Preclinical, Multi-Aspect Assessment of the Reliability of a Photoplethysmography-Based Telemonitoring System to Track Cardiovascular Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
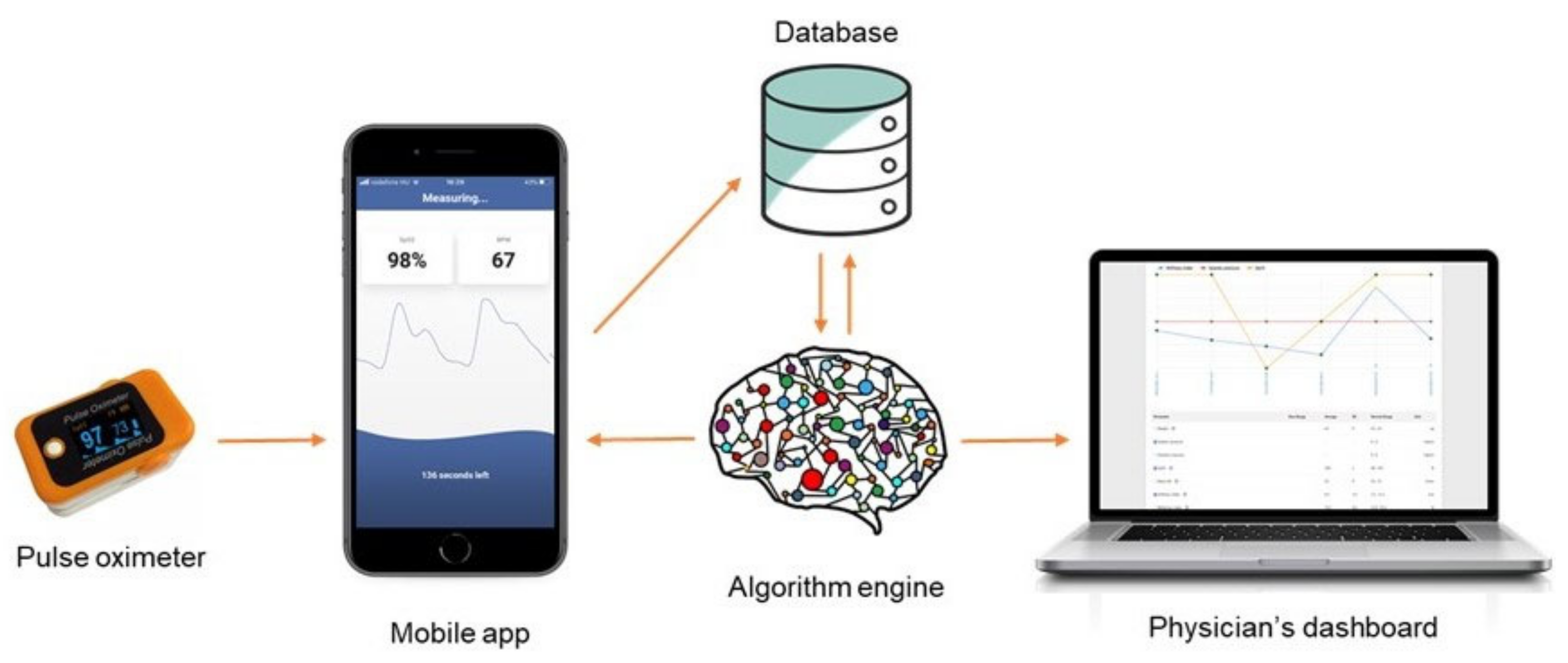
2.2. Measurements with the SCN4ALL System
2.3. Protocols
2.3.1. Measurement Reliability of the Telemedicine System
2.3.2. Reliability of Human Pulse Wave Measurements at Standard Conditions
2.3.3. Parallel Measurement on Four Fingers
2.4. Data Analysis and Statistics
3. Results
3.1. Measurement Reliability of the Telemedicine System
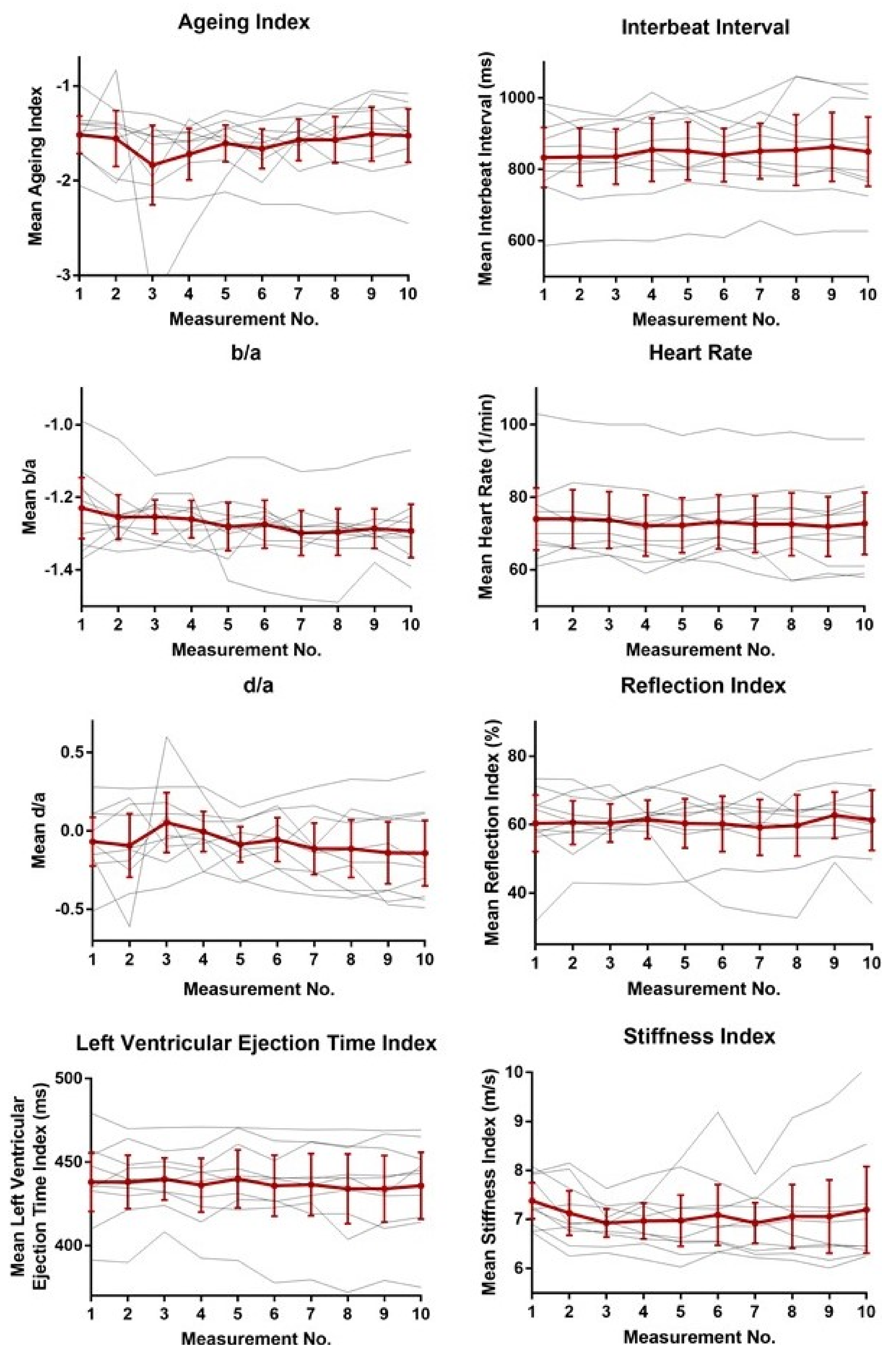
3.2. Reliability of Human Pulse Wave Measurements at Standard Conditions
3.3. Parallel Measurements on Four Fingers
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Ghamari, M. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgendi, M. On the Analysis of Fingertip Photoplethysmogram Signals. Curr. Cardiol. Rev. 2012, 8, 14–25. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Pauca, A.; Jiang, X.-J. Pulse wave analysis. Br. J. Clin. Pharmacol. 2001, 51, 507–522. [Google Scholar] [CrossRef]
- Millasseau, S.; Kelly, R.; Ritter, J.; Chowienczyk, P. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin. Sci. 2002, 103, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Wesselink, R. Blood Pressure Waveform Analysis in Cardiogenic Shock & Acute Myocardial Infarction. Master’s Thesis, University of Twente, Enschede, The Netherlands, 2016. Available online: https://essay.utwente.nl/69815/2/Wesselink_MA_TNW.pdf (accessed on 10 November 2020).
- Pilt, K.; Meigas, K.; Ferenets, R.; Temitski, K.; Viigimaa, M. Photoplethysmographic signal waveform index for detection of increased arterial stiffness. PubMed. Physiol. Meas. 2014, 35, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Smulyan, H.; Safar, M.E. Systolic Blood Pressure Revisited. J. Am. Coll. Cardiol. 1997, 29, 1407–1413. [Google Scholar] [CrossRef]
- Steppan, J.; Barodka, V.; Berkowitz, D.E.; Nyhan, D. Vascular Stiffness and Increased Pulse Pressure in the Aging Cardiovascular System. Cardiol. Res. Pr. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Avolio, A.; Butlin, M.; Walsh, A. Arterial blood pressure measurement and pulse wave analysis—their role in enhancing cardiovascular assessment. PubMed. Physiol. Meas. 2010, 31. [Google Scholar] [CrossRef]
- Inoue, N.; Kawakami, H.; Yamamoto, H.; Ito, C.; Fujiwara, S.; Sasaki, H.; Kihara, Y. Second derivative of the finger photoplethysmogram and cardiovascular mortality in middle-aged and elderly Japanese women. Hypertens. Res. 2016, 40, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Haiden, A.; Eber, B.; Weber, T. U-Shaped Relationship of Left Ventricular Ejection Time Index and All-Cause Mortality. Am. J. Hypertens. 2014, 27, 702–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millasseau, S.C.; Guigui, F.G.; Kelly, R.P.; Prasad, K.; Cockcroft, J.R.; Ritter, J.M.; Chowienczyk, P.J. Noninvasive Assessment of the Digital Volume Pulse. Hypertension 2000, 36, 952–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takazawa, K.; Tanaka, N.; Fujita, M.; Matsuoka, O.; Saiki, T.; Aikawa, M.; Tamura, S.; Ibukiyama, C. Assessment of Vasoactive Agents and Vascular Aging by the Second Derivative of Photoplethysmogram Waveform. Hypertension 1998, 32, 365–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirala, N.; Periyasamy, R.; Singh, B.K.; Kumar, A. Detection of type-2 diabetes using characteristics of toe photoplethysmogram by applying support vector machine. Biocybern. Biomed. Eng. 2019, 39, 38–51. [Google Scholar] [CrossRef]
- Paradkar, N.; Chowdhury, S.R. Coronary artery disease detection using photoplethysmography. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; Volume 2017, pp. 100–103. [Google Scholar] [CrossRef]
- Huotari, M.; Vehkaoja, A.; Määttä, K.; Kostamovaara, J. Photoplethysmography and its detailed pulse waveform analysis for arterial stiffness. J. Struct. Mech. 2011, 44, 345–362. [Google Scholar]
- Weber, T.; Auer, J.; O’Rourke, M.F.; Kvas, E.; Laßnig, E.; Berent, R.; Eber, B. Arterial Stiffness, Wave Reflections, and the Risk of Coronary Artery Disease. Circulation 2004, 109, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, I.; Yli-Hankala, A. Photoplethysmography and nociception. Acta Anaesthesiol. Scand. 2009, 53, 975–985. [Google Scholar] [CrossRef]
- Von Wowern, E.; Östling, G.; Nilsson, P.M.; Olofsson, P. Digital Photoplethysmography for Assessment of Arterial Stiffness: Repeatability and Comparison with Applanation Tonometry. PLoS ONE 2015, 10, e0135659. [Google Scholar] [CrossRef] [Green Version]
- Elgendi, M. Detection of c, d, and e waves in the acceleration photoplethysmogram. Comput. Methods Programs Biomed. 2014, 117, 125–136. [Google Scholar] [CrossRef]
- Jago, J.R.; Murray, A. Repeatability of peripheral pulse measurements on ears, fingers and toes using photoelectric plethysmography. Clin. Phys. Physiol. Meas. 1988, 9, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, L.A.; Blacher, J.; Kondo, T.; Takazawa, K.; Safar, M.E. Assessment of vascular aging and atherosclerosis in hypertensive subjects: second derivative of photoplethysmogram versus pulse wave velocity. Am. J. Hypertens. 2000, 13, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Imanaga, I.; Hara, H.; Koyanagi, S.; Tanaka, K. Correlation between Wave Components of the Second Derivative of Plethysmogram and Arterial Distensibility. Jpn. Hear. J. 1998, 39, 775–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, J.; Chonan, K.; Aoki, Y.; Nishimura, T.; Ohkubo, T.; Hozawa, A.; Suzuki, M.; Matsubara, M.; Michimata, M.; Araki, T.; et al. Pulse wave velocity and the second derivative of the finger photoplethysmogram in treated hypertensive patients. J. Hypertens. 2002, 20, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Kawada, T.; Katsumata, M.; Ibuki, C. Utility of Second Derivative of the Finger Photoplethysmogram for the Estimation of the Risk of Coronary Heart Disease in the General Population. Circ. J. 2006, 70, 304–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, H.J.; Kim, J.S.; Kim, Y.S.; Lee, H.B.; Park, K.S. Second Derivative of Photoplethysmography for Estimating Vascular Aging. In Proceedings of the 2007 6th International Special Topic Conference on Information Technology Applications in Biomedicine, Tokyo, Japan, 8–11 November 2007; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2007; pp. 70–72. [Google Scholar] [CrossRef]
- Pilt, K.; Ferenets, R.; Meigas, K.; Lindberg, L.-G.; Temitski, K.; Viigimaa, M. New Photoplethysmographic Signal Analysis Algorithm for Arterial Stiffness Estimation. Sci. World J. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Kips, J.; Trachet, B.; Swillens, A.; Vermeersch, S.; Mahieu, D.; Rietzschel, E.; De Buyzere, M.; Van Bortel, L. Limitations and pitfalls of non-invasive measurement of arterial pressure wave reflections and pulse wave velocity. Artery Res. 2009, 3, 79–88. [Google Scholar] [CrossRef]
- Takazawa, K. Second derivative of photoplethysmogram—Comment on Tanaka et al., page 43-48. Vasa 2015, 44, 3–4. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Sokal, R.R., Rohlf, F.J., Eds.; W.H. Freeman: New York, NY, USA, 2012; Volume 937, ISBN 9780716786047. [Google Scholar]
- Lee, J.; Yang, S.; Lee, S.; Kim, H.C. Analysis of Pulse Arrival Time as an Indicator of Blood Pressure in a Large Surgical Biosignal Database: Recommendations for Developing Ubiquitous Blood Pressure Monitoring Methods. J. Clin. Med. 2019, 8, 1773. [Google Scholar] [CrossRef] [Green Version]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. PubMed. Physiol. Meas 2007, 28, R1–R39. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, T.; Kawada, T.; Katsumata, M.; Ibuki, C.; Kusama, Y. Independent Determinants of Second Derivative of the Finger Photoplethysmogram among Various Cardiovascular Risk Factors in Middle-Aged Men. Hypertens. Res. 2007, 30, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inuma, J.; Murakoshi, M.; Kobayashi, T.; Io, H.; Kaneko, K.; Takahashi, T.; Hamada, C.; Horikoshi, S.; Tomino, Y. Relationship between acceleration plethysmography and aortic calcification index in chronic kidney disease patients. Hong Kong J. Nephrol. 2012, 14, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Padilla, J.M.; Berjano, E.; Sáiz, J.; Rodriguez, R.; Fácila, L. Pulse Wave Velocity and Digital Volume Pulse as Indirect Estimators of Blood Pressure: Pilot Study on Healthy Volunteers. Cardiovasc. Eng. 2009, 9, 104–112. [Google Scholar] [CrossRef]
- Padilla, J.M.; Berjano, E.J.; Saiz, J.; Facila, L.; Diaz, P.; Merce, S. Assessment of relationships between blood pressure, pulse wave velocity and digital volume pulse. In Proceedings of the 2006 Computers in Cardiology, Valencia, Spain, 17–20 September 2006; IEEE Conference Publication: New York, NY, USA, 2006; pp. 893–896. [Google Scholar]
- Alty, S.R.; Angarita-Jaimes, N.; Millasseau, S.C.; Chowienczyk, P.J. Predicting Arterial Stiffness from the Digital Volume Pulse Waveform. IEEE Trans. Biomed. Eng. 2007, 54, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Aiba, Y.; Ohshiba, S.; Horiguchi, S.; Morioka, I.; Miyashita, K.; Kiyota, I.; Endo, G.; Takada, H.; Iwata, H. Peripheral Hemodynamics Evaluated by Acceleration Plethysmography in Workers Exposed to Lead. Ind. Health 1999, 37, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Kohjitani, A.; Miyata, M.; Iwase, Y.; Sugiyama, K. Responses of the second derivative of the finger photoplethysmogram indices and hemodynamic parameters to anesthesia induction. Hypertens. Res. 2011, 35, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Millasseau, S.C.; Ritter, J.M.; Takazawa, K.; Chowienczyk, P.J. Contour analysis of the photoplethysmographic pulse measured at the finger. J. Hypertens. 2006, 24, 1449–1456. [Google Scholar] [CrossRef]
- Elgendi, M.; Norton, I.; Brearley, M.; Abbott, D.; Schuurmans, D. Detection of a and b waves in the acceleration photoplethysmogram. Biomed. Eng. Online 2014, 13, 139. [Google Scholar] [CrossRef] [Green Version]

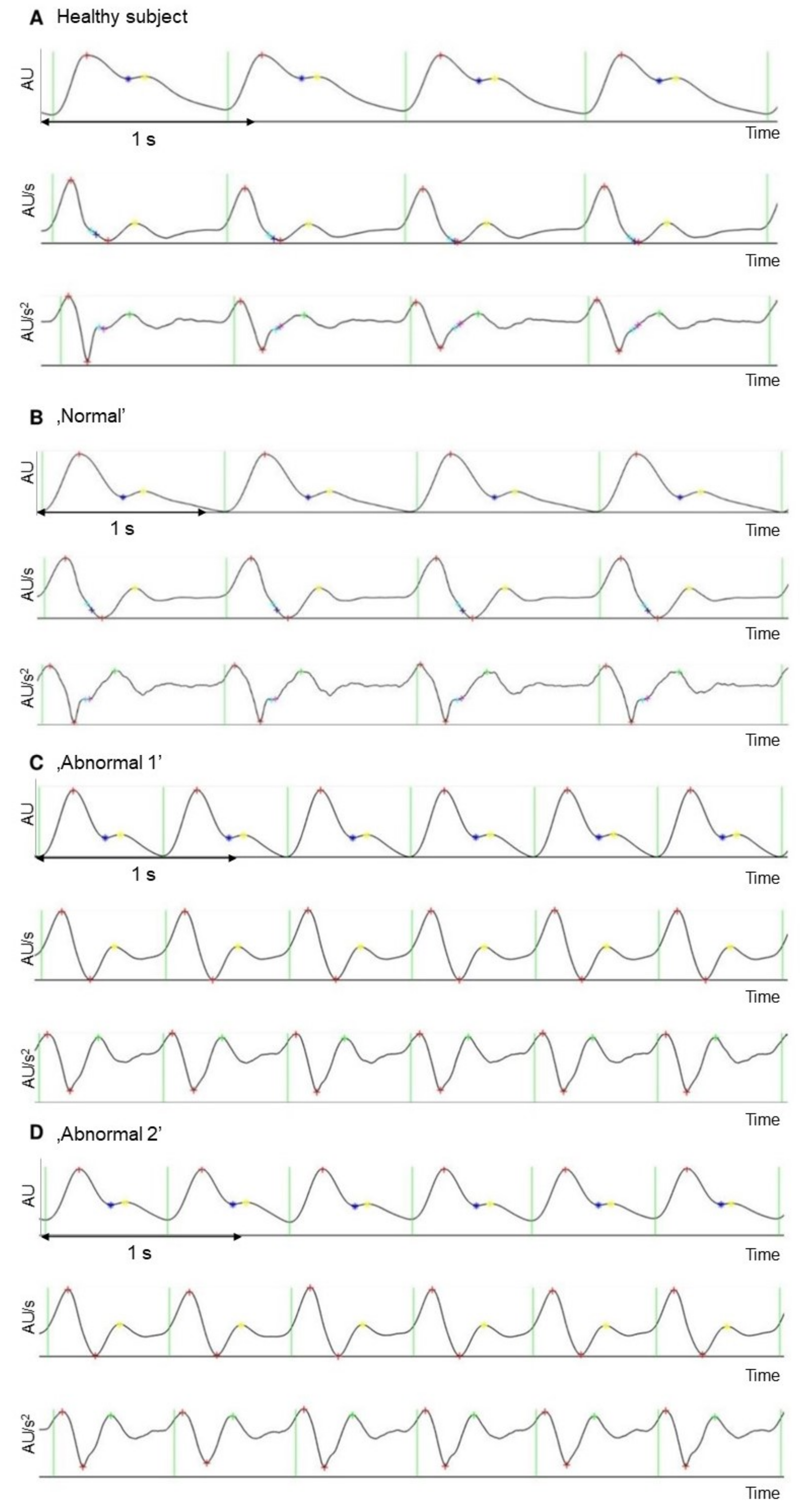
| Normal | Abnormal 1 | Abnormal 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | n = 25 | n = 5 | n = 25 | n = 5 | n = 25 | |||||||
| Variables | Mean [CI] | CV (%) | Mean [CI] | CV (%) | Mean [CI] | CV (%) | Mean [CI] | CV (%) | Mean [CI] | CV (%) | Mean [CI] | CV (%) |
| Aging index | −1.13 [−1.14; −1.12] | 0.41 | −1.14 [−1.14; −1.13] | 0.57 | −3.37 [−4.45; −2.29] | 27.1 | −3.12 [−3.46; −2.79] | 26.1 | −3.71 [−4.60; −2.81] | 20.4 | −3.84 [−4; −3.69] | 9.9 |
| b/a | −1.78 [−1.79; −1.78] | 0.26 | −1.79 [−1.79; −1.78] | 0.32 | −1.59 [−1.59; −1.59] | 0.29 | −1.59 [−1.60; −1.59] | 0.32 | −1.60 [−1.60; −1.59] | 0.36 | −1.60 [−1.59; −1.56] | 0.33 |
| c-d point detection ratio (%) | 100 [100; 100] | 0 | 100 [100; 100] | 0 | 0.60 [0.08; 1.28] | 95.9 | 0.44 [0.23; 0.65] | 116 | 2 [0.48; 3.52] | 64.3 | 2.70 [2.25; 3.19] | 42.2 |
| d/a | −0.75 [−0.75; −0.74] | 0.77 | −0.75 [−0.75; −0.75] | 0.37 | −0.48 [−1.01; −0.06] | 95.9 | −0.35 [0.18–0.52] | 116 | −0.64 [−1.09; −0.20] | 58.7 | −0.71 [−0.79; −0.63] | 26.9 |
| Left ventricular ejection time index (ms) | 552 [552; 554] | 0.22 | 553 [552; 553] | 0.27 | 462 [461; 462] | 0.06 | 462 [462; 462] | 0.05 | 462 [462; 463] | 0.06 | 462 [462; 463] | 0.07 |
| Heart rate (1/min) | 55 [55; 55] | 0 | 55 [55; 55] | 0 | 95 [95; 95] | 0 | 95 [95; 95] | 0 | 95 [95; 95] | 0 | 95 [95; 95] | 0 |
| Interbeat interval (ms) | 1089 [1089; 1089] | 0 | 1089 [1088; 1090] | 0.21 | 631 [631; 631] | 0 | 631 [631; 631] | 0.19 | 630 [630; 631] | 0.07 | 631 [631; 632] | 0.18 |
| Reflection index (%) | 35.5 [35.5; 35.6] | 0.13 | 35.5 [35.5; 35.6] | 0.11 | 32.7 [32.7; 32.8] | 0.12 | 32.7 [32.7; 32.8] | 0.13 | 32.8 [32.6; 32.9] | 0.35 | 32.8 [32.7; 32.8] | 0.42 |
| Stiffness index (m/s) | 4.62 [4.62; 4.63] | 0.10 | 4.62 [4.62; 4.63] | 0.26 | 7.34 [7.34; 7.34] | 0 | 7.34 [7.33; 7.34] | 0.18 | 7.34 [7.33; 7.36] | 0.16 | 7.34 [7.33; 7.35] | 0.34 |
| Pulse Contour Variables | CV % [CI] |
|---|---|
| Aging index | 13.6 [4.78; 22.5] |
| b/a | 3.84 [2.13; 5.55] |
| c–d point detection ratio (%) | 33.6 [17.1; 50.1] |
| d/a | 83.9 [9.5; 177] |
| Left ventricular ejection time (ms) | 1.30 [0.75; 1.84] |
| Heart rate (1/min) | 3.19 [1.99; 4.39] |
| Interbeat interval (ms) | 3.23 [2.11; 4.35] |
| Reflection index (%) | 7.43 [2.79; 12.1] |
| Stiffness index (m/s) | 4.34 [2.20; 6.48] |
| Left Index Finger | Left Ring Finger | Right Index Finger | Right Ring Finger | ICC | |
|---|---|---|---|---|---|
| Pulse contour variables | n = 25 | n = 25 | n = 25 | n = 25 | |
| Mean [CI] | Mean [CI] | Mean [CI] | Mean [CI] | ||
| Aging index | −1.29 [−1.46; −1.13] | −1.30 [−1.47; −1.13] | −1.34 [−1.15; −1.12] | −1.47 [−1.17; −1.25] | 0.81 |
| b/a | −1.21 [−1.26; −1.152] | −1.22 [−1.29; −1.16] | −1.25 [−1.312; −1.20] | −1.24 [−1.30; −1.17] | 0.83 |
| c–d point detection ratio (%) | 33.8 [25.3; 42.4] | 31.3 [23.1; 39.5] | 31.9 [22.9; 40.8] | 32.3 [23.9; 40.78] | 0.90 |
| d/a | −0.15 [−0.24; −0.06] | −0.16 [−0.26; −0.07] | −0.17 [−0.29; −0.06] | −0.10 [−0.21; −0.01] | 0.82 |
| Left ventricular ejection time index (ms) | 148 [56; 240] | 148 [57; 240] | 147 [56; 238] | 147 [56; 237] | >0.99 |
| Heart rate (1/min) | 70.6 [67.1; 74.2] | 71.0 [67.5; 74.2] | 70.9 [67.4; 74.4] | 71.0 [67.4; 74.5] | >0.99 |
| Interbeat interval (ms) | 862 [817; 906] | 862 [818; 908] | 862 [816; 907] | 861 [817; 907] | >0.99 |
| Reflection index (%) | 62.2 [59.2; 65.1] | 60.8 [57; 64.6] | 61.5 [58.4; 64.5] | 61.3 [57.6; 65.0] | 0.81 |
| Stiffness index (ms) | 7.74 [7.37; 8.10] | 7.71 [7.32; 8.10] | 7.58 [7.20; 7.97] | 7.59 [7.13; 8.05] | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulin, D.; Antali, F.; Kulin, S.; Wafa, D.; Lucz, K.I.; Veres, D.S.; Miklós, Z. Preclinical, Multi-Aspect Assessment of the Reliability of a Photoplethysmography-Based Telemonitoring System to Track Cardiovascular Status. Appl. Sci. 2020, 10, 7977. https://doi.org/10.3390/app10227977
Kulin D, Antali F, Kulin S, Wafa D, Lucz KI, Veres DS, Miklós Z. Preclinical, Multi-Aspect Assessment of the Reliability of a Photoplethysmography-Based Telemonitoring System to Track Cardiovascular Status. Applied Sciences. 2020; 10(22):7977. https://doi.org/10.3390/app10227977
Chicago/Turabian StyleKulin, Dániel, Flóra Antali, Sándor Kulin, Dina Wafa, Konrád István Lucz, Dániel Sándor Veres, and Zsuzsanna Miklós. 2020. "Preclinical, Multi-Aspect Assessment of the Reliability of a Photoplethysmography-Based Telemonitoring System to Track Cardiovascular Status" Applied Sciences 10, no. 22: 7977. https://doi.org/10.3390/app10227977
APA StyleKulin, D., Antali, F., Kulin, S., Wafa, D., Lucz, K. I., Veres, D. S., & Miklós, Z. (2020). Preclinical, Multi-Aspect Assessment of the Reliability of a Photoplethysmography-Based Telemonitoring System to Track Cardiovascular Status. Applied Sciences, 10(22), 7977. https://doi.org/10.3390/app10227977






