Understanding Biotic Stress and Hormone Signalling in Cassava (Manihot esculenta): Potential for Using Hyphenated Analytical Techniques
Abstract
1. Introduction
1.1. Cassava Mosaic Disease
1.2. Cassava Bacterial Blight Disease
1.3. Cassava Brown Streak Disease
1.4. Cassava Green Mites
1.5. Cassava Mealybug
2. Plant–Insect/Pathogen Interactions
3. Direct Defences
3.1. Jasmonic Acid (JA)
3.2. Ethylene (ET)
3.3. Salicylic Acid (SA)
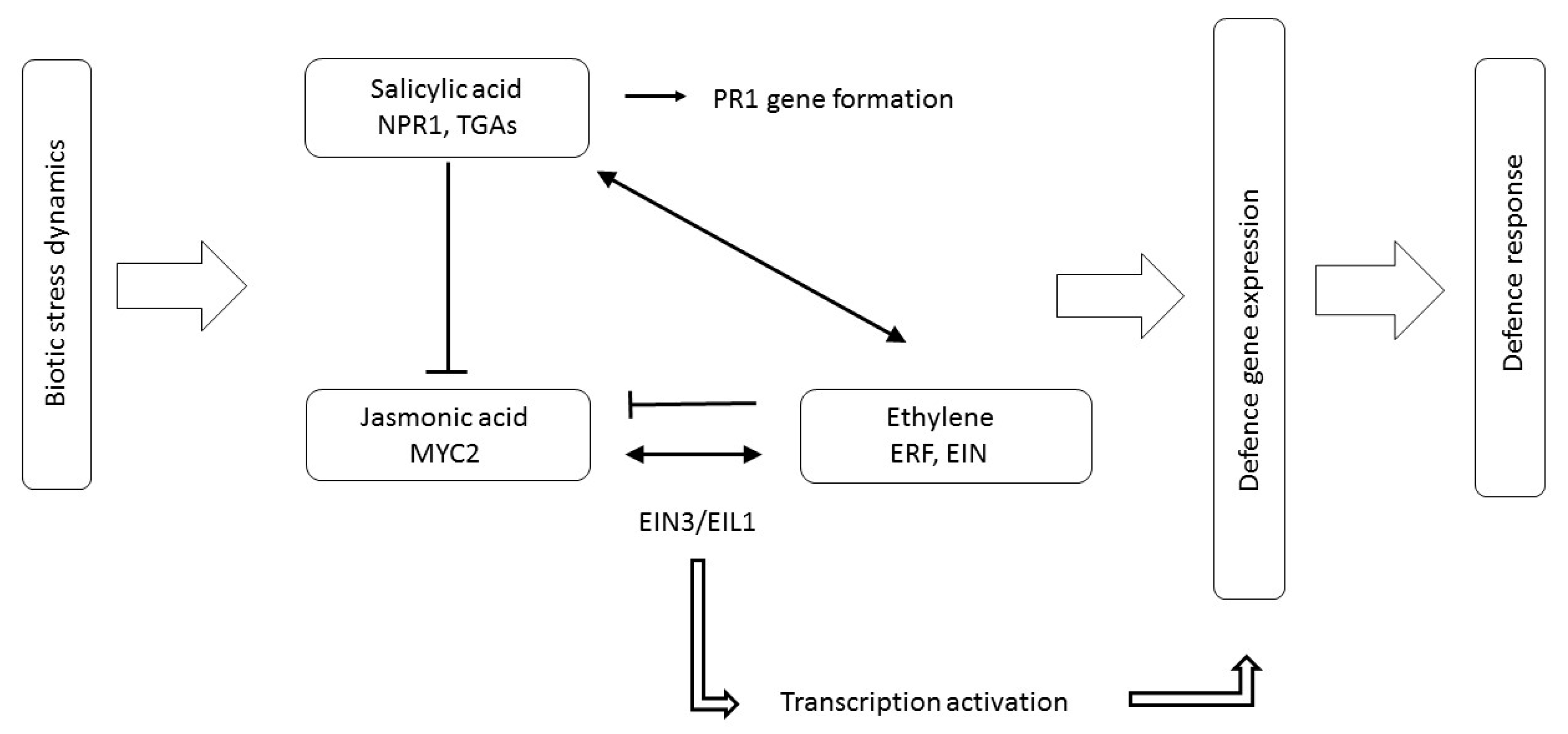
3.4. JA, ET and SA Interactions
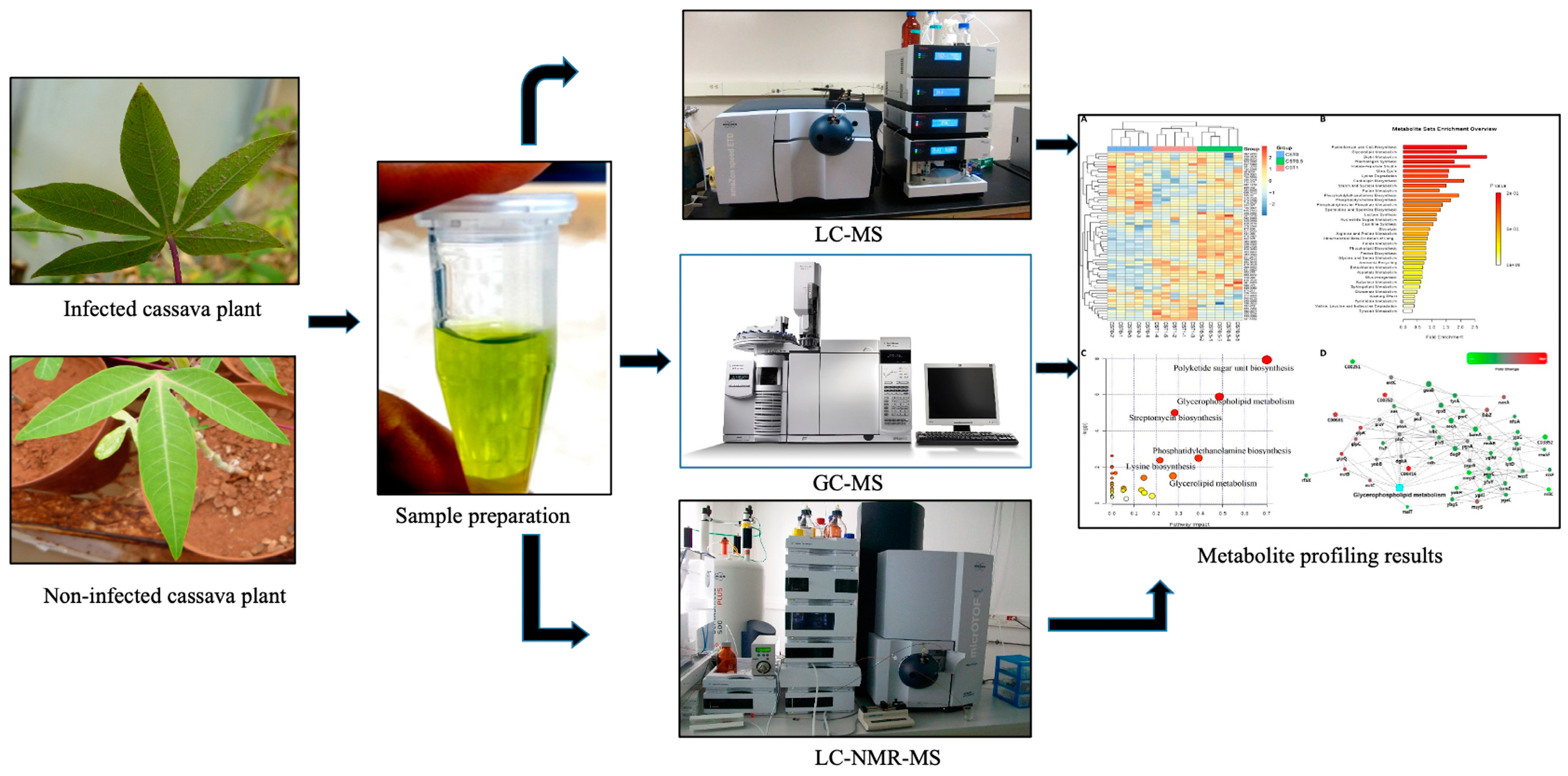
4. What Are Hyphenated Techniques?
5. How Have Hyphenated Techniques Been Used to Study Cassava? Biotic Stress Response and Hormonal Response?
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food Outlook. Biannual Report on Global Food Markets; FAO: Rome, Italy, 2018. [Google Scholar]
- Ceballos, H.; Ramirez, J.; Bellotti, A.C.; Jarvis, A.; Alvarez, E. Adaptation of cassava to changing climates. Crop Adapt. Clim. Chang. 2011, 26, 411–425. [Google Scholar]
- Anjanappa, R.B.; Mehta, D.; Maruthi, M.; Kanju, E.; Gruissem, W.; Vanderschuren, H. Characterization of Brown Streak Virus–Resistant Cassava. Mol. Plant-Microbe Interact. 2016, 29, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Mbewe, W.; Winter, S.; Mukasa, S.; Tairo, F.; Sseruwagi, P.; Ndunguru, J.; Duffy, S. Deep sequencing reveals a divergent Ugandan cassava brown streak virus isolate from Malawi. Genome Announc. 2017, 5, e00818-17. [Google Scholar] [CrossRef] [PubMed]
- Rogans, S.J.; Allie, F.; Tirant, J.E.; Rey, M.E. Small RNA and methylation responses in susceptible and tolerant landraces of cassava infected with South African cassava mosaic virus. Virus Res. 2016, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, G.Q.; Zhou, Q.; Lu, W.; Ma, J.Q.; Huang, J.H. Transcriptomic and proteomic response of Manihot esculenta to Tetranychus urticae infestation at different densities. Exp. Appl. Acarol. 2019, 78, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Camañes, G.; Scalschi, L.; Vicedo, B.; González-Bosch, C.; García-Agustín, P. An untargeted global metabolomic analysis reveals the biochemical changes underlying basal resistance and priming in Solanum lycopersicum, and identifies 1-methyltryptophan as a metabolite involved in plant responses to Botrytis cinerea and Pseudomonas syringae. Plant J. 2015, 84, 125–139. [Google Scholar]
- Drapal, M.; de Carvalho, E.B.; Ovalle Rivera, T.M.; Becerra Lopez-Lavalle, L.A.; Fraser, P.D. Capturing biochemical diversity in cassava (Manihot esculenta Crantz) through the application of metabolite profiling. J. Agric. Food Chem. 2019, 67, 986–993. [Google Scholar] [CrossRef]
- Gazola, D.; Zucareli, C.; Ringenberg, R.; de Oliveira, M.C.N.; da Graça, J.P.; de Oliveira Nunes, E.; Hoffmann-Campo, C.B. Secondary metabolite contents in different parts of cassava plants infested by Phenacoccus manihoti Matile-Ferrero (Hemiptera: Pseudococcidae). Arthropod-Plant Interact. 2019, 13, 359–366. [Google Scholar] [CrossRef]
- Jones, O.A.H.; Maguire, M.L.; Griffin, J.L.; Jung, Y.H.; Shibato, J.; Rakwal, R.; Agrawal, G.K.; Jwa, N.S. Using metabolic profiling to assess plant–pathogen interactions: An example using rice (Oryza sativa) and the blast pathogen Magnaporthe grisea. Eur. J. Plant Pathol. 2011, 129, 539–554. [Google Scholar] [CrossRef]
- Perez-Fons, L.; Bohorquez-Chaux, A.; Irigoyen, M.L.; Garceau, D.C.; Morreel, K.; Boerjan, W.; Walling, L.L.; Lopez-Lavalle, L.A.B.; Fraser, P.D. A metabolomics characterisation of natural variation in the resistance of cassava to whitefly. BMC Plant Biol. 2019, 19, 518. [Google Scholar] [CrossRef]
- Amuge, T.; Berger, D.K.; Katari, M.S.; Myburg, A.A.; Goldman, S.L.; Ferguson, M.E. A time series transcriptome analysis of cassava (Manihot esculenta Crantz) varieties challenged with Ugandan cassava brown streak virus. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Anjanappa, R.B.; Mehta, D.; Okoniewski, M.J.; Szabelska, A.; Gruissem, W.; Vanderschuren, H. Early transcriptome analysis of the brown streak virus–cassava pathosystem provides molecular insights into virus susceptibility and resistance. BioRxiv 2017, 100552. [Google Scholar] [CrossRef]
- Mahungu, N.; Dixon, A.G.; Kumbira, J. Breeding cassava for multiple pest resistance in Africa. Afr. Crop Sci. J. 2009, 2, 539–552. [Google Scholar]
- Nassar, N.; Ortiz, R. Cassava improvement: Challenges and impacts. J. Agric. Sci. 2007, 145, 163–171. [Google Scholar] [CrossRef]
- Allie, F.; Pierce, E.J.; Okoniewski, M.J.; Rey, C. Transcriptional analysis of South African cassava mosaic virus-infected susceptible and tolerant landraces of cassava highlights differences in resistance, basal defense and cell wall associated genes during infection. BMC Genom. 2014, 15, 1006–1035. [Google Scholar] [CrossRef] [PubMed]
- Hillocks, R.J. Cassava in Africa. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing, CIAT: Cali, Colombia; University of Greenwich: Kent, UK, 2002; pp. 41–54. [Google Scholar]
- Rauwane, M.E.; Odeny, D.A.; Millar, I.; Rey, C.; Rees, J. The early transcriptome response of cassava (Manihot esculenta Crantz) to mealybug (Phenacoccus manihoti) feeding. PLoS ONE 2018, 13, e0202541. [Google Scholar] [CrossRef]
- Calvert, L.; Thresh, J.M. The viruses and virus diseases of cassava. In Cassava: Biology, Production and Utilization; CIAT: Cali, Colombia; University of Greenwich: Kent, UK, 2002; pp. 237–260. [Google Scholar]
- Otim-Nape, G.W.; Bua, A.; Baguma, Y. Accelerating the transfer of improved production technologies: Controlling African cassava mosaic virus disease epidemics in Uganda. Afr. Crop Sci. J. 1994, 2, 479–495. [Google Scholar]
- Rogans, S.J.; Rey, C. Unveiling the micronome of Cassava (Manihot esculenta Crantz). PLoS ONE 2016, 11, e0147251. [Google Scholar] [CrossRef]
- Thresh, J.; Cooter, R. Strategies for controlling cassava mosaic virus disease in Africa. Plant Pathol. 2005, 54, 587–614. [Google Scholar] [CrossRef]
- Legg, J. Emergence, spread and strategies for controlling the pandemic of cassava mosaic virus disease in east and central Africa. Crop Prot. 1999, 18, 627–637. [Google Scholar] [CrossRef]
- Legg, J.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava mosaic virus disease in east and central Africa: Epidemiology and management of a regional pandemic. Adv. Virus Res. 2006, 67, 355–418. [Google Scholar] [PubMed]
- Lopez, C.; Soto, M.; Restrepo, S.; Piégu, B.; Cooke, R.; Delseny, M.; Tohme, J.; Verdier, V. Gene expression profile in response to Xanthomonas axonopodis pv. manihotis infection in cassava using a cDNA microarray. Plant Mol. Biol. 2005, 57, 393–410. [Google Scholar] [CrossRef]
- López, C.E.; Bernal, A.J. Cassava bacterial blight: Using genomics for the elucidation and management of an old problem. Trop. Plant Biol. 2012, 5, 117–126. [Google Scholar] [CrossRef]
- Boher, B.; Verdier, V. Cassava bacterial blight in Africa: The state of knowledge and implications for designing control strategies. Afr. Crop Sci. J. 2009, 2, 505–509. [Google Scholar]
- Wydra, K.; Verdier, V. Occurrence of cassava diseases in relation to environmental, agronomic and plant characteristics. Agric. Ecosyst. Environ. 2002, 93, 211–226. [Google Scholar] [CrossRef]
- Jorge, V.; Fregene, M.A.; Duque, M.; Bonierbale, M.W.; Tohme, J.; Verdier, V. Genetic mapping of resistance to bacterial blight disease in cassava (Manihot esculenta Crantz). Theor. Appl. Genet. 2000, 101, 865–872. [Google Scholar] [CrossRef][Green Version]
- Fokunang, C.; Ikotun, T.; Dixon, A.; Akem, C. Field reaction of cassava genotypes to anthracnose, bacterial blight, cassava mosaic disease and their effects on yield. Afr. Crop Sci. J. 2000, 8, 179–186. [Google Scholar] [CrossRef]
- Hillocks, R.; Jennings, D. Cassava brown streak disease: A review of present knowledge and research needs. Int. J. Pest Manag. 2003, 49, 225–234. [Google Scholar] [CrossRef]
- Mbanzibwa, D.; Tian, Y.; Tugume, A.; Patil, B.; Yadav, J.; Bagewadi, B.; Abarshi, M.; Alicai, T.; Changadeya, W.; Mkumbira, J. Evolution of cassava brown streak disease-associated viruses. J. Gen. Virol. 2011, 92, 974–987. [Google Scholar] [CrossRef]
- Mbanzibwa, D.; Tian, Y.; Tugume, A.; Mukasa, S.; Tairo, F.; Kyamanywa, S.; Kullaya, A.; Valkonen, J.P. Genetically distinct strains of Cassava brown streak virus in the Lake Victoria basin and the Indian Ocean coastal area of east Africa. Arch. Virol. 2009, 154, 353–359. [Google Scholar] [CrossRef]
- Maruthi, M.; Hillocks, R.; Mtunda, K.; Raya, M.; Muhanna, M.; Kiozia, H.; Rekha, A.; Colvin, J.; Thresh, J. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 2005, 153, 307–312. [Google Scholar] [CrossRef]
- Wasswa, P.; Alicai, A.; Mukasa, S. Optimisation of in vitro techniques for Cassava brown streak virus elimination from infected cassava clones. Afr. Crop Sci. J. 2010, 18, 235–241. [Google Scholar] [CrossRef]
- Patil, B.L.; Ogwok, E.; Wagaba, H.; Mohammed, I.U.; Yadav, J.S.; Bagewadi, B.; Taylor, N.J.; Kreuze, J.F.; Maruthi, M.; Alicai, T. RNAi-mediated resistance to diverse isolates belonging to two virus species involved in Cassava brown streak disease. Mol. Plant Pathol. 2011, 12, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Yaninek, J.; Wermelinger, B.; Herren, H.; Ellis, C. Analysis of biological control of cassava pests in Africa. III. Cassava green mite Mononychellus tanajoa. J. Appl. Ecol. 1988, 25, 941–950. [Google Scholar] [CrossRef]
- Mutisya, D.L.; El-Banhawy, E.; Khamala, C.; Kariuki, C.W. Management of cassava green mite Mononychellus progresivus (Acari: Tetranychidae) in different agro-ecological zones of Kenya. Syst. Appl. Acarol. 2015, 20, 39–50. [Google Scholar]
- Yaninek, S.; Hanna, R. Cassava Green Mite in Africa—A unique example of successful classical biological control of a mite pest on a continental scale. In Biological Control in IPM Systems in Africa; Neuenschwander, C.B., Langewald, J., Eds.; CABI: Oxon, UK, 2003; pp. 61–76. [Google Scholar]
- Aidoo, R.; Osekre, E.A.; Logah, V.; Bakang, J.-E.A. Economic benefits of biological control of cassava green mite (CGM) in Ghana. J. Dev. Agric. Econ. 2016, 8, 172–185. [Google Scholar]
- Mutisya, D.L.; Wambua, J.M.; Miano, D.W.; Kariuki, C.W. Farmer perceptions of cassava green mite pest impact in eastern Kenya. J. Entomol. Zool. Stud. 2015, 3, 354–358. [Google Scholar]
- Miller, D.R.; Miller, G.L.; Hodges, G.S.; Davidson, J.A. Introduced scale insects (Hemiptera: Coccoidea) of the United States and their impact on US agriculture. Proc. Entomol. Soc. Wash. 2005, 107, 123–158. [Google Scholar]
- Miller, D.R.; Miller, G.L.; Watson, G.W. Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to US agriculture. Proc. Entomol. Soc. Wash. 2002, 104, 825–836. [Google Scholar]
- Miller, D.R.; Rossman, A.Y. Systematics, biodiversity, and agriculture. BioScience 1995, 45, 680–686. [Google Scholar] [CrossRef]
- Daane, K.M.; Almeida, R.P.; Bell, V.A.; Walker, J.T.; Botton, M.; Fallahzadeh, M.; Mani, M.; Miano, J.L.; Sforza, R.; Walton, V.M. Biology and management of mealybugs in vineyards. In Arthropod Management in Vineyards; Bostanian, N.J., Isaacs, R., Vincent, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 271–307. [Google Scholar]
- Kuniyuki, H.; Gioria, R.; Rezende, J.A.M.; Willink, C.G.D.; Novo, J.P.S.; Yuki, V.A. Transmission of the Grapevine virus B by the mealybug Pseudococcus longispinus Targioni-Tozzetti (Hemiptera: Pseudococcidae) in Brazil. Summa Phytopathol. 2006, 32, 151–155. [Google Scholar] [CrossRef]
- Meyer, J.; Kasdorf, G.; Nel, L.; Pietersen, G. Transmission of activated-episomal banana streak ol (badna) virus (bsolv) to cv. Williams banana (musa sp.) by three mealybug species. Plant Dis. 2008, 92, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Nakaune, R.; Toda, S.; Mochizuki, M.; Nakano, M. Identification and characterization of a new vitivirus from grapevine. Arch. Virol. 2008, 153, 1827–1832. [Google Scholar] [CrossRef]
- Sforza, R.; Boudon-Padieu, E.; Greif, C. New mealybug species vectoring Grapevine leafroll-associated viruses-1 and-3 (GLRaV-1 and-3). Eur. J. Plant Pathol. 2003, 109, 975–981. [Google Scholar] [CrossRef]
- Calatayud, P.A.; Rahbé, Y.; Tjallingii, W.; Tertuliano, M.; Rü, B. Electrically recorded feeding behaviour of cassava mealybug on host and non-host plants. Entomol. Exp. Appl. 1994, 72, 219–232. [Google Scholar] [CrossRef]
- Bellotti, A.C.; Smith, L.; Lapointe, S.L. Recent advances in cassava pest management. Annu. Rev. Entomol. 1999, 44, 343–370. [Google Scholar] [CrossRef]
- Neuenschwander, P. Biological control of the cassava mealybug in Africa: A review. Biol. Control 2001, 21, 214–229. [Google Scholar] [CrossRef]
- Akinbo, O.; Labuschagne, M.; Fregene, M. Introgression of whitefly (Aleurotrachelus socialis) resistance gene from F1 inter-specific hybrids into commercial cassava. Euphytica 2012, 183, 19–26. [Google Scholar] [CrossRef]
- Carabalí, A.; Bellotti, A.; Montoya-Lerma, J.; Fregene, M. Resistance to the whitefly, Aleurotrachelus socialis, in wild populations of cassava, Manihot tristis. J. Insect Sci. 2010, 10, 170–180. [Google Scholar] [CrossRef][Green Version]
- Carabalí, A.; Bellotti, A.C.; Montoya-Lerma, J.; Fregene, M. Manihot flabellifolia Pohl, wild source of resistance to the whitefly Aleurotrachelus socialis Bondar (Hemiptera: Aleyrodidae). Crop Prot. 2010, 29, 34–38. [Google Scholar] [CrossRef]
- Morante, N.; Marín, J.A.; Gutiérrez, A.J.P.; Sánchez, T.; Ospina, C.; Bellotti, A.C.; Fregene, M.A. Mining the Primary Gene Pool of Cassava: Introgression of Resistance to the Cassava Green Mite and High Root Protein from Accessions of Manihot Esculenta Sub spp Flabellifolia and Manihot Tristis into Cassava; CIAT: Cali, Colombia, 2004; Available online: http://ciat-library.ciat.cgiar.org/Articulos_ciat/cbn/Posters-PDF/PS-2/N_Morante.pdf (accessed on 22 May 2020).
- Gatehouse, J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Arnaiz, A.; Velasco-Arroyo, B.; Grbic, V.; Diaz, I.; Martinez, M. Arabidopsis response to the spider mite Tetranychus urticae depends on the regulation of reactive oxygen species homeostasis. Sci. Rep. UK 2018, 8, 9432. [Google Scholar] [CrossRef] [PubMed]
- Korth, K.L. Profiling the response of plants to herbivorous insects. Genome Biol. 2003, 4, 211–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Poecke, R.M. Arabidopsis-insect interactions. The Arabidopsis Book. Am. Soc. Plant Biol. 2007, 5, 107–140. [Google Scholar]
- Wei, J.; Wang, L.; Zhu, J.; Zhang, S.; Nandi, O.I.; Kang, L. Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2007, 2, 852. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangle, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Sergeant, K.; Renaut, J. Plant biotic stress and proteomics. Curr. Proteom. 2010, 7, 275–297. [Google Scholar] [CrossRef]
- Arimura, G.-I.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- Chen, M.S. Inducible direct plant defense against insect herbivores: A review. Insect Sci. 2008, 15, 101–114. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, X.; Yan, F.; Li, R.; Cheng, J.; Lou, Y. Genome-wide transcriptional changes and defence-related chemical profiling of rice in response to infestation by the rice striped stem borer Chilo suppressalis. Physiol. Plant. 2011, 143, 21–40. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef]
- Kessler, A.; Halitschke, R.; Baldwin, I.T. Silencing the jasmonate cascade: Induced plant defenses and insect populations. Science 2004, 305, 665–668. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Snoeren, T.A.; Dicke, M.; Vosman, B. Exploiting natural variation to identify insect-resistance genes. Plant Biotechnol. J. 2011, 9, 819–825. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Duffey, S.S.; Stout, M.J. Antinutritive and toxic components of plant defense against insects. Arch. Insect Biochem. Physiol. 1996, 32, 3–37. [Google Scholar] [CrossRef]
- Steppuhn, A.; Baldwin, I.T. Resistance management in a native plant: Nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol. Lett. 2007, 10, 499–511. [Google Scholar] [CrossRef]
- Klingler, J.; Creasy, R.; Gao, L.; Nair, R.M.; Calix, A.S.; Jacob, H.S.; Edwards, O.R.; Singh, K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005, 137, 1445–1455. [Google Scholar] [CrossRef]
- Bellotti, A.C.; Riis, L. Cassava cyanogenic potential and resistance to pests and diseases. In Proceedings of the International Workshop on Cassava Safety, Ibadan, Nigeria, 1–4 March 1994; Volume 375, pp. 141–152. [Google Scholar]
- Riis, L.; Bellotti, A.C.; Bonierbale, M.; O’Brien, G.M. Cyanogenic potential in cassava and its influence on a generalist insect herbivore Cyrtomenus bergi (Hemiptera: Cydnidae). J. Econ. Entomol. 2003, 96, 1905–1914. [Google Scholar] [CrossRef]
- Calatayud, P.A.; Le Rü, B.P. Cassava-Mealybug Interactions; Institut De Recherche, Ed.; CIAT: Cali, Colombia, 2006; pp. 11–21. [Google Scholar]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Agudelo-Romero, P.; Erban, A.; Rego, C.; Carbonell-Bejerano, P.; Nascimento, T.; Sousa, L.; Martínez-Zapater, J.M.; Kopka, J.; Fortes, A.M. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 2015, 66, 1769–1785. [Google Scholar] [CrossRef]
- Artico, S.; Ribeiro-Alves, M.; Oliveira-Neto, O.B.; de Macedo, L.L.; Silveira, S.; Grossi-de-Sa, M.F.; Martinelli, A.P.; Alves-Ferreira, M. Transcriptome analysis of Gossypium hirsutum flower buds infested by cotton boll weevil (Anthonomus grandis) larvae. BMC Genom. 2014, 15, 854–877. [Google Scholar] [CrossRef]
- Chahtane, H.; Füller, T.N.; Allard, P.M.; Marcourt, L.; Queiroz, E.F.; Shanmugabalaji, V.; Falquet, J.; Wolfender, J.L.; Lopez-Molina, L. The plant pathogen Pseudomonas aeruginosa triggers a DELLA-dependent seed germination arrest in Arabidopsis. Elife 2018, 7, e37082. [Google Scholar] [CrossRef]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Van Loon, L.; Dicke, M. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef]
- Gutsche, A.; Heng-Moss, T.; Sarath, G.; Twigg, P.; Xia, Y.; Lu, G.; Mornhinweg, D. Gene expression profiling of tolerant barley in response to Diuraphis noxia (Hemiptera: Aphididae) feeding. Bull. Entomol. Res. 2009, 99, 163–173. [Google Scholar] [CrossRef]
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. Jasmonate-Insensitive1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef]
- Liu, X.M.; Smith, C.M.; Gill, B.S. Allelic relationships among Russian wheat aphid resistance genes. Crop Sci. 2005, 45, 2273–2280. [Google Scholar] [CrossRef]
- Cooper, W.; Goggin, F. Effects of jasmonate-induced defenses in tomato on the potato aphid, Macrosiphum euphorbiae. Entomol. Exp. Appl. 2005, 115, 107–115. [Google Scholar] [CrossRef]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef]
- Park, S.-J.; Huang, Y.; Ayoubi, P. Identification of expression profiles of sorghum genes in response to greenbug phloem-feeding using cDNA subtraction and microarray analysis. Planta 2006, 223, 932–947. [Google Scholar] [CrossRef]
- Timm, A.E.; Reineke, A. First insights into grapevine transcriptional responses as a result of vine mealybug Planococcus ficus feeding. Arthropod. Plant Interact. 2014, 8, 495–505. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, X.; Huang, F.; Liu, Y.; Zhang, J.; Lu, Y.; Ruan, Y. Suppression of jasmonic acid-dependent defense in cotton plant by the mealybug Phenacoccus solenopsis. PLoS ONE 2011, 6, e22378. [Google Scholar] [CrossRef]
- Gutzat, R.; Scheid, O.M. Epigenetic responses to stress: Triple defense? Curr. Opin. Plant Biol. 2012, 15, 568–573. [Google Scholar] [CrossRef]
- De Vos, M.; Van Zaanen, W.; Koornneef, A.; Korzelius, J.P.; Dicke, M.; Van Loon, L.; Pieterse, C.M. Herbivore-induced resistance against microbial pathogens in Arabidopsis. Plant Physiol. 2006, 142, 352–363. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Li, Z.; Zhang, H.; Ye, J.; Li, G. Gene Expression Changes during the gummosis development of peach shoots in response to Lasiodiplodia theobromae infection using RNA-Seq. Front. Physiol. 2016, 7, 1–12. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Zou, J.; Li, M.; Bilgin, D.D.; Vodkin, L.O.; Hartman, G.L.; Clough, S.J. Soybean defense responses to the soybean aphid. New Phytol. 2008, 179, 185–195. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Kurotani, A.; Yoshida, T.; Mochida, K.; Matsui, A.; Ishitani, M.; Sraphet, S.; Whankaew, S.; Asvarak, T. Cassava (Manihot esculenta) transcriptome analysis in response to infection by the fungus Colletotrichum gloeosporioides using an oligonucleotide-DNA microarray. J. Plant Res. 2016, 129, 711–726. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vidigal, P.; Amâncio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Dolgikh, V.A.; Pukhovaya, E.M.; Zemlyanskaya, E.V. Shaping ethylene response: The role of EIN3/EIL1 transcription factors. Front. Plant Sci. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef]
- Hilfiker, O.; Groux, R.; Bruessow, F.; Kiefer, K.; Zeier, J.; Reymond, P. Insect eggs induce a systemic acquired resistance in Arabidopsis. Plant J. 2014, 80, 1085–1094. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Halitschke, R.; Baldwin, I.T. Jasmonates and related compounds in plant-insect interactions. J. Plant Growth Regul. 2004, 23, 238–245. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef]
- Verhage, A.; van Wees, S.C.; Pieterse, C.M. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [PubMed]
- Dubey, N.K.; Goel, R.; Ranjan, A.; Idris, A.; Singh, S.K.; Bag, S.K.; Chandrashekar, K.; Pandey, K.D.; Singh, P.K.; Sawant, S.V. Comparative transcriptome analysis of Gossypium hirsutum L. in response to sap sucking insects: Aphid and whitefly. BMC Genom. 2013, 14, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cano, F.; Soto, J.; Restrepo, S.; Bernal, A.; López-Kleine, L.; López, C.E. Gene co-expression network for Xanthomonas-challenged cassava reveals key regulatory elements of immunity processes. Eur. J. Plant Pathol. 2019, 153, 1083–1104. [Google Scholar] [CrossRef]
- Irigoyen, M.L.; Garceau, D.C.; Bohorquez-Chaux, A.; Lopez-Lavalle, L.A.B.; Perez-Fons, L.; Fraser, P.D.; Walling, L.L. Genome-wide analyses of cassava Pathogenesis-related (PR) gene families reveal core transcriptome responses to whitefly infestation, salicylic acid and jasmonic acid. BMC Genom. 2020, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bodnar, A.; Perez-Quintero, A.L.; Gomez-Cano, F.; Gil, J.; Michelmore, R.; Bernal, A.; Szurek, B.; Lopez, C. RNAseq analysis of cassava reveals similar plant responses upon infection with pathogenic and non-pathogenic strains of Xanthomonas axonopodis pv. manihotis. Plant Cell Rep. 2014, 33, 1901–1912. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Li, B.; Liu, G.; Wei, Y.; He, C.; Shi, H. Identification and functional analysis of cassava DELLA proteins in plant disease resistance against cassava bacterial blight. Plant Physiol. Biochem. 2018, 124, 70–76. [Google Scholar] [CrossRef]
- Chang, K.N.; Zhong, S.; Weirauch, M.T.; Hon, G.; Pelizzola, M.; Li, H.; Huang, S.-S.C.; Schmitz, R.J.; Urich, M.A.; Kuo, D. Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. Elife 2013, 2, 1–20. [Google Scholar] [CrossRef]
- Guo, H.; Ecker, J.R. The ethylene signaling pathway: New insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef]
- McManus, M.T. The plant hormone ethylene. Annu. Plant Rev. 2012, 44, 1–17. [Google Scholar]
- Von Dahl, C.C.; Baldwin, I.T. Deciphering the role of ethylene in plant–herbivore interactions. J. Plant Growth Regul. 2007, 26, 201–209. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef]
- Ouaked, F.; Rozhon, W.; Lecourieux, D.; Hirt, H. A MAPK pathway mediates ethylene signaling in plants. Embo J. 2003, 22, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E. Ethylene, a metabolic product of diseased and injured plants. Phytopathology 1950, 40, 205–208. [Google Scholar]
- Winz, R.A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol. 2001, 125, 2189–2202. [Google Scholar]
- Huang, X.Z.; Chen, J.Y.; Xiao, H.J.; Xiao, Y.T.; Wu, J.; Wu, J.X.; Zhou, J.-J.; Zhang, Y.-J.; Guo, Y.Y. Dynamic transcriptome analysis and volatile profiling of Gossypium hirsutum in response to the cotton bollworm Helicoverpa armigera. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithofer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef]
- Seo, S.; Sano, H.; Ohashi, Y. Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 1999, 11, 289–298. [Google Scholar] [CrossRef]
- Ehlting, J.; Chowrira, S.G.; Mattheus, N.; Aeschliman, D.S.; Arimura, G.-I.; Bohlmann, J. Comparative transcriptome analysis of Arabidopsis thaliana infested by diamond back moth (Plutella xylostella) larvae reveals signatures of stress response, secondary metabolism, and signalling. BMC Genom. 2008, 9, 154–173. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Leon Reyes, H.; Does, D.; Verhage, A.; Koornneef, A.; van Pelt, J.A.; van Wees, S.C. Networking by small-molecule hormones in plant immunity. Iobc-Wprs Bull. 2012, 83, 77–80. [Google Scholar] [CrossRef]
- Ryals, J.A.; Neuenschwander, U.H.; Willits, M.G.; Molina, A.; Steiner, H.Y.; Hunt, M.D. Systemic acquired resistance. Plant Cell 1996, 8, 1809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Wei, X.; Qiu, Y.; Song, J.; Wang, H.; Shen, D.; Agerbirk, N.; Li, X. Expression patterns, molecular markers and genetic diversity of insect-susceptible and resistant Barbarea genotypes by comparative transcriptome analysis. BMC Genom. 2015, 16, 486. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Upadhyay, P.; Rai, A.; Kumar, R.; Singh, M.; Sinha, B. Differential expression of pathogenesis related protein genes in tomato during inoculation with A. solani. J. Plant Pathol. Microbiol. 2014, 5, 217–223. [Google Scholar]
- de Ilarduya, O.M.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant-Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Anjanappa, R.B.; Mehta, D.; Okoniewski, M.J.; Szabelska-Berȩsewicz, A.; Gruissem, W.; Vanderschuren, H. Molecular insights into cassava brown streak virus susceptibility and resistance by profiling of the early host response. Mol. Plant Pathol. 2018, 19, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.; Van Wees, S.C. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Zhang, P.J.; Broekgaarden, C.; Zheng, S.J.; Snoeren, T.A.; Loon, J.J.; Gols, R.; Dicke, M. Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol. 2013, 197, 1291–1299. [Google Scholar] [CrossRef]
- Beckers, G.; Spoel, S. Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol. 2006, 8, 1–10. [Google Scholar] [CrossRef]
- Spoel, S.H.; Johnson, J.S.; Dong, X. Regulation of tradeoffs between plant defenses against pathogens with different lifestyles. Proc. Natl. Acad. Sci. USA 2007, 104, 18842–18847. [Google Scholar] [CrossRef]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hao, F.; Hu, J.; Zhang, W.; Wan, L.; Zhu, L.; Tang, H.; He, G. Revealing different systems responses to brown planthopper infestation for pest susceptible and resistant rice plants with the combined metabonomic and gene-expression analysis. J. Proteome Res. 2010, 9, 6774–6785. [Google Scholar] [CrossRef]
- Li, F.; Wu, C.; Dewer, Y.; Li, D.; Qu, C.; Luo, C. Changes in Gene Expression and Metabolite Profiles in Platanus acerifolia Leaves in Response to Feeding Damage Caused by Corythucha ciliata. Int. J. Mol. Sci. 2019, 20, 3465. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Faubert, D.; Jabaji, S. A metabolic profiling strategy for the dissection of plant defense against fungal pathogens. PLoS ONE 2014, 9, e111930. [Google Scholar] [CrossRef]
- Ghosh, S.; Kanwar, P.; Jha, G. Alterations in rice chloroplast integrity, photosynthesis and metabolome associated with pathogenesis of Rhizoctonia solani. Sci. Rep. 2017, 7, 41610. [Google Scholar] [CrossRef]
- Suharti, W.S.; Nose, A.; Zheng, S.H. Metabolite profiling of sheath blight disease resistance in rice: In the case of positive ion mode analysis by CE/TOF-MS. Plant Prod. Sci. 2016, 19, 279–290. [Google Scholar] [CrossRef][Green Version]
- Prochnik, S.; Marri, P.R.; Desany, B.; Rabinowicz, P.D.; Kodira, C.; Mohiuddin, M.; Rodriguez, F.; Fauquet, C.; Tohme, J.; Harkins, T. The cassava genome: Current progress, future directions. Trop. Plant Biol. 2012, 5, 88–94. [Google Scholar] [CrossRef]
- Bredeson, J.V.; Lyons, J.B.; Prochnik, S.E.; Wu, G.A.; Ha, C.M.; Edsinger-Gonzales, E.; Grimwood, J.; Schmutz, J.; Rabbi, I.Y.; Egesi, C. Sequencing wild and cultivated cassava and related species reveals extensive interspecific hybridization and genetic diversity. Nat. Biotechnol. 2016, 34, 562–570. [Google Scholar] [CrossRef]
- Obata, T.; Klemens, P.A.; Rosado-Souza, L.; Schlereth, A.; Gisel, A.; Stavolone, L.; Zierer, W.; Morales, N.; Mueller, L.A.; Zeeman, S.C.; et al. Metabolic profiles of six African cultivars of cassava (Manihot esculenta Crantz) highlight bottlenecks of root yield. Plant J. 2020, 102, 1202–1219. [Google Scholar] [CrossRef] [PubMed]
- Omondi, J.O.; Lazarovitch, N.; Rachmilevitch, S.; Kukew, T.; Yermiyahu, U.; Yasuor, H. Potassium and storage root development: Focusing on photosynthesis, metabolites and soluble carbohydrates in cassava. Physiol. Plant. 2020, 169, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Olayide, P.; Large, A.; Stridh, L.; Rabbi, I.; Baldermann, S.; Stavolone, L.; Alexandersson, E. Gene Expression and Metabolite Profiling of Thirteen Nigerian Cassava Landraces to Elucidate Starch and Carotenoid Composition. Agronomy 2020, 10, 424. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauwane, M.; Ntushelo, K. Understanding Biotic Stress and Hormone Signalling in Cassava (Manihot esculenta): Potential for Using Hyphenated Analytical Techniques. Appl. Sci. 2020, 10, 8152. https://doi.org/10.3390/app10228152
Rauwane M, Ntushelo K. Understanding Biotic Stress and Hormone Signalling in Cassava (Manihot esculenta): Potential for Using Hyphenated Analytical Techniques. Applied Sciences. 2020; 10(22):8152. https://doi.org/10.3390/app10228152
Chicago/Turabian StyleRauwane, Molemi, and Khayalethu Ntushelo. 2020. "Understanding Biotic Stress and Hormone Signalling in Cassava (Manihot esculenta): Potential for Using Hyphenated Analytical Techniques" Applied Sciences 10, no. 22: 8152. https://doi.org/10.3390/app10228152
APA StyleRauwane, M., & Ntushelo, K. (2020). Understanding Biotic Stress and Hormone Signalling in Cassava (Manihot esculenta): Potential for Using Hyphenated Analytical Techniques. Applied Sciences, 10(22), 8152. https://doi.org/10.3390/app10228152





