Study on Preparation and Properties of Intrinsic Super-Hydrophobic Foamed Magnesium Oxychloride Cement Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
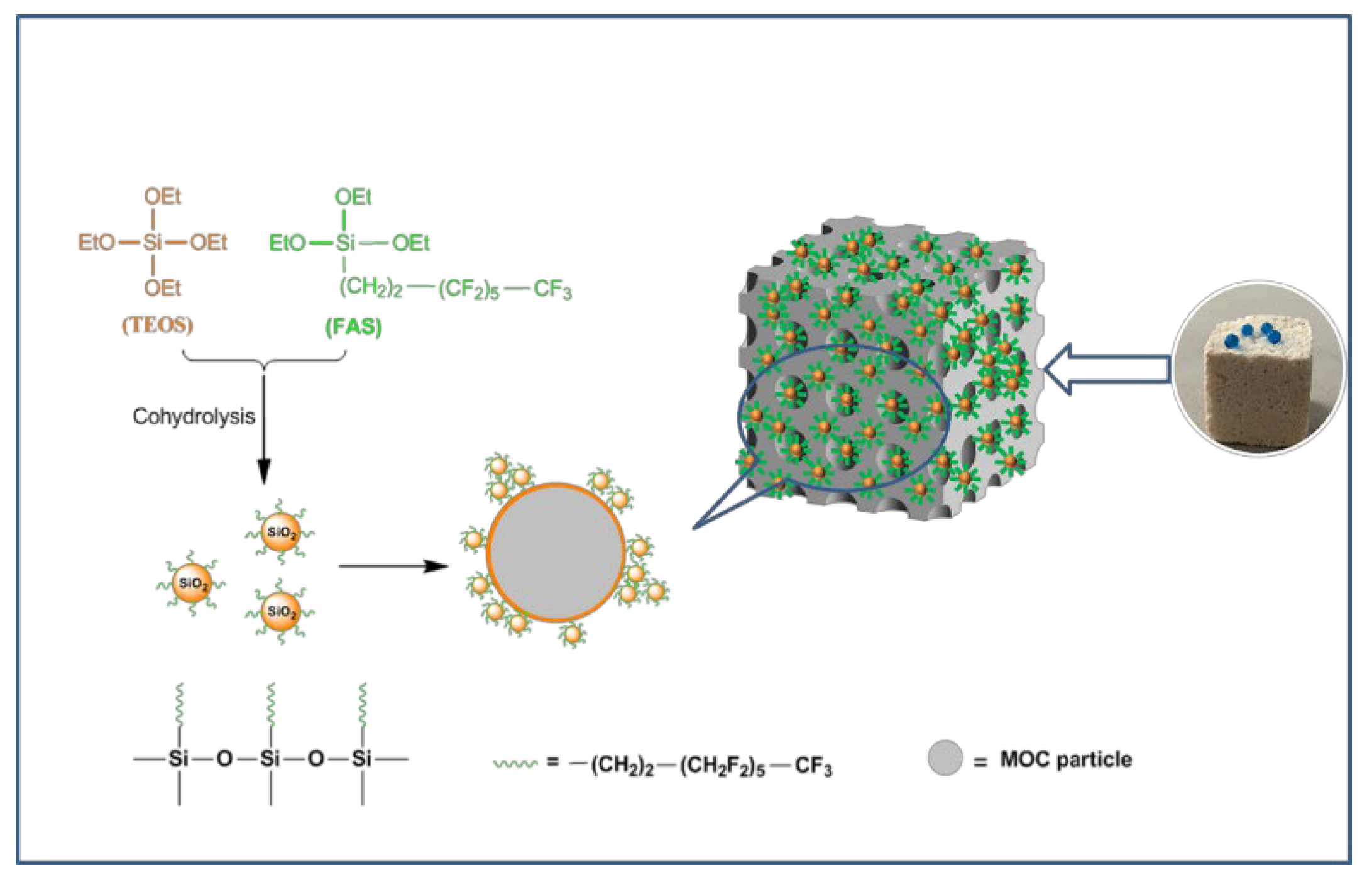
2.2. Preparation of Foamed Hydrophobic Magnesium Oxychloride Cement Test Block
2.3. Characterization and Test Methods of Samples
2.3.1. Characterization of Pore Structure
2.3.2. Bulk Density Test
2.3.3. Compressive Strength Test
2.3.4. X-Ray Diffractometer (XRD) Test
2.3.5. X-Ray Photoelectron Spectroscopy (XPS) Test
2.3.6. Fourier Infrared Spectrometer (FTIR) Test
2.3.7. Water Contact Angle, Mechanical Abrading, and Chemical Corrosion Test
2.3.8. Moisture Resistance and Dehydration Performance Test
3. Results and Discussion
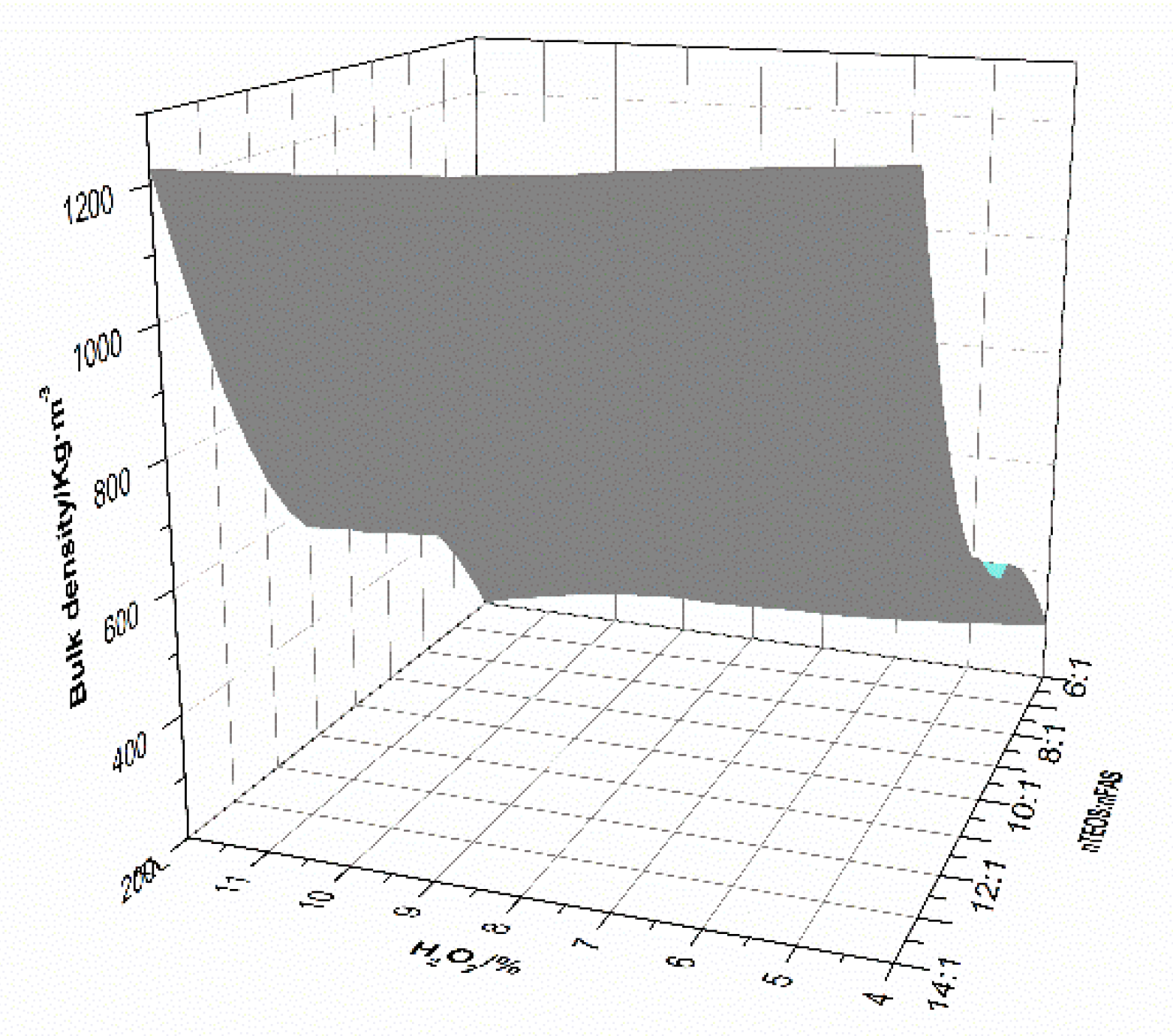
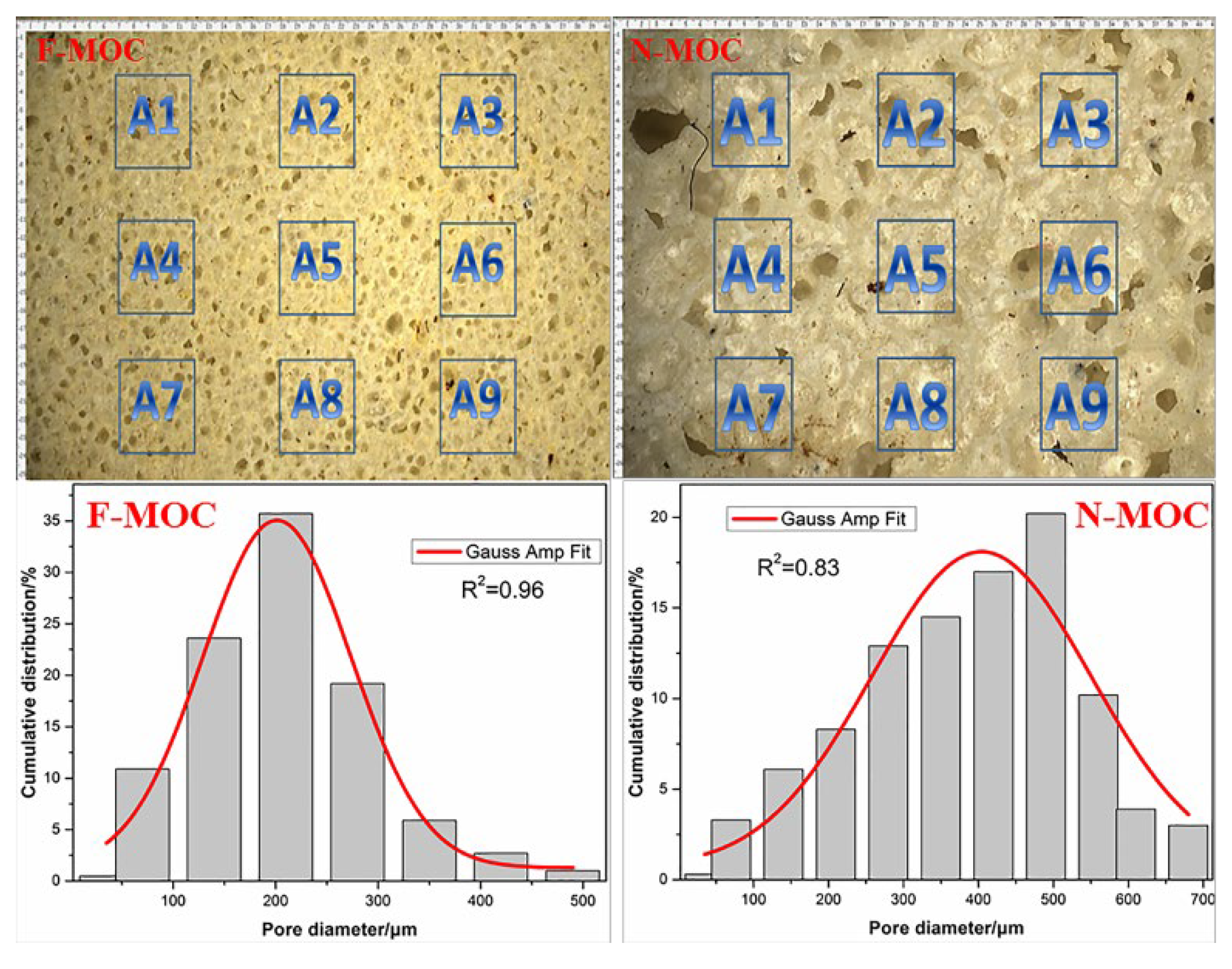
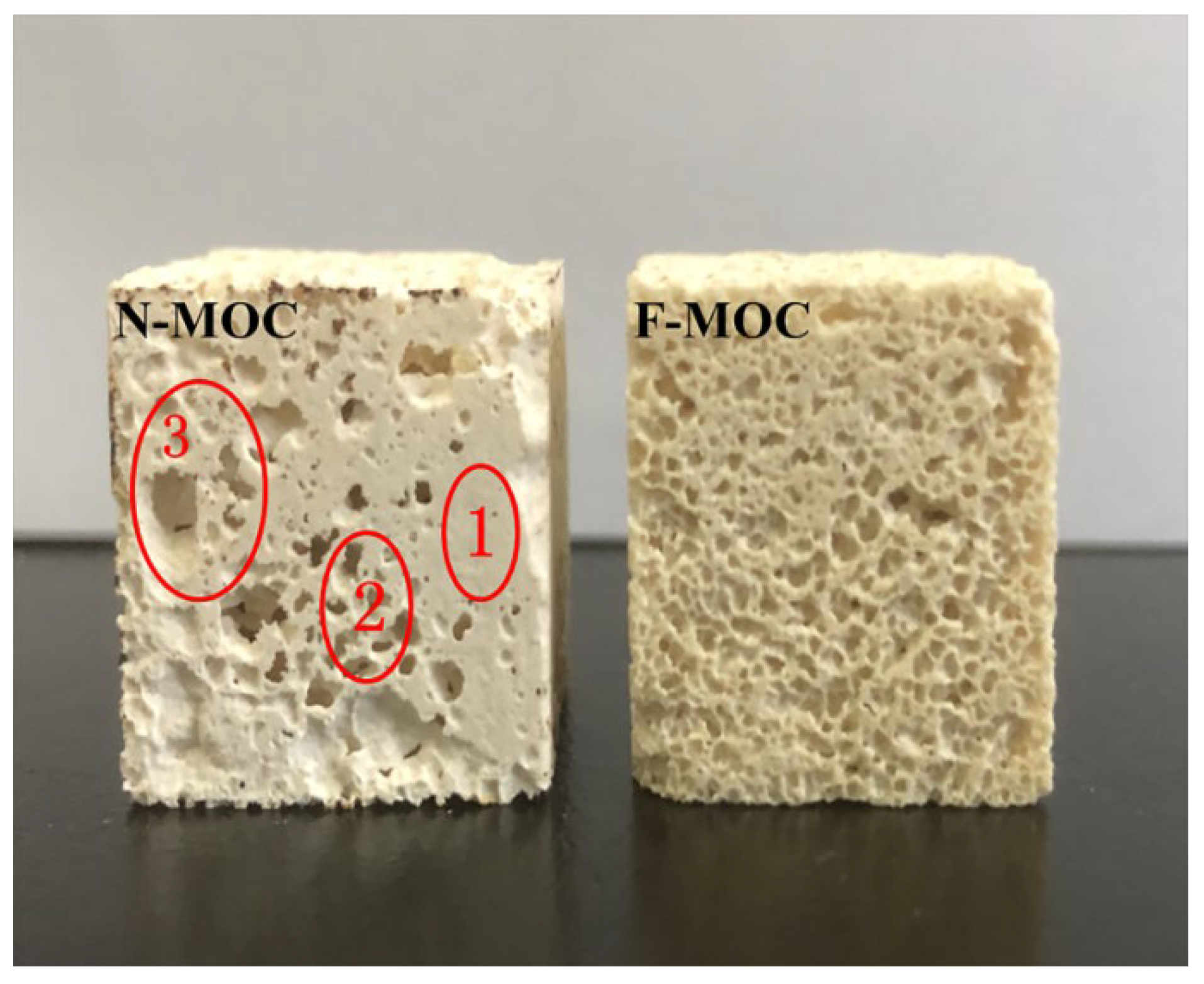
3.1. The Bulk Density and Pore Structure of Foam Material
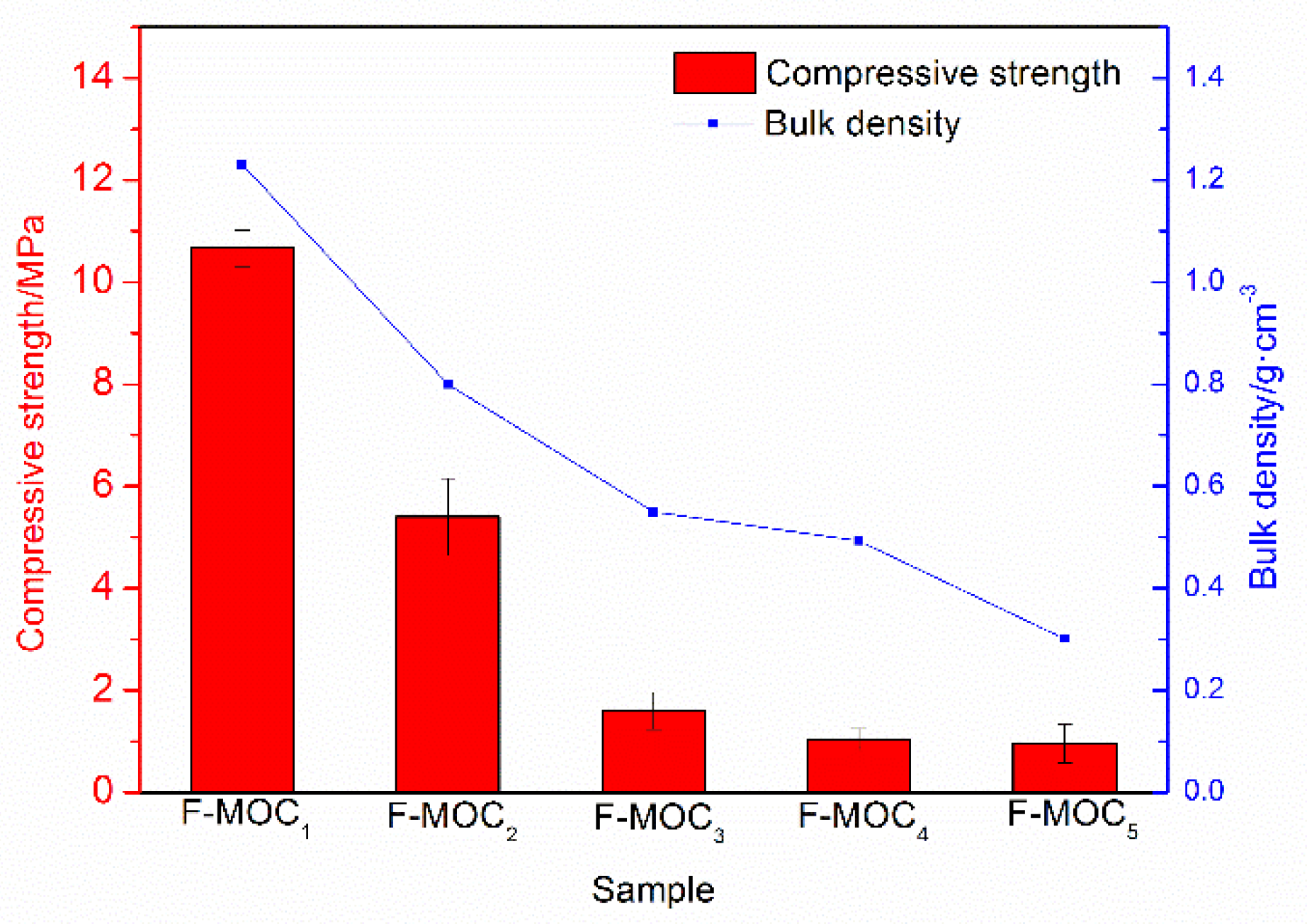
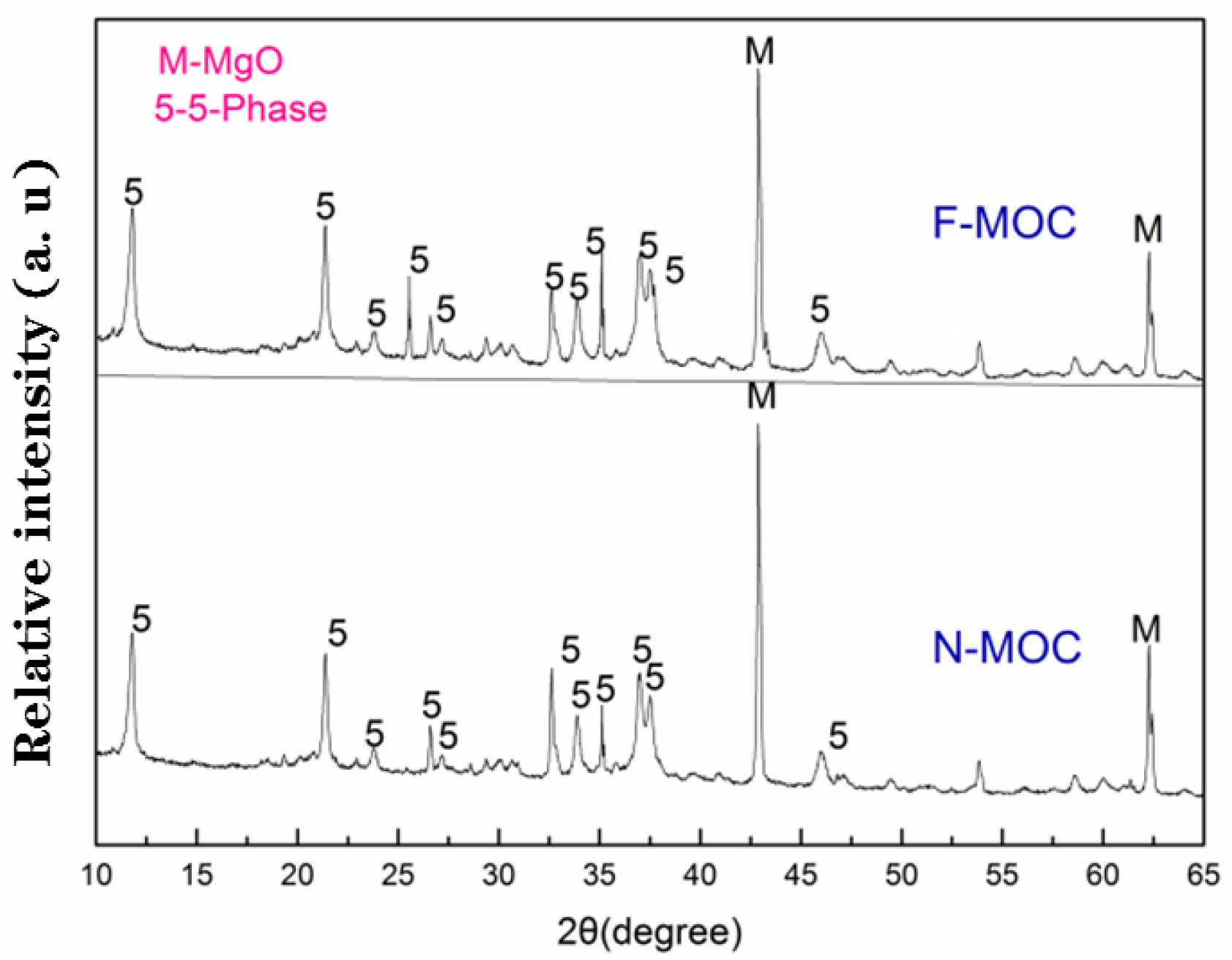
3.2. Mechanical Properties and Phase Composition of Super-Hydrophobic Foamed MOC Materials
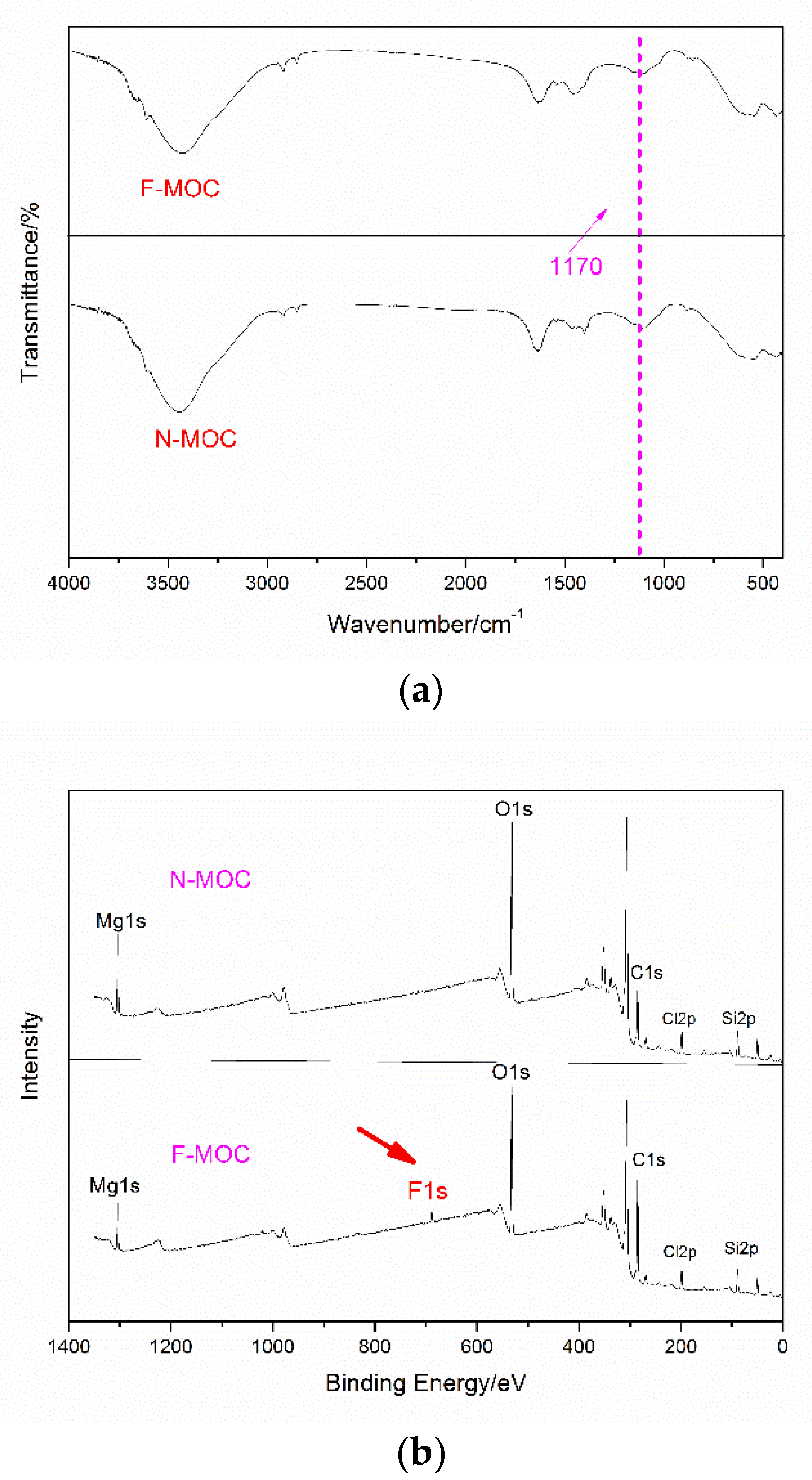
3.3. The Presence of Organosilane in MOC Foam Materials
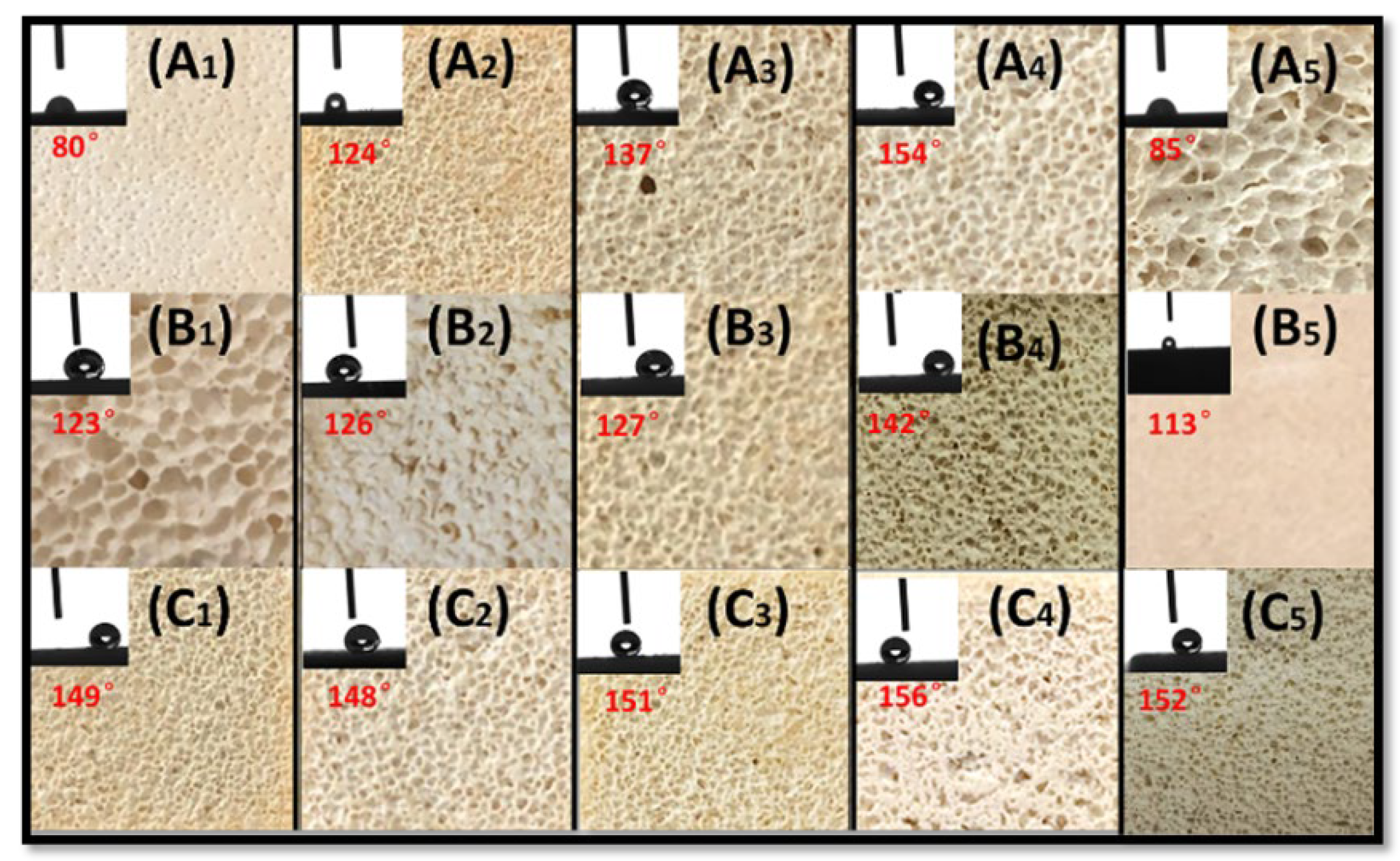
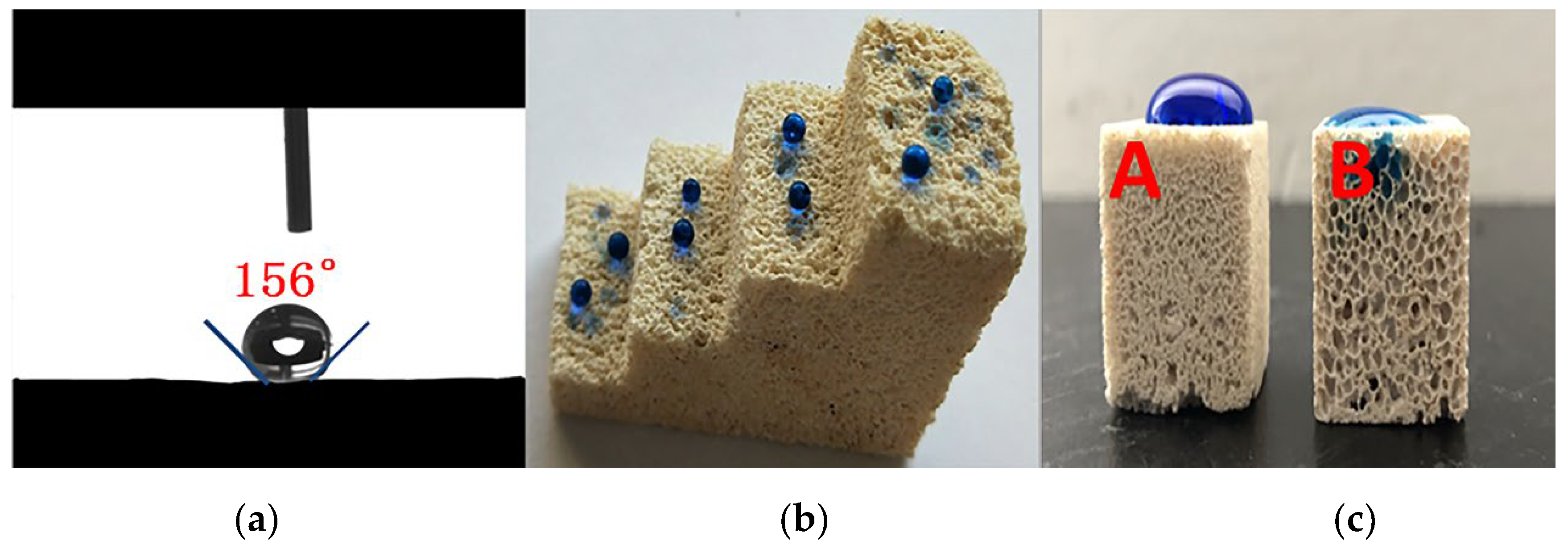
3.4. Hydrophobicity of Foamed MOC Materials
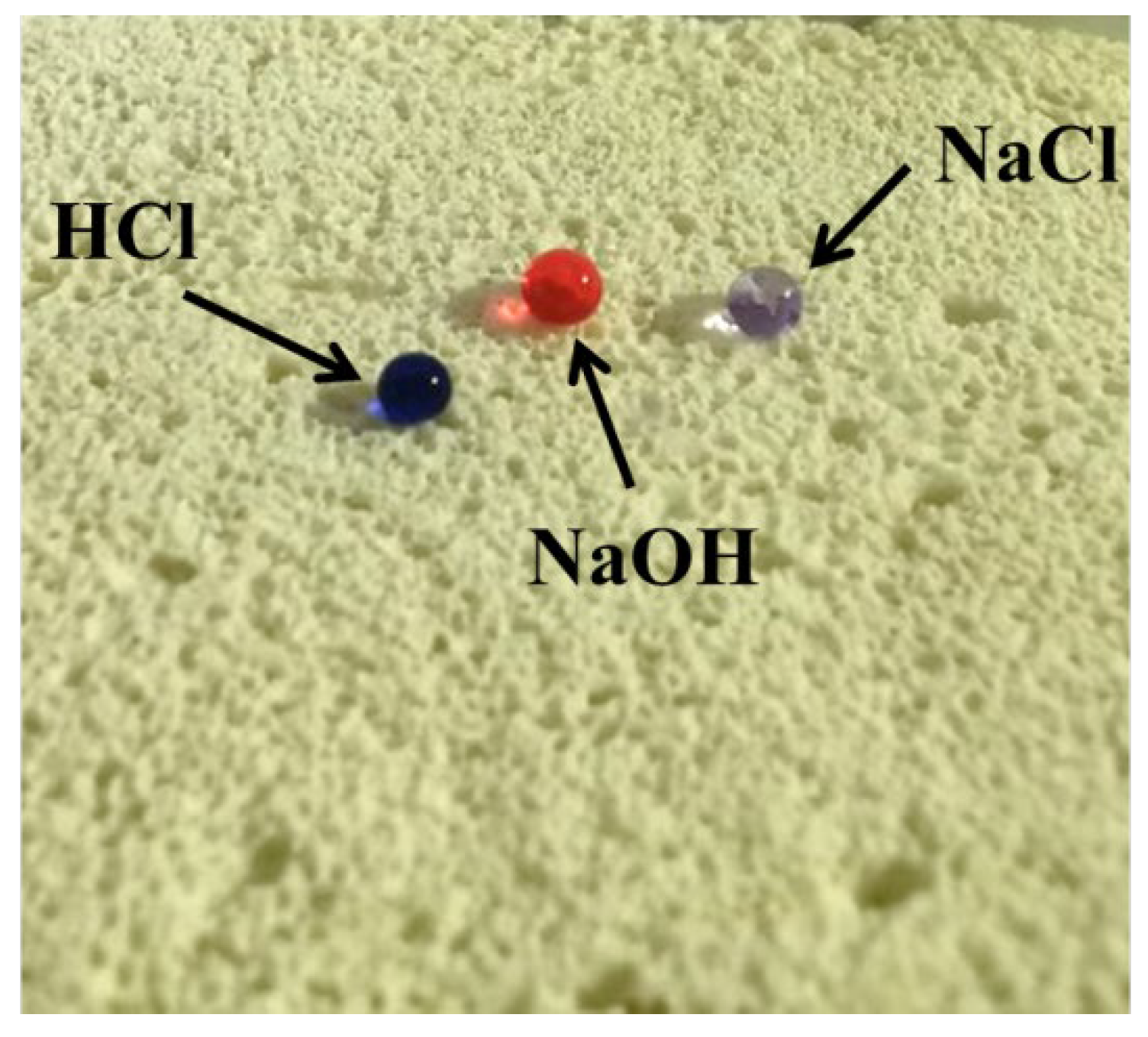
3.5. Engineering Durability of Foamed MOC Materials
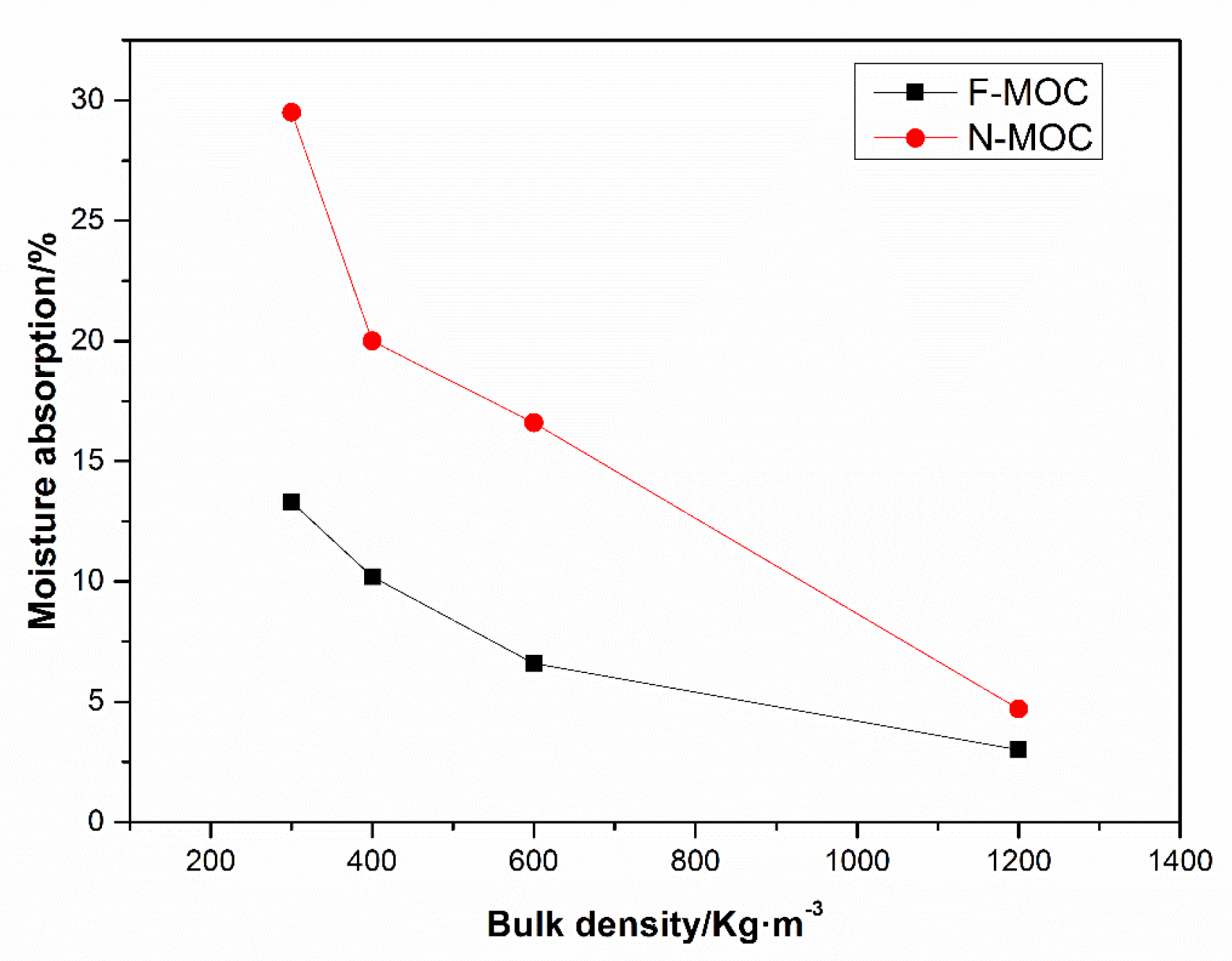
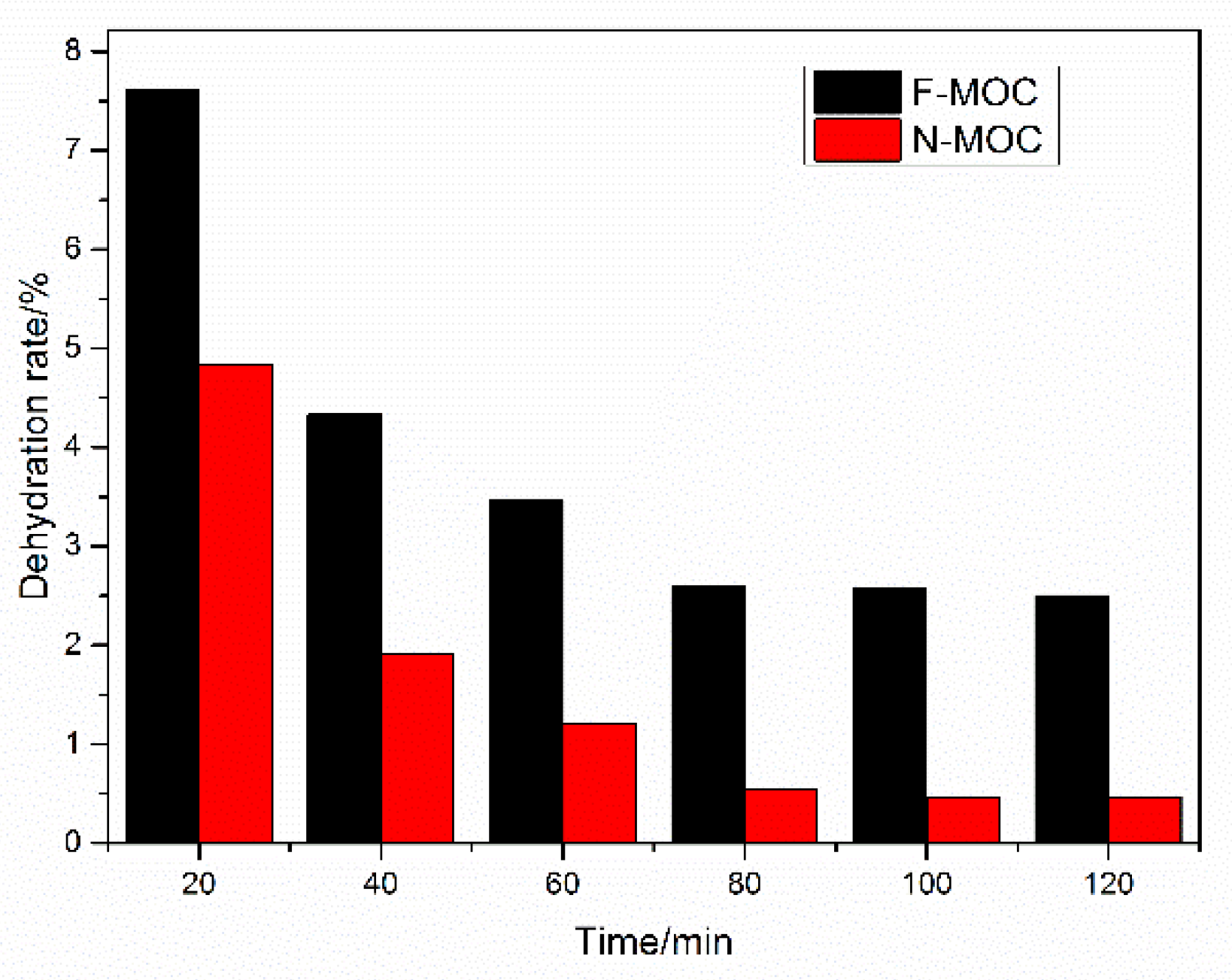
3.6. The Moisture Absorption Rate and Dehumidification Rate of Foamed MOC Material
4. Conclusions
- Firstly, we pre-hydrolyzed TEOS and FAS in the MgCl2 solution. When the MgO and the MgCl2 solutions were mixed, the pH of the system rose to weakly alkaline, which further catalyzed the hydrolysis condensation of TEOS and FAS, and the XPS results demonstrate that the fluoroalkyl chain was successfully introduced into F-MOC. The presence of TEOS made the F-MOC normal pore size distribution curve obtained by adding the same H2O2 foaming agent content thinner than the N-MOC, and the pore structure of the material was more uniform.
- The overall hydrophobic F-MOC with a bulk density of 300 kg·m−3–1200 kg·m−3 and a water contact angle of 156° was prepared by doping with an organosilane. The XRD result and the calculation of the relative content of five-phase show that the five-phase of F-MOC was the main strength phase, as its relative content was only 3.5% lower than that of N-MOC. Based on compressive strength retention, it prevented moisture intrusion and increased its resistance to water. It fundamentally solved the poor water-resistance of the foamed MOC.
- With decreasing bulk density, although the moisture absorption rate of F-MOC increased, it was less than that of N-MOC. When the bulk density was 300 Kg·m−3, the moisture absorption rate of F-MOC was 13.3%, while in that of N-MOC, the moisture absorption rate of MOC was as high as 29.5%. In the high-temperature dehydration experiment, the F-MOC mass could be restored to 98.1% of the original mass, while the N-MOC mass could only be restored to 83.2%, indicating that the moisture invading the sample was more evaporated in the form of free water and did not participate in the MOC hydration reaction, effectively preventing the strength loss caused by the collapse of the pore structure caused by hydration.
- The influence of mechanical polishing and chemical corrosion on the hydrophobic properties of F-MOC show that the composite material has good engineering durability and broad application prospects.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guan, X.; Zhou, G.; Cui, Y.; Fei, J.; Fan, Y. Effect of different-sizes of hydroxyapatite on the water resistance of magnesium oxychloride cement for bone repair. RSC Adv. 2019, 9, 38619–38628. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Hu, C.; Li, Z. Influence of cenospheres on properties of magnesium oxychloride cement-based composites. Mater. Struct. 2015, 49, 1319–1326. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- Li, P.; Wu, H.; Liu, Y.; Yang, J.; Fang, Z.; Lin, B. Preparation and optimization of ultra-light and thermal insulative aerogel foam concrete. Constr. Build. Mater. 2019, 205, 529–542. [Google Scholar] [CrossRef]
- Azimi, A.H. Experimental investigations on the physical and rheological characteristics of sand–foam mixtures. J. Non-Newton. Fluid Mech. 2015, 221, 28–39. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Bui, H.H.; Ngo, T.D.; Nguyen, G.D. Experimental and numerical investigation of influence of air-voids on the compressive behaviour of foamed concrete. Mater. Des. 2017, 130, 103–119. [Google Scholar] [CrossRef]
- He, X.; Zhou, J.; Wang, Z.; Zhang, L. Study on mechanics and water transport characteristics of sea-sand concrete based on the volume analysis of each solid composition. Constr. Build. Mater. 2020, 257, 119591. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, H.; Li, Z.; Li, H. Simulation of the properties of MgO-MgfCl2-H2O system by thermodynamic method. Cem. Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Pei, H.; Yu, H. The influence of FeSO4 and KH2PO4 on the performance of magnesium oxychloride cement. Constr. Build. Mater. 2016, 102, 233–238. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, C. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Eiras, J.; Segovia, F.; Borrachero, M.; Monzó, J.; Bonilla, M.; Payá, J. Physical and mechanical properties of foamed Portland cement composite containing crumb rubber from worn tires. Mater. Des. 2014, 59, 550–557. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Pivák, A.; Sedmidubský, D. Towards novel building materials: High-strength nanocomposites based on graphene, graphite oxide and magnesium oxychloride. Appl. Mater. Today 2020, 20, 100766. [Google Scholar] [CrossRef]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Jankovský, O. Synthesis, Structure, and Thermal Stability of Magnesium Oxychloride 5Mg(OH)2∙MgCl2∙8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef]
- Pavlíková, M.; Pivák, A.; Záleská, M.; Jankovský, O.; Reiterman, P.; Pavlík, Z. Magnesium Oxychloride Cement Composites Lightened with Granulated Scrap Tires and Expanded Glass. Appl. Sci. 2020, 13, 4828. [Google Scholar] [CrossRef]
- Hsu, K.-L. Development of waste expanded polystyrene flexible coating material in concrete waterproofing. IOP Conf. Ser. Earth Environ. Sci. 2019, 351, 012021. [Google Scholar] [CrossRef]
- Geng, Y.; Li, S.; Hou, D.; Zhang, W.; Jin, Z.; Li, Q.; Luo, J. Fabrication of superhydrophobicity on foamed concrete surface by GO/silane coating. Mater. Lett. 2020, 265, 127423. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Lim, T.-K.; Jeong, S.-M.; Yang, K.-H. Thermal transfer and moisture resistances of nano-aerogel-embedded foam concrete. Constr. Build. Mater. 2020, 236, 117575. [Google Scholar] [CrossRef]
- Song, J.; Zhao, D.; Han, Z.; Xu, W.; Lu, Y.; Liu, X.; Liu, B.; Carmalt, C.J.; Deng, X.; Parkin, I.P. Super-robust superhydrophobic concrete. J. Mater. Chem. A 2017, 5, 14542–14550. [Google Scholar] [CrossRef]
- Zhang, X.; Ge, S.; Wang, H.; Chen, R. Effect of 5-phase seed crystal on the mechanical properties and microstructure of magnesium oxychloride cement. Constr. Build. Mater. 2017, 150, 409–417. [Google Scholar] [CrossRef]
- Kus, H.; Carlsson, T. Microstructural investigations of naturally and artificially weathered autoclaved aerated concrete. Cem. Concr. Res. 2003, 33, 1423–1432. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, G.; Van Breugel, K. Characterization of pore structure in cement-based materials using pressurization–depressurization cycling mercury intrusion porosimetry (PDC-MIP). Cem. Concr. Res. 2010, 40, 1120–1128. [Google Scholar] [CrossRef]
- Sun, Z.; Scherer, G.W. Pore size and shape in mortar by thermoporometry. Cem. Concr. Res. 2010, 40, 740–751. [Google Scholar] [CrossRef]
- Amran, Y.M.; Farzadnia, N.; Ali, A.A.A. Properties and applications of foamed concrete; a review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Kaddami, A.; Pitois, O. A physical approach towards controlling the microstructure of metakaolin-based geopolymer foams. Cem. Concr. Res. 2019, 124. [Google Scholar] [CrossRef]
- She, W.; Zheng, Z.; Zhang, Q.; Zuo, W.; Yang, J.; Zhang, Y.; Zheng, L.; Hong, J.; Miao, C. Predesigning matrix-directed super-hydrophobization and hierarchical strengthening of cement foam. Cem. Concr. Res. 2020, 131, 106029. [Google Scholar] [CrossRef]
- She, W.; Zhang, Y.; Miao, C.; Hong, J.; Mu, S. Water transport in foam concrete: Visualisation and numerical modelling. Mag. Concr. Res. 2020, 72, 734–746. [Google Scholar] [CrossRef]
- Mousavi, M.; Hassanajili, S.; Rahimpour, M. Synthesis of fluorinated nano-silica and its application in wettability alteration near-wellbore region in gas condensate reservoirs. Appl. Surf. Sci. 2013, 273, 205–214. [Google Scholar] [CrossRef]
- Wang, H.; Fang, J.; Cheng, T.; Ding, J.; Qu, L.; Dai, L.; Wang, X.; Lin, T. One-step coating of fluoro-containing silicananoparticles for universal generation of surface superhydrophobicity. Chem. Commun. 2008, 7, 877–879. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Wei, L.; Yonggang, W.; Shukun, S.; Shaofei, S.; Shukun, S. Ultraviolet Light Catalyzed Gelation of 3-Methacryloxypropyltrimethoxysilane via Altered Silicate Spatial Structure. J. Phys. Chem. B 2016, 120, 9513–9522. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, W.; Zhang, W.; Li, J.; Chen, H. Tuning the phase structure and mechanical performance of magnesium oxychloride cements by curing temperature and H2O/MgCl2 ratio. Constr. Build. Mater. 2018, 179, 413–419. [Google Scholar] [CrossRef]
- Huang, Q.-Z.; Fang, Y.-Y.; Liu, P.-Y.; Zhu, Y.-Q.; Shi, J.-F.; Xu, G. A novel strategy for durable superhydrophobic coating on glass substrate via using silica chains to fix silica particles. Chem. Phys. Lett. 2018, 692, 33–37. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]


| Components | MgO | SiO2 | CaO | Fe2O3 | Al2O3 | Loss on Ignition |
|---|---|---|---|---|---|---|
| Mass fraction: % | 84.27 | 6.25 | 1.68 | 0.28 | 0.57 | 6.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Ge, S.; Wang, H.; Chen, R. Study on Preparation and Properties of Intrinsic Super-Hydrophobic Foamed Magnesium Oxychloride Cement Material. Appl. Sci. 2020, 10, 8134. https://doi.org/10.3390/app10228134
Huang J, Ge S, Wang H, Chen R. Study on Preparation and Properties of Intrinsic Super-Hydrophobic Foamed Magnesium Oxychloride Cement Material. Applied Sciences. 2020; 10(22):8134. https://doi.org/10.3390/app10228134
Chicago/Turabian StyleHuang, Jiaxin, Shaojin Ge, Hongning Wang, and Ruoyu Chen. 2020. "Study on Preparation and Properties of Intrinsic Super-Hydrophobic Foamed Magnesium Oxychloride Cement Material" Applied Sciences 10, no. 22: 8134. https://doi.org/10.3390/app10228134
APA StyleHuang, J., Ge, S., Wang, H., & Chen, R. (2020). Study on Preparation and Properties of Intrinsic Super-Hydrophobic Foamed Magnesium Oxychloride Cement Material. Applied Sciences, 10(22), 8134. https://doi.org/10.3390/app10228134





