
- Yang, C.; Tashiro, Y.; Lynch, A.; Fu, F.; Anderst, W. Kinematics and arthrokinematics in the chronic ACL-deficient knee are altered even in the absence of instability symptoms. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 1406–1413. [Google Scholar] [CrossRef]
- Leardini, A.; Belvedere, C.; Nardini, F.; Sancisi, N.; Conconi, M.; Parenti-Castelli, V. Kinematic models of lower limb joints for musculo-skeletal modelling and optimization in gait analysis. J. Biomech. 2017, 62, 77–86. [Google Scholar] [CrossRef]
- Zheng, L.; Carey, R.; Thorhauer, E.; Tashman, S.; Harner, C.; Zhang, X. In vivo tibiofemoral skeletal kinematics and cartilage contact arthrokinematics during decline walking after isolated meniscectomy. Med. Eng. Phys. 2018, 51, 41–48. [Google Scholar] [CrossRef]
- Hume, D.R.; Kefala, V.; Harris, M.D.; Shelburne, K.B. Comparison of Marker-Based and Stereo Radiography Knee Kinematics in Activities of Daily Living. Ann. Biomed. Eng. 2018, 46, 1806–1815. [Google Scholar] [CrossRef]
- Barré, A.; Jolles, B.M.; Theumann, N.; Aminian, K. Soft tissue artifact distribution on lower limbs during treadmill gait: Influence of skin markers’ location on cluster design. J. Biomech. 2015, 48, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-D.; Lu, T.-W.; Lin, C.-C.; Kuo, M.-Y.; Hsu, H.-C.; Shen, W.-C. Soft tissue artefacts of skin markers on the lower limb during cycling: Effects of joint angles and pedal resistance. J. Biomech. 2017, 62, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Cerciello, T.; Romano, M.; Bifulco, P.; Cesarelli, M.; Allen, R. Advanced template matching method for estimation of intervertebral kinematics of lumbar spine. Med. Eng. Phys. 2011, 33, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, D.; Lindblom, M.; Quick, P.; Gauffin, H. Quantitative analysis of the patellofemoral motion pattern using semi-automatic processing of 4D CT data. Int. J. Comput. Assist. Radiol. Surg. 2016, 11, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Krohn, S.; Gersdorff, N.; Wassmann, T.; Merboldt, K.-D.; Joseph, A.A.; Buergers, R.; Frahm, J. Real-time MRI of the temporomandibular joint at 15 frames per second-A feasibility study. Eur. J. Radiol. 2016, 85, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Bradford, R.; Johnson, K.; Wieben, O.; Thelen, D.G. Measurement of tibiofemoral kinematics using highly accelerated 3D radial sampling. Magn. Reson. Med. 2013, 69, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Sipprell Iii, W.H.; Wible, E.E.; Sheehan, F.T. A methodology to accurately quantify patellofemoral cartilage contact kinematics by combining 3D image shape registration and cine-PC MRI velocity data. J. Biomech. 2012, 45, 1117–1122. [Google Scholar] [CrossRef]
- Barrance, P.J.; Williams, G.N.; Novotny, J.E.; Buchanan, T.S. A method for measurement of joint kinematics in vivo by registration of 3-D geometric models with cine phase contrast magnetic resonance imaging data. J. Biomech. Eng.-Trans. ASME 2005, 127, 829–837. [Google Scholar] [CrossRef]
- Lin, C.-C.; Zhang, S.; Hsu, C.-Y.; Frahm, J.; Lu, T.-W.; Shih, T.-F. Measuring three-dimensional tibiofemoral kinematics using dual-slice real-time magnetic resonance imaging. Med. Phys. 2019, 46, 4588–4599. [Google Scholar] [CrossRef]
- Kaiser, J.; Monawer, A.; Chaudhary, R.; Johnson, K.M.; Wieben, O.; Kijowski, R.; Thelen, D.G. Accuracy of model-based tracking of knee kinematics and cartilage contact measured by dynamic volumetric MRI. Med. Eng. Phys. 2016, 38, 1131–1135. [Google Scholar] [CrossRef]
- List, R.; Postolka, B.; Schütz, P.; Hitz, M.; Schwilch, P.; Gerber, H.; Ferguson, S.J.; Taylor, W.R. A moving fluoroscope to capture tibiofemoral kinematics during complete cycles of free level and downhill walking as well as stair descent. PLoS ONE 2017, 12, e0185952. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Gray, H.A.; Keynejad, F.; Pandy, M.G. Mobile Biplane X-Ray Imaging System for Measuring 3D Dynamic Joint Motion During Overground Gait. IEEE Trans. Med. Imaging 2016, 35, 326–336. [Google Scholar] [CrossRef]
- Postolka, B.; List, R.; Thelen, B.; Schütz, P.; Taylor, W.R.; Zheng, G. Evaluation of an intensity-based algorithm for 2D/3D registration of natural knee videofluoroscopy data. Med. Eng. Phys. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Lu, T.W.; Chen, C.M.; Kuo, M.Y.; Hsu, H.C. A volumetric model-based 2D to 3D registration method for measuring kinematics of natural knees with single-plane fluoroscopy. Med. Phys. 2010, 37, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Flood, P.D.L.; Banks, S.A. Automated Registration of 3-D Knee Implant Models to Fluoroscopic Images Using Lipschitzian Optimization. IEEE Trans. Med. Imaging 2018, 37, 326–335. [Google Scholar] [CrossRef]
- Ellingson, A.M.; Mozingo, J.D.; Magnuson, D.J.; Pagnano, M.W.; Zhao, K.D. Characterizing fluoroscopy based kinematic accuracy as a function of pulse width and velocity. J. Biomech. 2016, 49, 3741–3745. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, G. An automatic 2D–3D image matching method for reproducing spatial knee joint positions using single or dual fluoroscopic images. Comput. Methods Biomech. Biomed. Eng. 2011, 15, 1245–1256. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lu, T.-W.; Wang, T.-M.; Hsu, C.-Y.; Shih, T.-F. Comparisons of surface vs. volumetric model-based registration methods using single-plane vs. bi-plane fluoroscopy in measuring spinal kinematics. Med. Eng. Phys. 2014, 36, 267–274. [Google Scholar] [CrossRef]
- You, B.M.; Siy, P.; Anderst, W.; Tashman, S. In vivo measurement of 3-D skeletal kinematics from sequences of biplane radiographs: Application to knee kinematics. IEEE Trans. Med. Imaging 2001, 20, 514–525. [Google Scholar] [CrossRef]
- Anderst, W.; Zauel, R.; Bishop, J.; Demps, E.; Tashman, S. Validation of three-dimensional model-based tibio-femoral tracking during running. Med. Eng. Phys. 2009, 31, 10–16. [Google Scholar] [CrossRef]
- Giphart, J.E.; Zirker, C.A.; Myers, C.A.; Pennington, W.W.; LaPrade, R.F. Accuracy of a contour-based biplane fluoroscopy technique for tracking knee joint kinematics of different speeds. J. Biomech. 2012, 45, 2935–2938. [Google Scholar] [CrossRef] [PubMed]
- Stentz-Olesen, K.; Nielsen, E.T.; De Raedt, S.; Jørgensen, P.B.; Sørensen, O.G.; Kaptein, B.L.; Andersen, M.S.; Stilling, M. Validation of static and dynamic radiostereometric analysis of the knee joint using bone models from CT data. Bone Jt. Res. 2017, 6, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Brainerd, E.L.; Baier, D.B.; Gatesy, S.M.; Hedrick, T.L.; Metzger, K.A.; Gilbert, S.L.; Crisco, J.J. X-ray reconstruction of moving morphology (XROMM): Precision, accuracy and applications in comparative biomechanics research. J. Exp. Zool. A Ecol. Genet. Physiol. 2010, 313, 262–279. [Google Scholar] [CrossRef]
- Ivester, J.C.; Cyr, A.J.; Harris, M.D.; Kulis, M.J.; Rullkoetter, P.J.; Shelburne, K.B. A Reconfigurable High-Speed Stereo-Radiography System for Sub-Millimeter Measurement of In Vivo Joint Kinematics. J. Med. Devices 2015, 9. [Google Scholar] [CrossRef]
- Li, G.; Van de Velde, S.K.; Bingham, J.T. Validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J. Biomech. 2008, 41, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Barré, A.; Aminian, K. Error performances of a model-based biplane fluoroscopic system for tracking knee prosthesis during treadmill gait task. Med. Biol. Eng. Comput. 2018, 56, 307–316. [Google Scholar] [CrossRef]
- Mozingo, J.D.; Akbari Shandiz, M.; Marquez, F.M.; Schueler, B.A.; Holmes, D.R.; McCollough, C.H.; Zhao, K.D. Validation of imaging-based quantification of glenohumeral joint kinematics using an unmodified clinical biplane fluoroscopy system. J. Biomech. 2018, 71, 306–312. [Google Scholar] [CrossRef]
- Cross, J.A.; McHenry, B.; Schmidt, T.G. Quantifying cross-scatter contamination in biplane fluoroscopy motion analysis systems. JMIOBU 2015, 2, 043503. [Google Scholar] [CrossRef][Green Version]
- Akbari-Shandiz, M.; Mozingo, J.D.; Holmes Iii, D.R.; Zhao, K.D. An interpolation technique to enable accurate three-dimensional joint kinematic analyses using asynchronous biplane fluoroscopy. Med. Eng. Phys. 2018, 60, 109–116. [Google Scholar] [CrossRef]
- Lin, C.-C.; Li, J.-D.; Lu, T.-W.; Kuo, M.-Y.; Kuo, C.-C.; Hsu, H.-C. A model-based tracking method for measuring 3D dynamic joint motion using an alternating biplane x-ray imaging system. Med. Phys. 2018, 45, 3637–3649. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lu, T.-W.; Shih, T.-F.; Tsai, T.-Y.; Wang, T.-M.; Hsu, S.-J. Intervertebral anticollision constraints improve out-of-plane translation accuracy of a single-plane fluoroscopy-to-CT registration method for measuring spinal motion. Med. Phys. 2013, 40, 031912. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, B.L.; Shelburne, K.B.; Torry, M.R.; Erik Giphart, J. A comparison of calibration methods for stereo fluoroscopic imaging systems. J. Biomech. 2011, 44, 2511–2515. [Google Scholar] [CrossRef]
- Rafael, C.; Gonzalez, R.E.W. Digital Image Processing, 4th ed.; Pearson Education Limited: London, UK, 2017. [Google Scholar]
- De Bruin, P.W.; Kaptein, B.L.; Stoel, B.C.; Reiber, J.H.C.; Rozing, P.M.; Valstar, E.R. Image-based RSA: Roentgen stereophotogrammetric analysis based on 2D–3D image registration. J. Biomech. 2008, 41, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lorensen, W.E.; Cline, H.E. Marching Cubes: A High Resolution 3D Surface Construction Algorithm. Comput. Graph. (ACM) 1987, 21, 163–169. [Google Scholar] [CrossRef]
- Miranda, D.L.; Rainbow, M.J.; Leventhal, E.L.; Crisco, J.J.; Fleming, B.C. Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. J. Biomech. 2010, 43, 1623–1626. [Google Scholar] [CrossRef] [PubMed]
- Arulampalam, M.S.; Maskell, S.; Gordon, N.; Clapp, T. A tutorial on particle filters for online nonlinear/non-Gaussian Bayesian tracking. IEEE Trans. Signal Process. 2002, 50, 174–188. [Google Scholar] [CrossRef]
- Moré, J.J. The Levenberg-Marquardt Algorithm: Implementation and Theory; Springer: Berlin/Heidelberg, Germany, 1978; pp. 105–116. [Google Scholar]
- Jiefu, Z.; Yu, K.; Jiang, L.; Shipeng, L. A low complexity motion compensated frame interpolation method. In Proceedings of the International Symposium on Circuits and Systems (ISCAS 2005), Kobe, Japan, 23–26 May 2005; Volume 4925, pp. 4927–4930. [Google Scholar]
- Byung-Tae, C.; Sung-Hee, L.; Sung-Jea, K. New frame rate up-conversion using bi-directional motion estimation. IEEE Trans. Consum. Electron. 2000, 46, 603–609. [Google Scholar] [CrossRef]
- Markelj, P.; Tomaževič, D.; Likar, B.; Pernuš, F. A review of 3D/2D registration methods for image-guided interventions. Med. Image Anal. 2012, 16, 642–661. [Google Scholar] [CrossRef]
- Otake, Y.; Armand, M.; Armiger, R.S.; Kutzer, M.D.; Basafa, E.; Kazanzides, P.; Taylor, R.H. Intraoperative image-based multiview 2D/3D registration for image-guided orthopaedic surgery: Incorporation of fiducial-based C-arm tracking and GPU-acceleration. IEEE Trans. Med. Imaging 2012, 31, 948–962. [Google Scholar] [CrossRef]
- Goldberg, D.E. Genetic Algorithms in Search, Optimization, and Machine Learning; Addison-Wesley: Boston, MA, USA, 1989. [Google Scholar]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder--Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, C.-L.; Lu, M.; Lu, T.-W.; Wu, C.-H. Quantification of three-dimensional soft tissue artifacts in the canine hindlimb during passive stifle motion. BMC Vet. Res. 2018, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Söderkvist, I.; Wedin, P.-Å. Determining the movements of the skeleton using well-configured markers. J. Biomech. 1993, 26, 1473–1477. [Google Scholar] [CrossRef]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng.-Trans. ASME 1983, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Van de Kraats, E.B.; Penney, G.P.; Tomazevic, D.; van Walsum, T.; Niessen, W.J. Standardized evaluation methodology for 2-D-3-D registration. IEEE Trans. Med. Imaging 2005, 24, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Ohnishi, T.; Suzuki, M.; Nawata, A.; Naomoto, S.; Iwasaki, T.; Haneishi, H. Three-dimensional motion study of femur, tibia, and patella at the knee joint from bi-plane fluoroscopy and CT images. Radiol. Phys. Technol. 2010, 3, 151–158. [Google Scholar] [CrossRef]
- Balkovec, C.; Veldhuis, J.H.; Baird, J.W.; Brodland, G.W.; McGill, S.M. A videofluoroscopy-based tracking algorithm for quantifying the time course of human intervertebral displacements. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 794–802. [Google Scholar] [CrossRef]
- Breen, A.; Breen, A. Accuracy and repeatability of quantitative fluoroscopy for the measurement of sagittal plane translation and finite centre of rotation in the lumbar spine. Med. Eng. Phys. 2016, 38, 607–614. [Google Scholar] [CrossRef]
- Bifulco, P.; Cesarelli, M.; Romano, M.; Fratini, A.; Sansone, M. Measurement of intervertebral cervical motion by means of dynamic x-ray image processing and data interpolation. Int. J. Biomed. Imaging 2013, 2013, 152920. [Google Scholar] [CrossRef]
- Bey, M.J.; Kline, S.K.; Tashman, S.; Zauel, R. Accuracy of biplane x-ray imaging combined with model-based tracking for measuring in-vivo patellofemoral joint motion. J. Orthop. Surg. Res. 2008, 3, 38. [Google Scholar] [CrossRef]
- Pitcairn, S.; Lesniak, B.; Anderst, W. In vivo validation of patellofemoral kinematics during overground gait and stair ascent. Gait Posture 2018, 64, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, D.; Jampani, V.; Yang, M.; Learned-Miller, E.; Kautz, J. Super SloMo: High Quality Estimation of Multiple Intermediate Frames for Video Interpolation. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 9000–9008. [Google Scholar]

| Component | Error Metric | Femoral FE | Tibial FE | Tibiofemoral FE | |
|---|---|---|---|---|---|
| Femur | Tibia | Femur | Tibia | ||
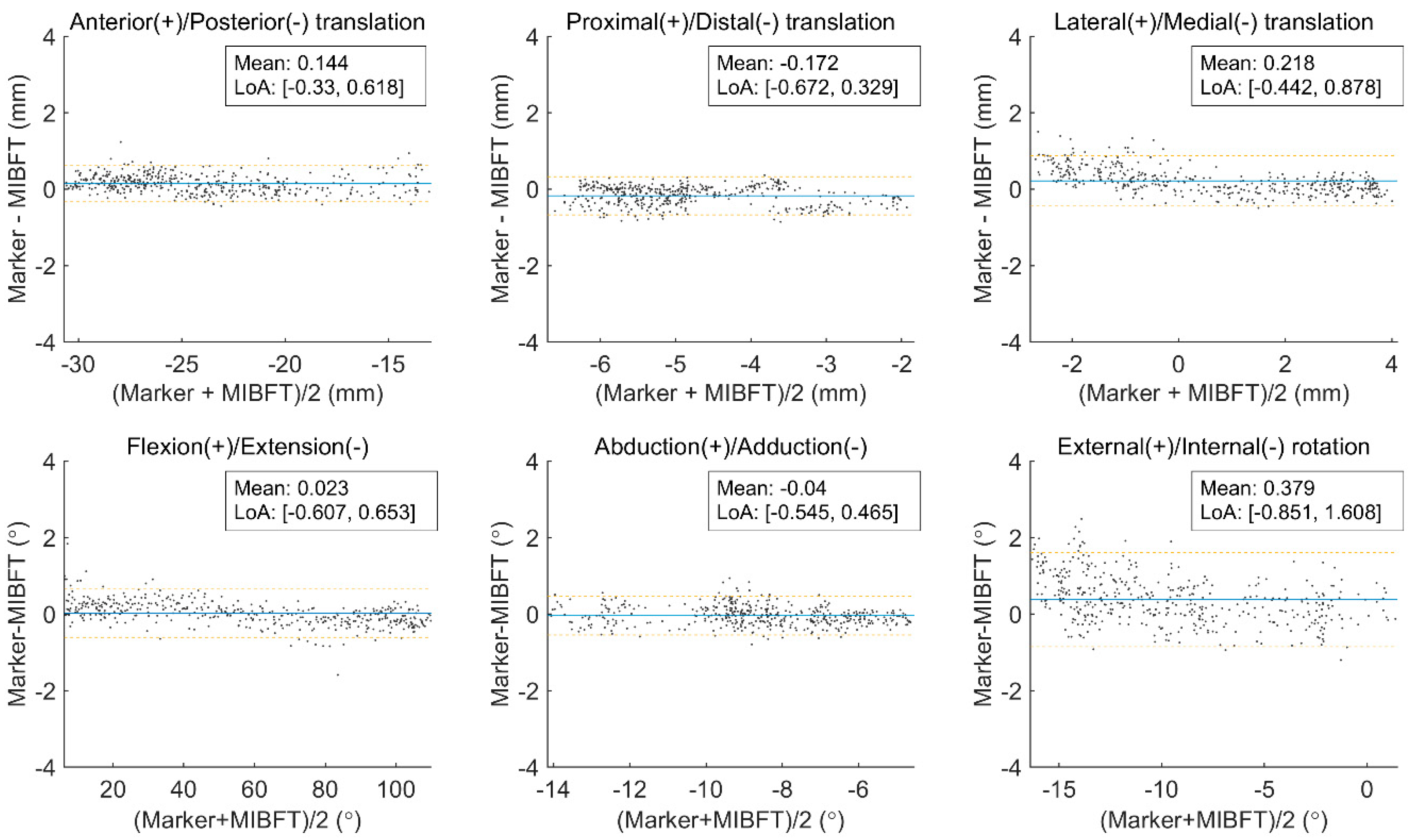
| Tx (mm) | Rms error | 0.28 (0.04) | 0.23 (0.03) | 0.15 (0.03) | 0.18 (0.05) |
| Bias ± precision | −0.07 ± 0.28 | 0.08 ± 0.22 | −0.07 ± 0.13 | 0.08 ± 0.16 | |
| Ty (mm) | Rms error | 0.35 (0.04) | 0.22 (0.03) | 0.11 (0.01) | 0.20 (0.04) |
| Bias ± precision | 0.27 ± 0.23 | 0.12 ± 0.18 | 0.02 ± 0.10 | 0.13 ± 0.15 | |
| Tz (mm) | Rms error | 0.29 (0.05) | 0.26 (0.05) | 0.18 (0.02) | 0.22 (0.04) |
| Bias ± precision | −0.13 ± 0.26 | 0.01 ± 0.26 | −0.04 ± 0.17 | 0.00 ± 0.22 | |
| θx (°) | Rms error | 0.20 (0.06) | 0.30 (0.07) | 0.19 (0.04) | 0.31 (0.13) |
| Bias ± precision | 0.03 ± 0.20 | 0.12 ± 0.27 | 0.04 ± 0.19 | 0.14 ± 0.27 | |
| θy (°) | Rms error | 0.28 (0.04) | 0.43 (0.05) | 0.47 (0.10) | 0.49 (0.11) |
| Bias ± precision | −0.03 ± 0.28 | 0.09 ± 0.42 | −0.23 ± 0.41 | 0.07 ± 0.46 | |
| θz (°) | Rms error | 0.20 (0.06) | 0.29 (0.05) | 0.18 (0.02) | 0.33 (0.14) |
| Bias ± precision | −0.03 ± 0.19 | 0.02 ± 0.29 | 0.03 ± 0.18 | 0.01 ± 0.33 | |
| Study | Equipment e | Activity | Add/Abd (°) (X-axis) | I/E Rot (°) (Y-axis) | Flex/Ext (°) (Z-axis) | AP Tran. (mm) (X-axis) | PD Tran. (mm) (Y-axis) | LM Tran. (mm) (Z-axis) |
|---|---|---|---|---|---|---|---|---|
| Li et al. [35] a | Synchronous BXI | Dynamic | 0.31 (0.72) | −0.16 (0.61) | 0.37 (0.91) | 0.24 (0.16) | −0.11 (0.18) | −0.13 (0.18) |
| Anderst et al. [30] | Synchronous BXI | Dynamic | −0.11 (0.30) [0.54] | 1.01 (0.62) [1.44] | −0.3 (0.94) [1.75] | −0.68 (0.74) [1.54] | −0.16 (0.37) [0.69] | −0.49 (0.31) [0.69] |
| Ohnishi et al. [60] b | Clinical BXI | Static | [0.34] | [0.55] | [0.62] | [0.51] | [0.49] | [0.53] |
| Giphart et al. [31] c | Synchronous BXI | Dynamic | −0.03 (0.61) | −0.05 (0.69) | 0.03 (0.43) | −0.02 (0.49) | 0.08 (0.60) | 0.27 (0.71) |
| Stentz-Olesen et al. [32] d | Synchronous BXI | Dynamic | 0.05 (0.71) | −0.17 (0.77) | −0.11 (0.45) | −0.05 (0.56) | −0.23 (0.38) | 0.16 (0.68) |
| Present study | Clinical BXI | Dynamic | −0.04 (0.26) [0.26] | 0.38 (0.63) [0.73] | 0.02 (0.32) [0.32] | 0.14 (0.24) [0.28] | −0.17 (0.26) [0.31] | 0.22 (0.34) [0.40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-C.; Lu, T.-W.; Li, J.-D.; Kuo, M.-Y.; Kuo, C.-C.; Hsu, H.-C. An Automated Three-Dimensional Bone Pose Tracking Method Using Clinical Interleaved Biplane Fluoroscopy Systems: Application to the Knee. Appl. Sci. 2020, 10, 8426. https://doi.org/10.3390/app10238426
Lin C-C, Lu T-W, Li J-D, Kuo M-Y, Kuo C-C, Hsu H-C. An Automated Three-Dimensional Bone Pose Tracking Method Using Clinical Interleaved Biplane Fluoroscopy Systems: Application to the Knee. Applied Sciences. 2020; 10(23):8426. https://doi.org/10.3390/app10238426
Chicago/Turabian StyleLin, Cheng-Chung, Tung-Wu Lu, Jia-Da Li, Mei-Ying Kuo, Chien-Chun Kuo, and Horng-Chuang Hsu. 2020. "An Automated Three-Dimensional Bone Pose Tracking Method Using Clinical Interleaved Biplane Fluoroscopy Systems: Application to the Knee" Applied Sciences 10, no. 23: 8426. https://doi.org/10.3390/app10238426
APA StyleLin, C.-C., Lu, T.-W., Li, J.-D., Kuo, M.-Y., Kuo, C.-C., & Hsu, H.-C. (2020). An Automated Three-Dimensional Bone Pose Tracking Method Using Clinical Interleaved Biplane Fluoroscopy Systems: Application to the Knee. Applied Sciences, 10(23), 8426. https://doi.org/10.3390/app10238426







