Iron-Rich Magnetic Coal Fly Ash Particles Induce Apoptosis in Human Bronchial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. CFA Collection and Processing
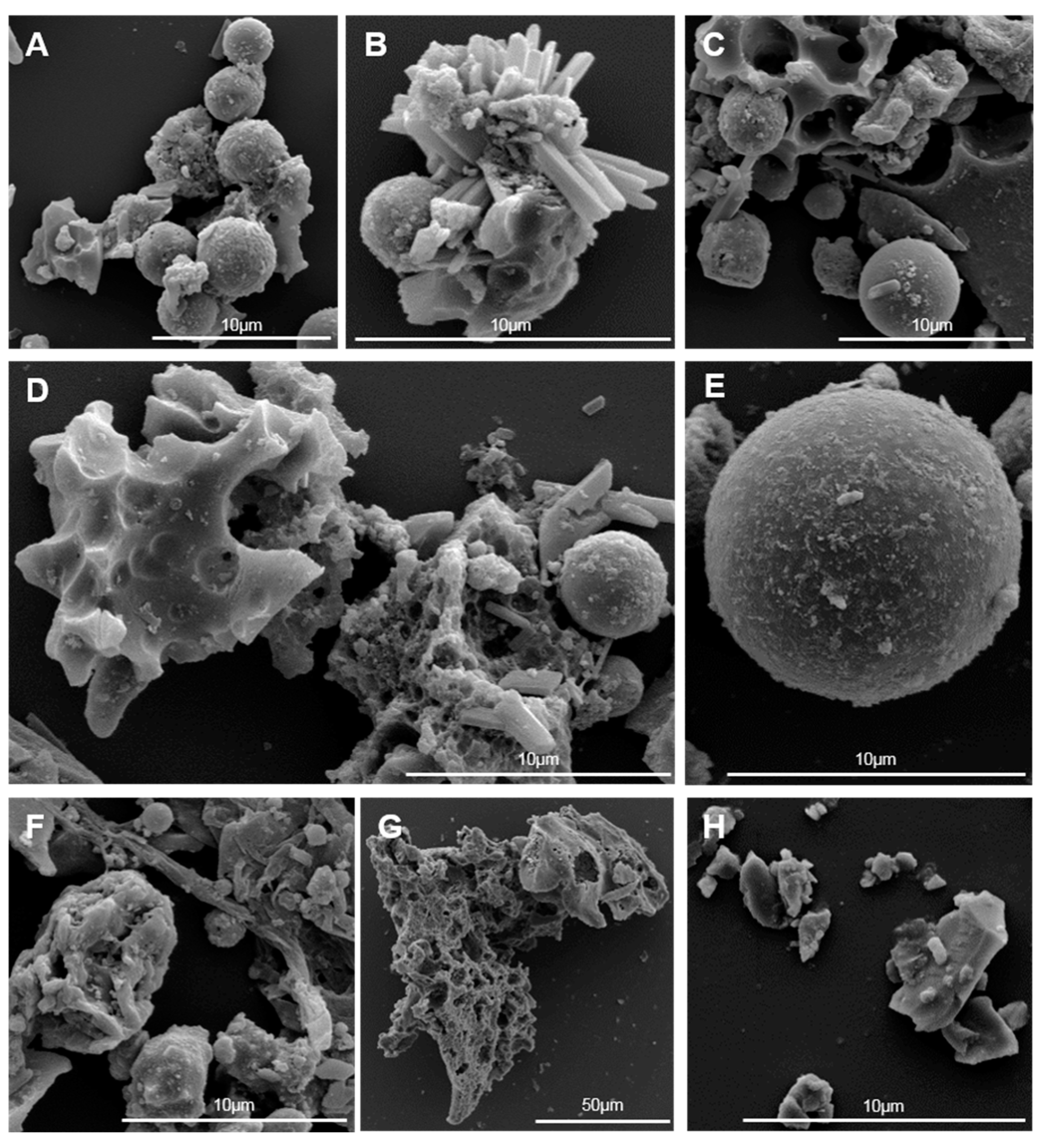
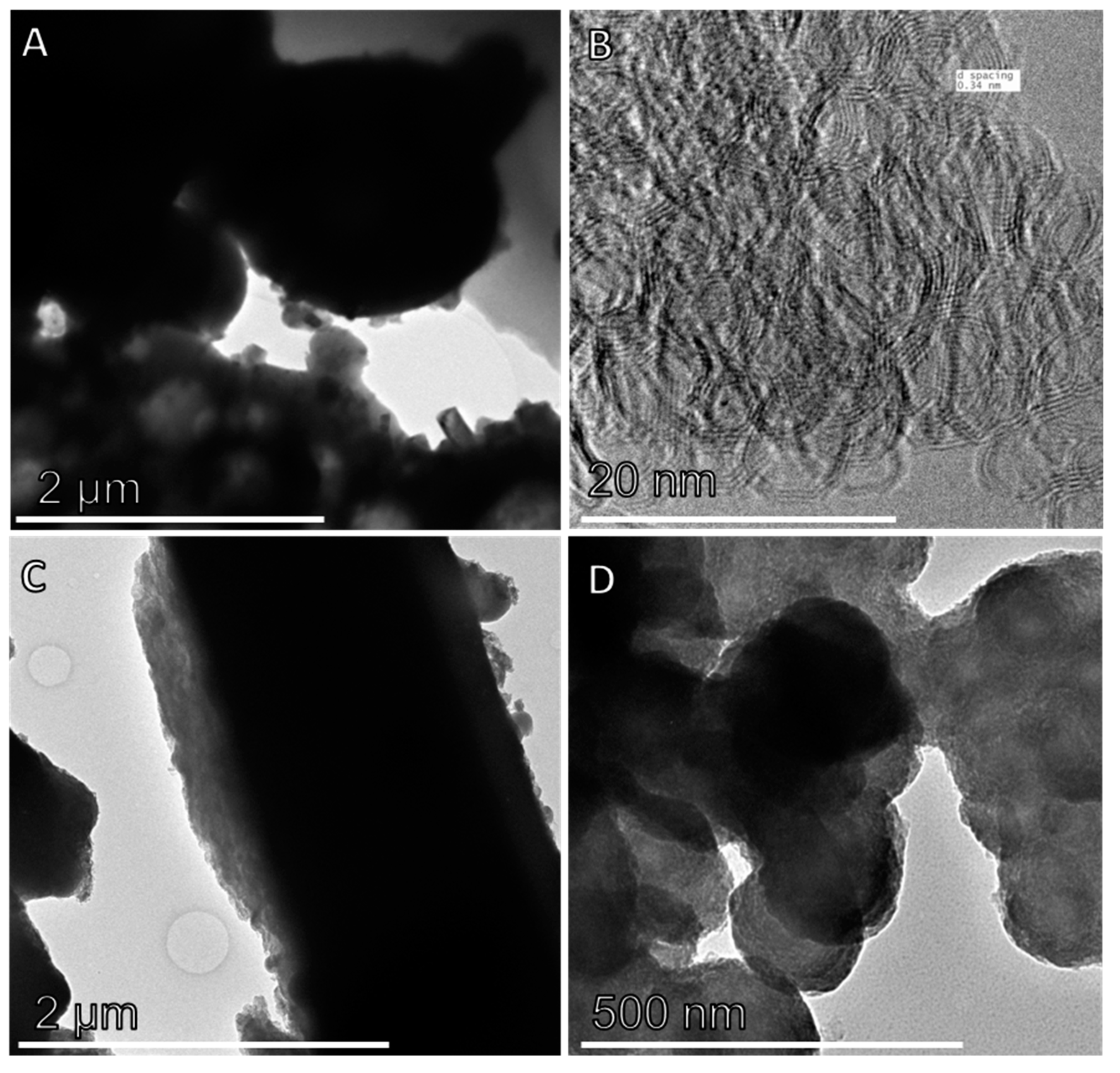
2.2. Electron Microscopy
2.3. Elemental Analysis
2.4. CFA Leachate Production
2.5. Plasma Scission Assay
2.6. CFA Haemolysis Assay
2.7. In Vitro Lung Cell Culture
2.8. Cell Viability Assay
2.9. Cytotoxicity Assay
2.10. Bradford Assay
2.11. Histology
3. Results
3.1. Physicochemical Characterisation of Size-Separated CFA
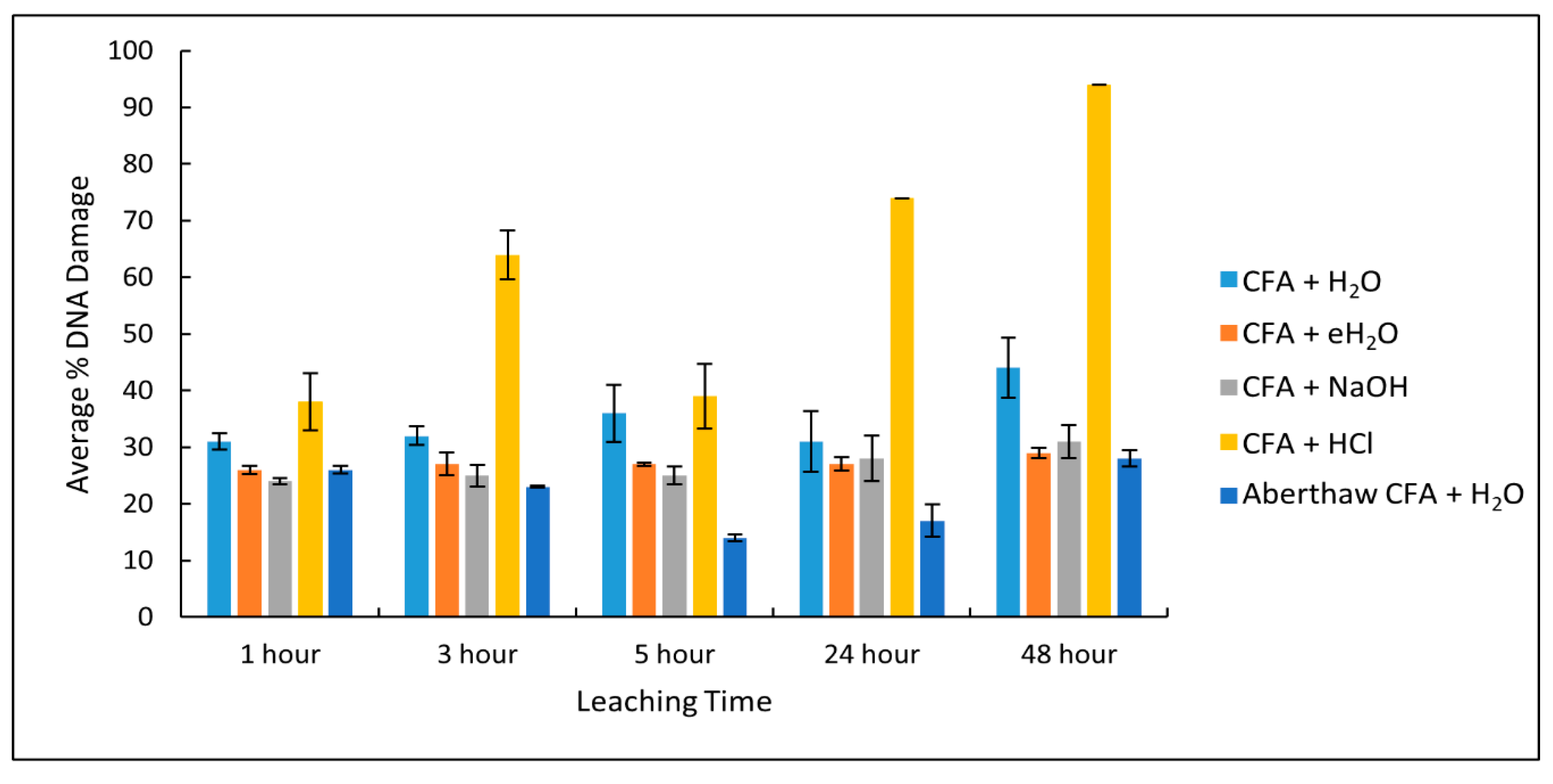
3.2. Manufacture and Analysis of Svalbard CFA Leachates
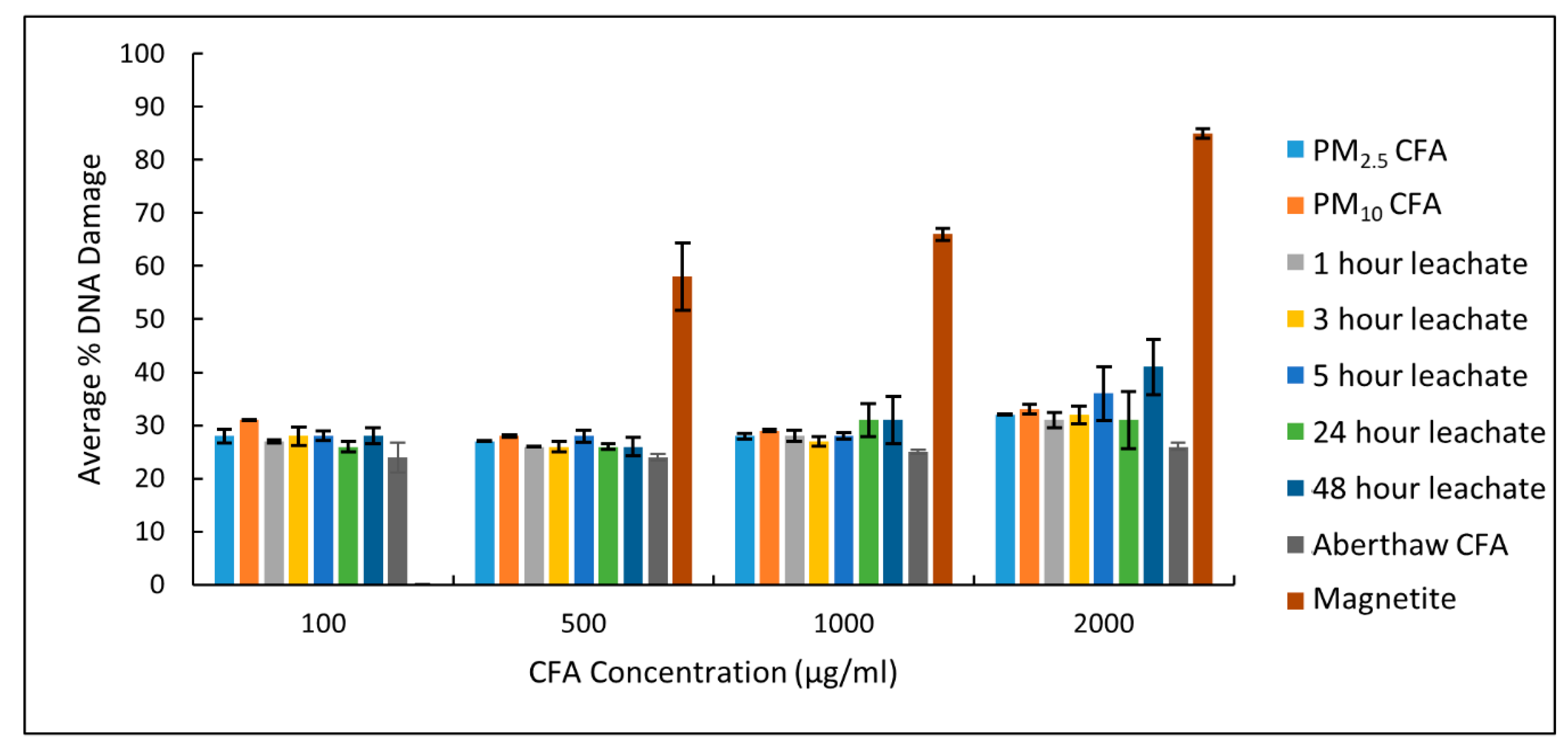
3.3. Plasmid Scission Assay
3.4. Haemolysis Assay
3.5. Exposure to Epi-Airway Bronchial Epithelial Cells
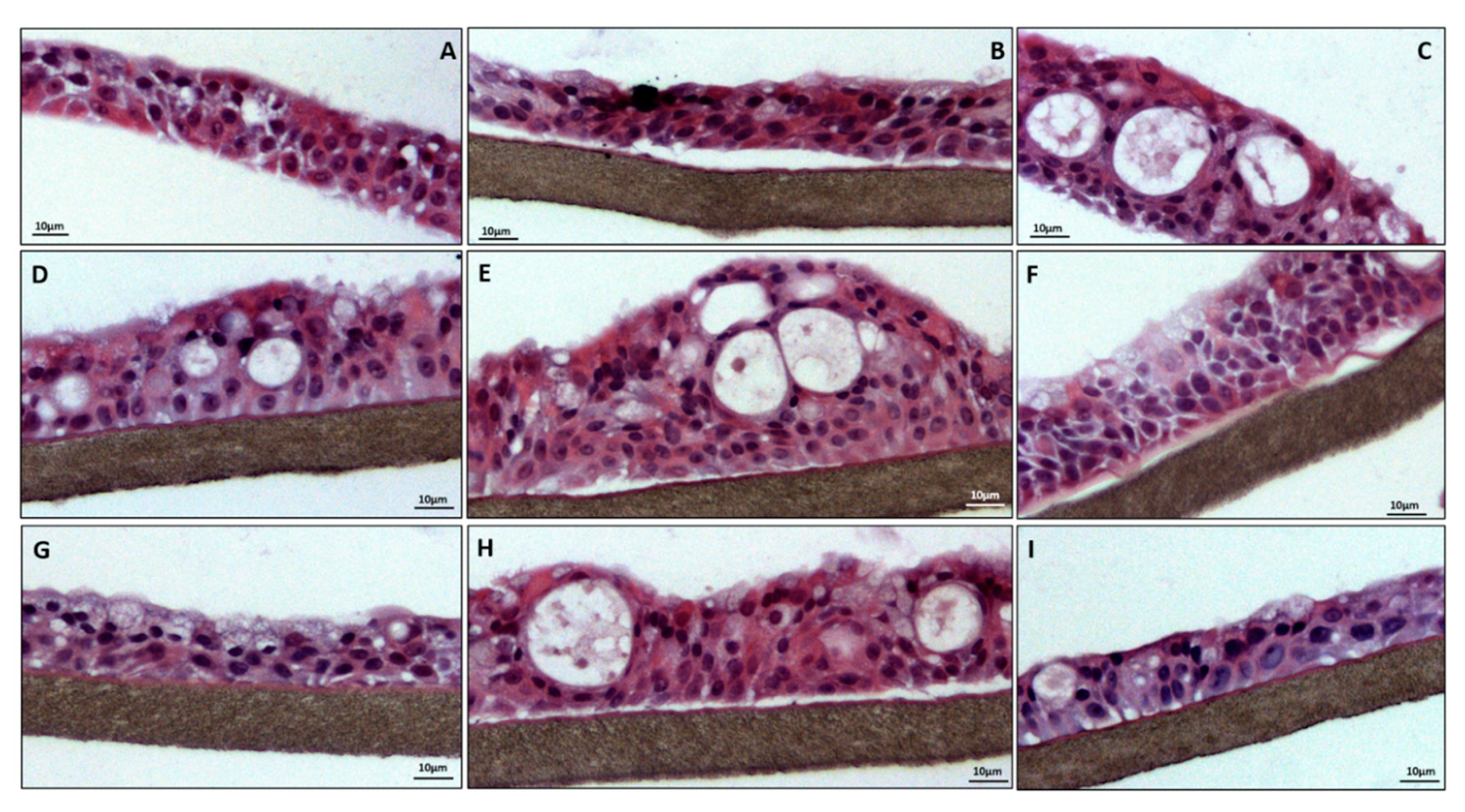
3.6. Histology
4. Discussion
4.1. Physicochemistry of Svalbard CFA
4.2. Bioreactivity of Svalbard CFA
4.3. Lung Cell Exposure to the Svalbard CFA/Leachates
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harland, W.B. The Geology of Svalbard; The Geologcial Society: London, UK, 1997. [Google Scholar]
- StoreNorske. Annual Report and Accounts 2013—Store Norske Spitsbergen Kulkompani AS; StoreNorske: Longyearbyen, Spitsbergen, 2013. [Google Scholar]
- Dallmann, W.K. Geology of the land and sea areas of northern Europe Norges Geologiske Undersøkelse Special Publication. Geol. Svalbard 2007, 10, 87–89. [Google Scholar]
- Smith, K.R.; Mehta, S. The burden of disease from indoor air pollution in developing countries: Comparison of estimates. Int. J. Hyg. Environ. Health 2003, 206, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Seames, W.S. An initial study of the fine fragmentation fly ash particle mode generated during pulverized coal combustion. Fuel Process. Technol. 2003, 81, 109–125. [Google Scholar] [CrossRef]
- Brown, P.D.C. The Physicochemistry, Bioreactivity and Toxicity of Respirable Coal Fly Ash from UK, Poland and China; Cardiff University: Cardiff, UK, 2011. [Google Scholar]
- Meij, R. Trace element behavior in coal-fired power plants. Fuel Process. Technol. 1994, 39, 199–217. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.L.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part. Fibre Toxicol. 2005, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Cohn, C.A.; Fisher, S.C.; Brownawell, B.J.; Schoonen, M.A. Adenine oxidation by pyrite-generated hydroxyl radicals. Geochem. Trans. 2010, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Brown, P.; BeruBe, K.; Wlodarczyk, A.; Longyi, S. The physicochemistry and toxicology of CFA particles. J. Toxicol. Environ. Health Part A Curr. Issues 2010, 73, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Rickardsson, A.; Thore, A. Continuous monitoring of ATP-converting reactions by purified firefly luciferase. Anal. Biochem. 1976, 75, 611–620. [Google Scholar] [CrossRef]
- An, J.; Yin, L.; Shang, Y.; Zhong, Y.; Zhang, X.; Wu, M.; Yu, Z.; Sheng, G.; Fu, J.; Huang, Y. The combined effects of BDE47 and BaP on oxidatively generated DNA damage in L02 cells and the possible molecular mechanism. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2011, 721, 192–198. [Google Scholar] [CrossRef]
- Jones, T.; Moreno, T.; BeruBe, K.; Richards, R. The physicochemical characterisation of microscopic airborne particles in south Wales: A review of the locations and methodologies. Sci. Total Environ. 2006, 360, 43–59. [Google Scholar] [CrossRef] [PubMed]
- BéruBé, K.; Prytherch, Z.; Job, C.; Hughes, T. Human primary bronchial lung cell constructs: The new respiratory models. Toxicology 2010, 278, 311–318. [Google Scholar] [CrossRef]
- Madsen, K.L. Interactions between Microbes and the Gut Epithelium. J. Clin. Gastroenterol. 2011, 45, S111–S114. [Google Scholar] [CrossRef]
- Hoogendoorn, B.; Bérubé, K.; Gregory, C.; Jones, T.; Sexton, K.; Brennan, P.; Brewis, I.A.; Murison, A.; Arthur, R.; Price, H.; et al. Gene and protein responses of human lung tissue explants exposed to ambient particulate matter of different sizes. Inhal. Toxicol. 2012, 24, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Ferruzza, S.; Scacchi, M.; Scarino, M.L.; Sambuy, Y. Iron and copper alter tight junction permeability in human intestinal Caco-2 cells by distinct mechanisms. Toxicol. Vitr. 2002, 16, 399–404. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of Tight Junction Permeability by Intestinal Bacteria and Dietary Components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Koshy, L.; Jones, T.; BeruBe, K. Characterization and bioreactivity of respirable airborne particles from a municipal landfill. Biomarkers 2009, 14, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-T.; Chuang, K.-J.; Chiang, L.-L.; Chen, C.-C.; Yeh, C.-T.; Wang, L.-S.; Gregory, C.; Jones, T.; Bérubé, K.; Lee, C.-N.; et al. Characterization of the interactions between protein and carbon black. J. Hazard. Mater. 2014, 264, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Koshy, L.; Paris, E.; Ling, S.; Jones, T.; BeruBe, K. Bioreactivity of leachate from municipal solid waste landfills-assessment of toxicity. Sci. Total Environ. 2007, 384, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, K.; Balharry, D.; Jones, T.; Moreno, T.; Hayden, P.; Sexton, K.; Hicks, M.; Merolla, L.; Timblin, C.; Shukla, A.; et al. Characterisation of airborne particulate matter and related mechanisms of toxicity: An experimental approach. Air Pollut. Rev. 2006, 3, 69–109. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, M.J.; Prytherch, Z.C.; Jones, T.P.; Adams, R.A.; BéruBé, K.A. Iron-Rich Magnetic Coal Fly Ash Particles Induce Apoptosis in Human Bronchial Cells. Appl. Sci. 2020, 10, 8368. https://doi.org/10.3390/app10238368
Lawson MJ, Prytherch ZC, Jones TP, Adams RA, BéruBé KA. Iron-Rich Magnetic Coal Fly Ash Particles Induce Apoptosis in Human Bronchial Cells. Applied Sciences. 2020; 10(23):8368. https://doi.org/10.3390/app10238368
Chicago/Turabian StyleLawson, Matthew J., Zoe C. Prytherch, Tim P. Jones, Rachel A. Adams, and Kelly A. BéruBé. 2020. "Iron-Rich Magnetic Coal Fly Ash Particles Induce Apoptosis in Human Bronchial Cells" Applied Sciences 10, no. 23: 8368. https://doi.org/10.3390/app10238368
APA StyleLawson, M. J., Prytherch, Z. C., Jones, T. P., Adams, R. A., & BéruBé, K. A. (2020). Iron-Rich Magnetic Coal Fly Ash Particles Induce Apoptosis in Human Bronchial Cells. Applied Sciences, 10(23), 8368. https://doi.org/10.3390/app10238368






