In Vitro Evidences of Different Fibroblast Morpho-Functional Responses to Red, Near-Infrared and Violet-Blue Photobiomodulation: Clues for Addressing Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. PBM Treatments
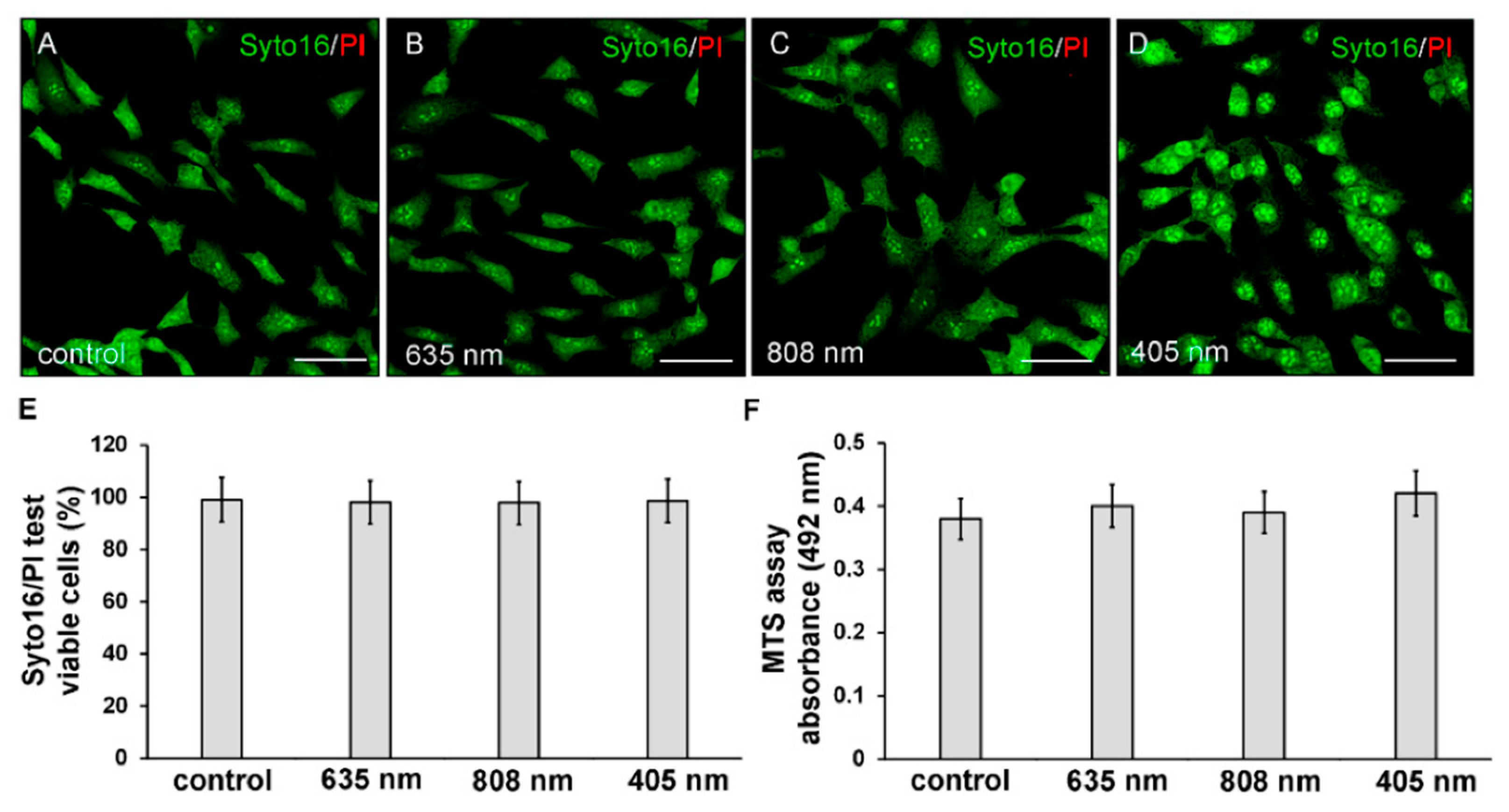
2.3. Cell Viability: Syto16/PI and MTS Assays
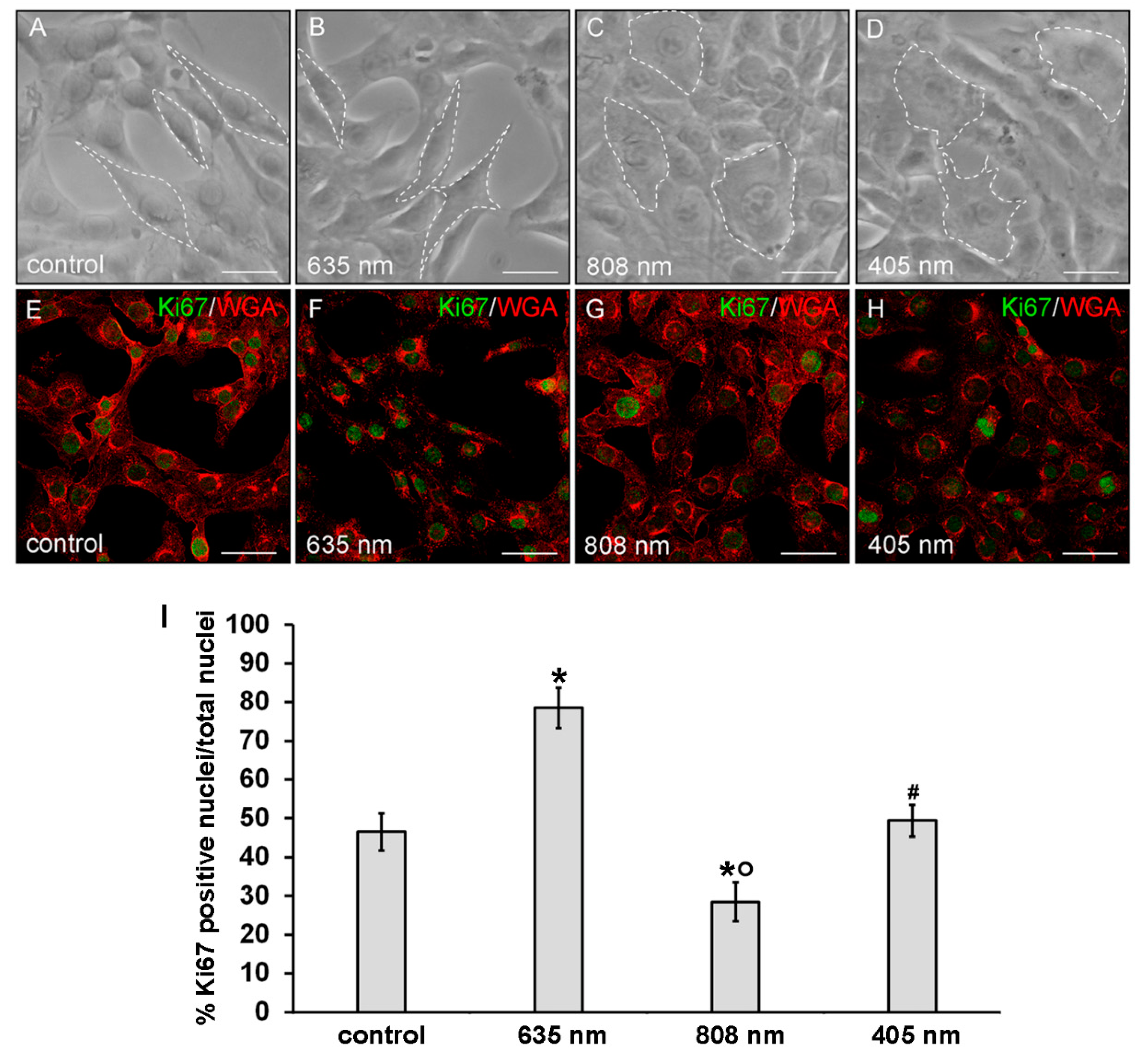
2.4. Phase Contrast Microscopy
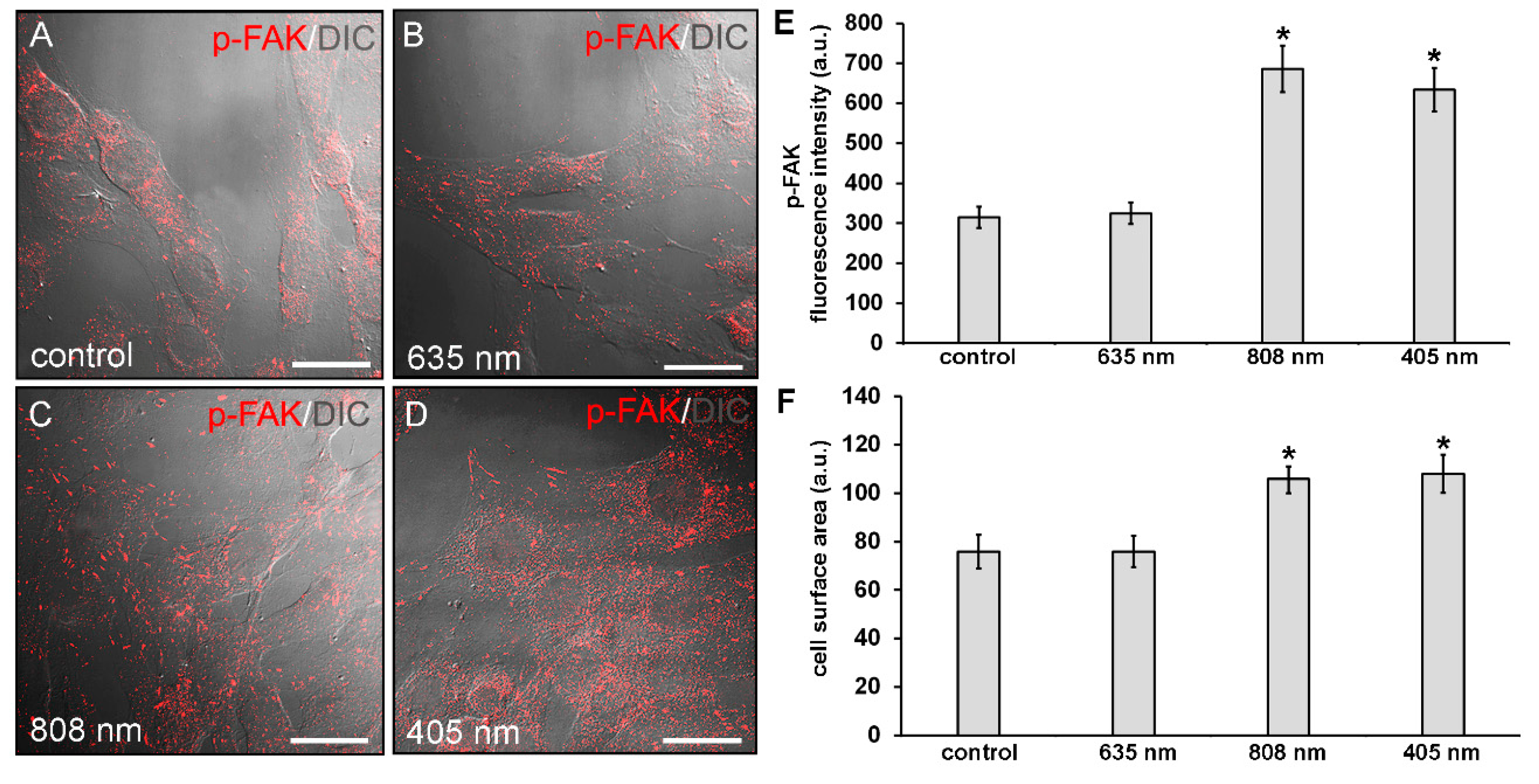
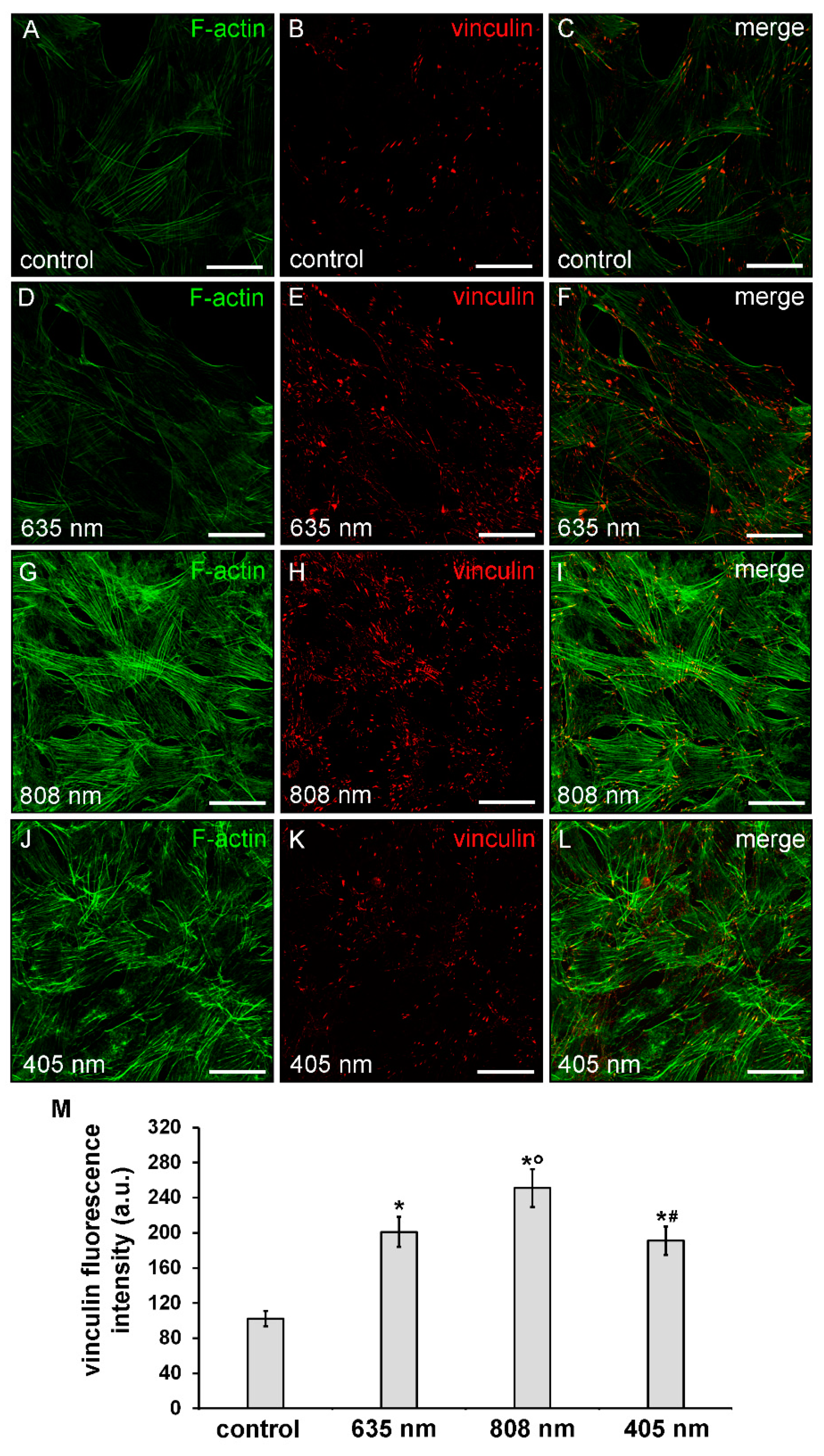
2.5. Confocal Laser Scanning Microscopy
2.6. Western Blotting
2.7. Gelatinase Assay
2.8. Statistical Analysis
3. Results
3.1. Effects of PBM Treatments on Fibroblast Cell Shape, Actin Cytoskeleton Assembly, Focal Adhesion Protein Expression/Aggregation and Proliferation
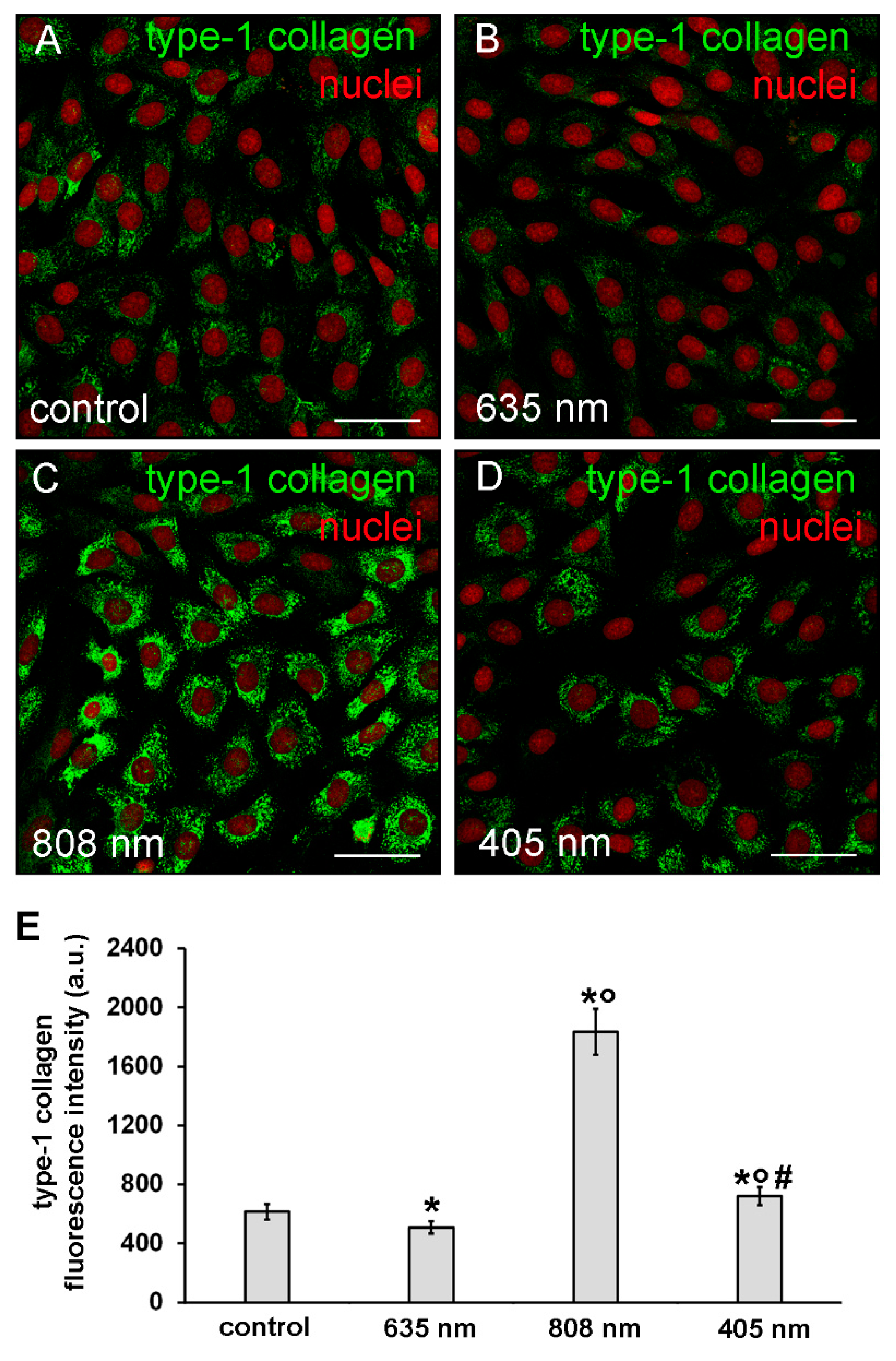
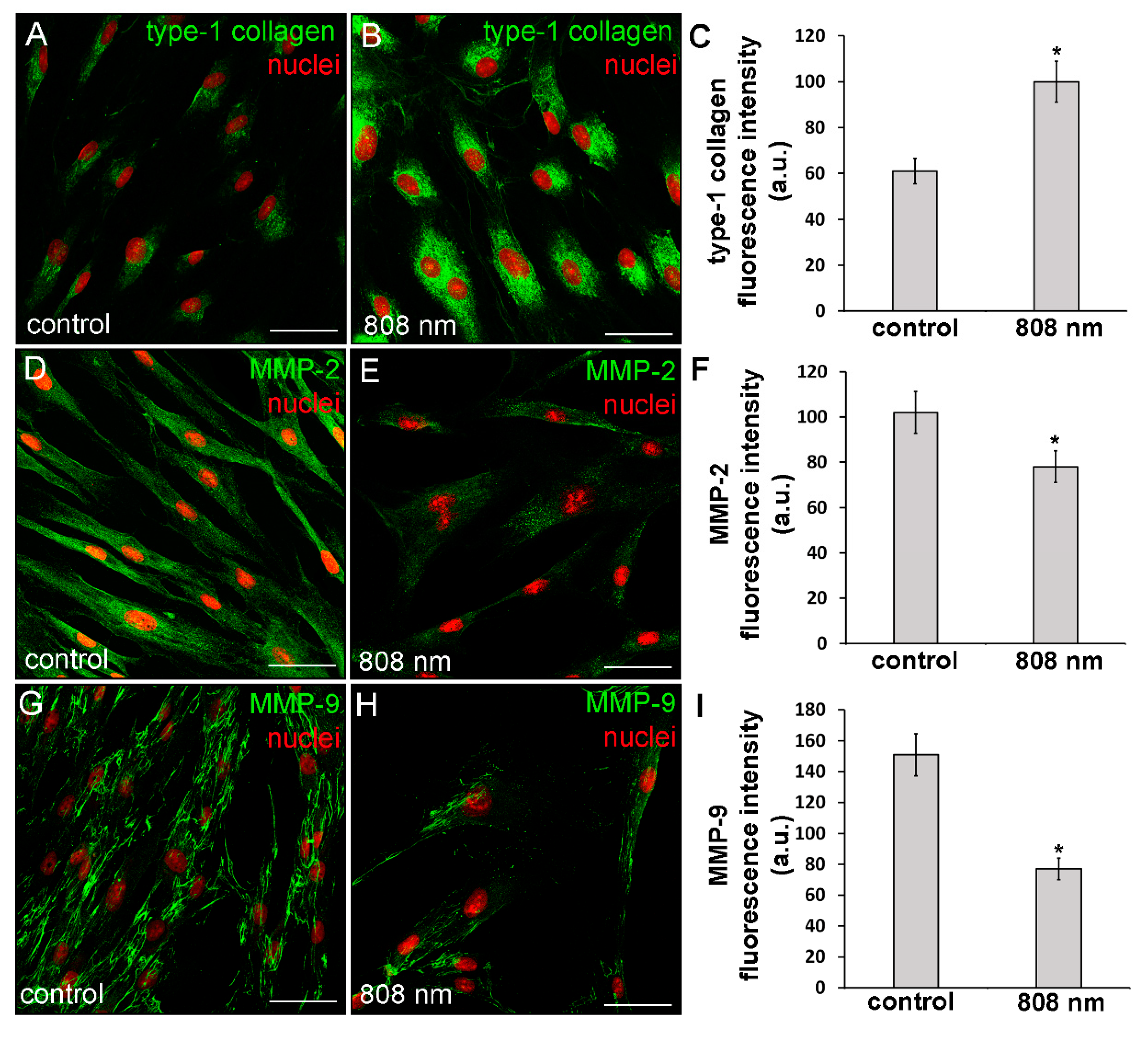
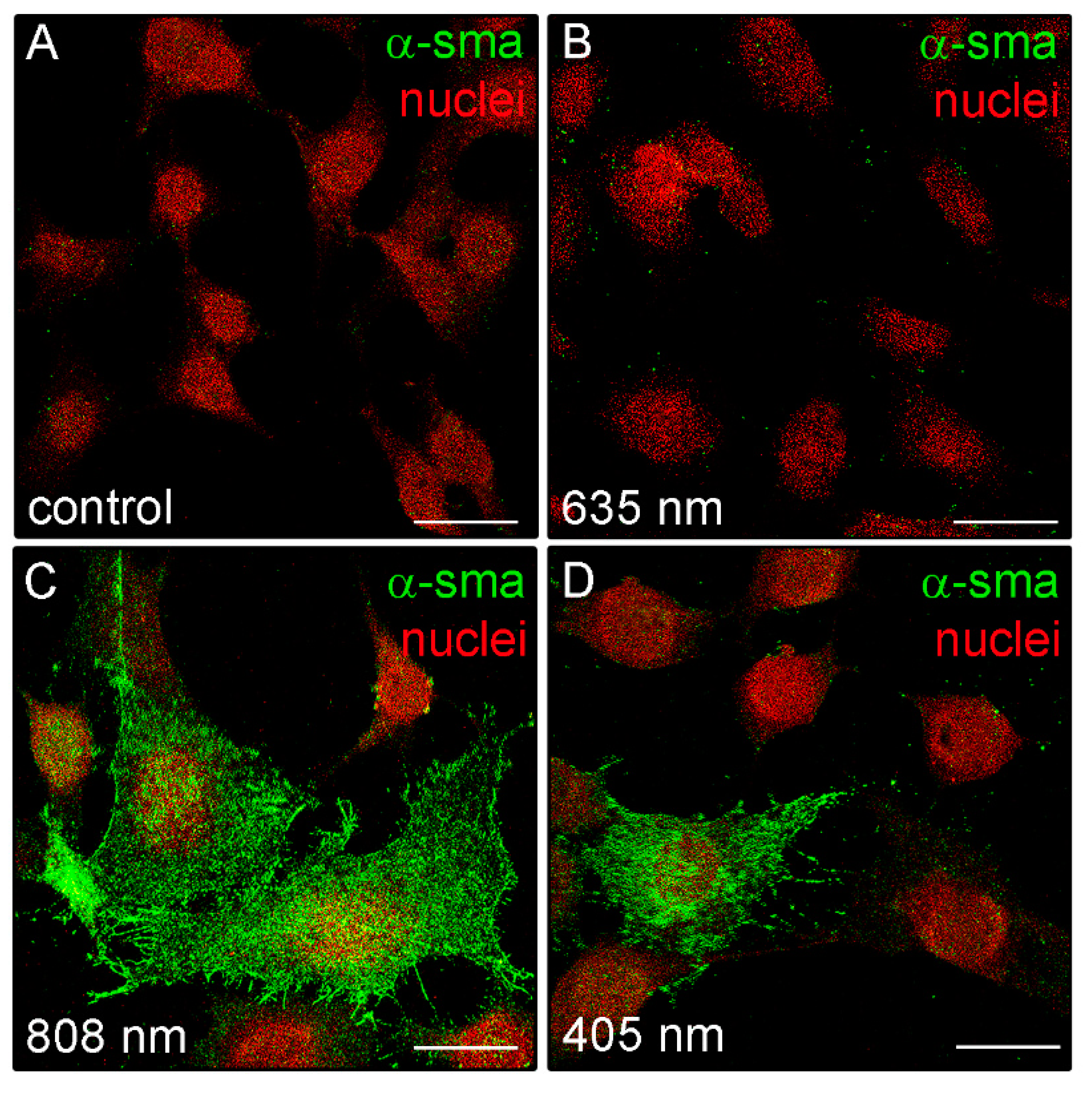
3.2. Effects of PBM Treatments on Fibroblast Type-1 Collagen and α-sma Expression
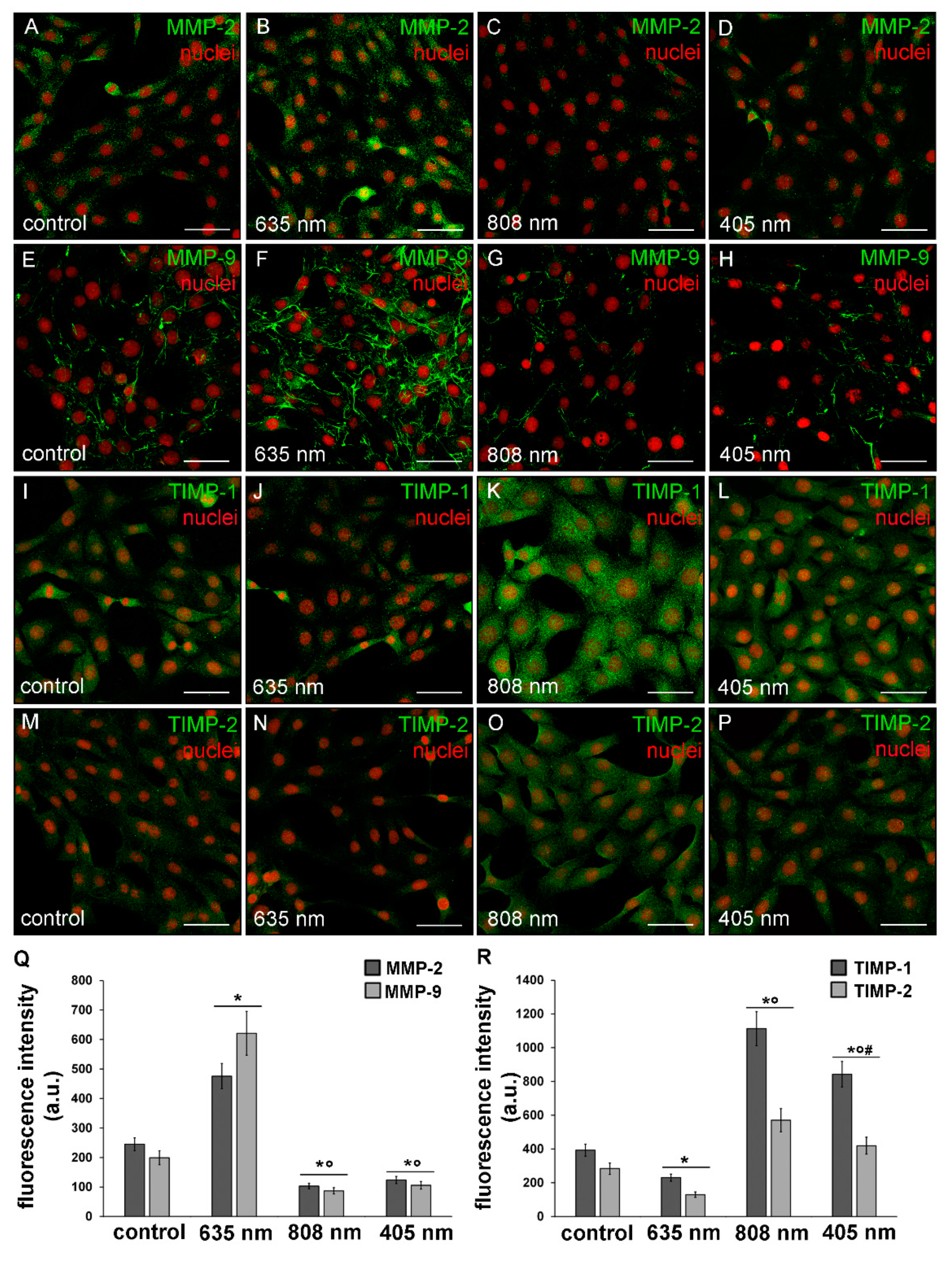
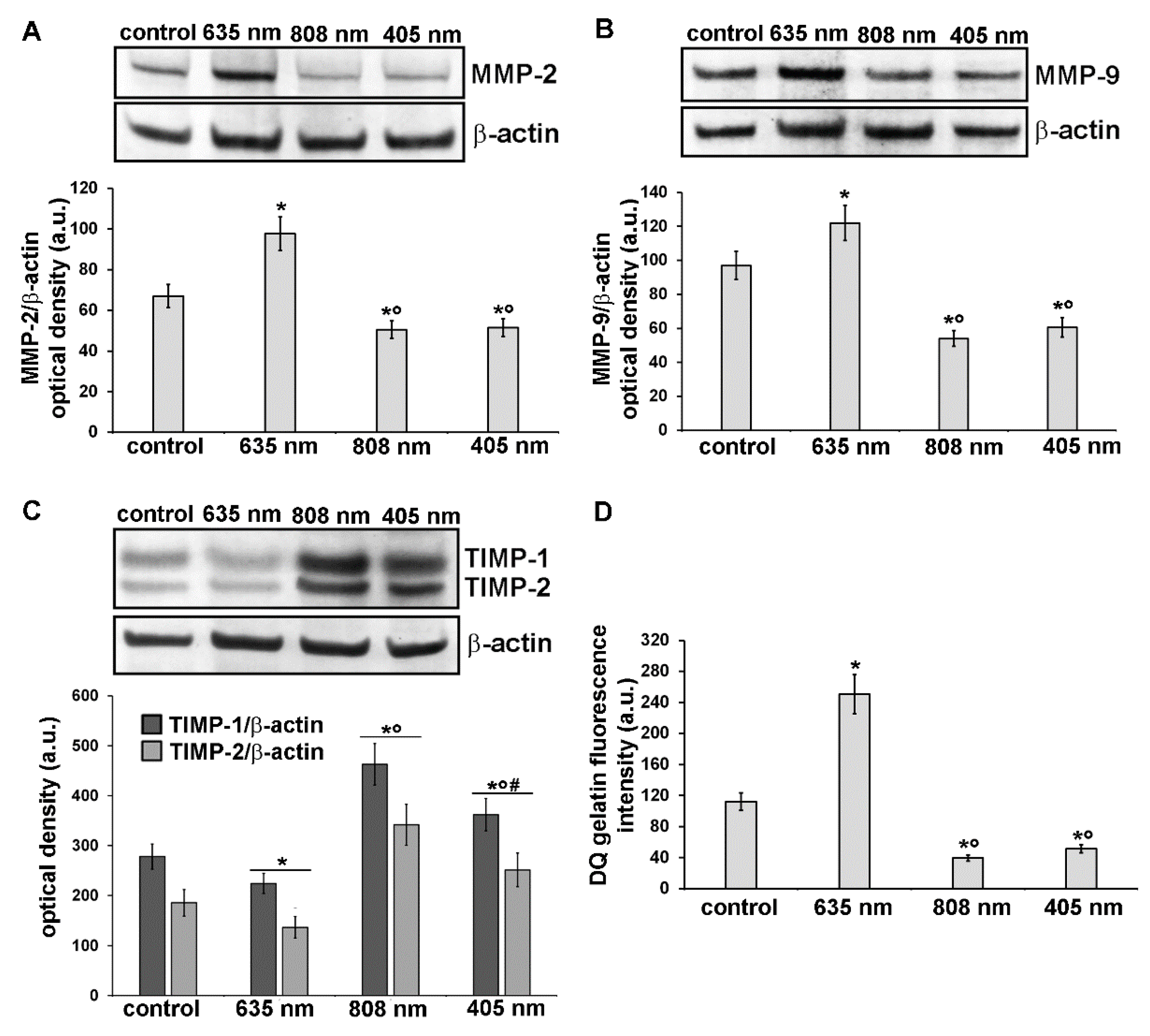
3.3. Effects of PBM Treatments on Fibroblast MMP-2, MMP-9, TIMP-1 and TIMP-2 Expression and on MMPs’ Functionality
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.; Arany, P. Low-Level Light/Laser Therapy Versus Photobiomodulation Therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Mosca, R.C.; Ong, A.A.; Albasha, O.; Bass, K.; Arany, P. Photobiomodulation Therapy for Wound Care. Adv. Ski. Wound Care 2019, 32, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Squecco, R.; Tani, A.; Idrizaj, E.; Nosi, D.; Giannelli, M.; Bsc, S.Z.; Zecchi-Orlandini, S. Low intensity 635 nm diode laser irradiation inhibits fibroblast–myofibroblast transition reducing TRPC1 channel expression/activity: New perspectives for tissue fibrosis treatment. Lasers Surg. Med. 2015, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Medrado, A.P.; Andrade, Z.A. Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg. Med. 2003, 32, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Medrado, A.P.; Soares, A.P.; Santos, E.T.; Reis, S.R.D.A.; Andrade, Z.A. Influence of laser photobiomodulation upon connective tissue remodeling during wound healing. J. Photochem. Photobiol. B: Biol. 2008, 92, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Albuquerque, R.L.C.; Ramalho, L.M.P.; Pinheiro, A.; Bonjardim, L.R.; Da Cunha, S.S. Immunohistochemical Assessment of Myofibroblasts and Lymphoid Cells During Wound Healing in Rats Subjected to Laser Photobiomodulation at 660 nm. Photomed. Laser Surg. 2009, 27, 49–55. [Google Scholar] [CrossRef]
- Chaves, M.E.D.A.; De Araújo, A.R.; Piancastelli, A.C.C.; Pinotti, M. Effects of low-power light therapy on wound healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Wagner, V.P.; Curra, M.; Webber, L.P.; Nör, C.; Matte, U.; Meurer, L.; Martins, M.D. Photobiomodulation regulates cytokine release and new blood vessel formation during oral wound healing in rats. Lasers Med. Sci. 2016, 31, 665–671. [Google Scholar] [CrossRef]
- Lima, F.J.C.; Neto, O.B.D.O.; Barbosa, F.T.; Galvão, A.M.D.N.; Ramos, F.W.S.; De Lima, C.C.F.; Sousa-Rodrigues, C.F. Is there a protocol in experimental skin wounds in rats using low-level diode laser therapy (LLDLT) combining or not red and infrared wavelengths? Systematic review. Lasers Med. Sci. 2016, 31, 779–787. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Mignon, C.; Uzunbajakava, N.E.; Castellano-Pellicena, I.; Botchkareva, N.V.; Tobin, D.J. Differential response of human dermal fibroblast subpopulations to visible and near-infrared light: Potential of photobiomodulation for addressing cutaneous conditions. Lasers Surg. Med. 2018, 50, 859–882. [Google Scholar] [CrossRef]
- Kouhkheil, R.; Fridoni, M.; Abdollhifar, M.-A.; Amini, A.; Bayat, S.; Ghoreishi, S.K.; Chien, S.; Kazemi, M.; Bayat, M. Impact of Photobiomodulation and Condition Medium on Mast Cell Counts, Degranulation, and Wound Strength in Infected Skin Wound Healing of Diabetic Rats. Photobiomodulation Photomed. Laser Surg. 2019, 37, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Lamaro-Cardoso, A.; Bachion, M.M.; Morais, J.M.; Fantinati, M.S.; Milhomem, A.C.; Almeida, V.L.; Vinaud, M.C.; Lino, R.S. Photobiomodulation associated to cellular therapy improve wound healing of experimental full thickness burn wounds in rats. J. Photochem. Photobiol. B Biol. 2019, 194, 174–182. [Google Scholar] [CrossRef]
- Gavish, L.; Hoffer, O.; Rabin, N.; Halak, M.; Shkilevich, S.; Shayovitz, Y.; Weizman, G.; Haim, O.; Gavish, B.; Gertz, S.D.; et al. Microcirculatory Response to Photobiomodulation—Why Some Respond and Others Do Not: A Randomized Controlled Study. Lasers Surg. Med. 2020, 52, 863–872. [Google Scholar] [CrossRef]
- Allendorf, J.D.F.; Bessler, M.; Huang, J.; Kayton, M.L.; Laird, D.; Nowygrod, R.; Treat, M.R. Helium-neon laser irradiation at fluences of 1, 2, and 4 J/cm2 failed to accelerate wound healing as assessed by both wound contracture rate and tensile strength. Lasers Surg. Med. 1997, 20, 340–345. [Google Scholar] [CrossRef]
- Lucas, C.; Criens-Poublon, L.; Cockrell, C.; De Haan, R. Wound healing in cell studies and animal model experiments by Low Level Laser Therapy; were clinical studies justified? a systematic review. Lasers Med. Sci. 2002, 17, 110–134. [Google Scholar] [CrossRef]
- Franek, A.; Król, P.; Kucharzewski, M. Does low output laser stimulation enhance the healing of crural ulceration? Some critical remarks. Med. Eng. Phys. 2002, 24, 607–615. [Google Scholar] [CrossRef]
- Lucas, C.; Van Gemert, M.J.C.; De Haan, R.J. Efficacy of low-level laser therapy in the management of stage III decubitus ulcers: A prospective, observer-blinded multicentre randomised clinical trial. Lasers Med. Sci. 2003, 18, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Msc, S.C.N.; Nogueira, G.E.C.; Ribeiro, M.S.; Msc, A.S.G.; Lage-Marques, J.L. He-Ne laser effects on blood microcirculation during wound healing: A method of in vivo study through laser Doppler flowmetry. Lasers Surg. Med. 2004, 35, 363–368. [Google Scholar] [CrossRef]
- Damante, C.A.; Sant’Ana, A.C.; Passanezi, E.; Taga, R. Histomorphometric study of the healing of human oral mucosa after gingivoplasty and low-level laser therapy. Lasers Surg. Med. 2004, 35, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kopera, D.; Kokol, R.; Berger, C.; Haas, J. Does the use of low-level laser influence wound healing in chronic venous leg ulcers? J. Wound Care 2005, 14, 391–394. [Google Scholar] [CrossRef]
- Houreld, N.N.; Abrahamse, H. Laser light influences cellular viability and proliferation in diabetic-wounded fibroblast cells in a dose- and wavelength-dependent manner. Lasers Med. Sci. 2007, 23, 11–18. [Google Scholar] [CrossRef]
- Machado, R.S.; Viana, S.; Sbruzzi, G. Low-level laser therapy in the treatment of pressure ulcers: Systematic review. Lasers Med. Sci. 2017, 32, 937–944. [Google Scholar] [CrossRef]
- Brassolatti, P.; De Andrade, A.L.M.; Bossini, P.S.; Otterço, A.N.; Parizotto, N.A. Evaluation of the low-level laser therapy application parameters for skin burn treatment in experimental model: A systematic review. Lasers Med. Sci. 2018, 33, 1159–1169. [Google Scholar] [CrossRef]
- Petz, F.D.F.C.; Felix, J.V.C.; Roehrs, H.; Pott, F.S.; Stocco, J.G.D.; Labat, R.; Meier, M.J. Effect of Photobiomodulation on Repairing Pressure Ulcers in Adult and Elderly Patients: A Systematic Review. Photochem. Photobiol. 2019, 96, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Tunér, J.; Jenkins, P.A. Parameter Reproducibility in Photobiomodulation. Photomed. Laser Surg. 2016, 34, 91–92. [Google Scholar] [CrossRef]
- Mignon, C.; Botchkareva, N.V.; Uzunbajakava, N.E.; Tobin, D.J. Photobiomodulation devices for hair regrowth and wound healing: A therapy full of promise but a literature full of confusion. Exp. Dermatol. 2016, 25, 745–749. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic Dose Response in Low Level Light Therapy—An Update. Dose-Response 2011, 9, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef]
- Desmouliere, A.; Darby, I.A.; Laverdet, B.; Bonté, F. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef]
- Tani, A.; Chellini, F.; Giannelli, M.; Nosi, D.; Zecchi-Orlandini, S.; Sassoli, C. Red (635 nm), Near-Infrared (808 nm) and Violet-Blue (405 nm) Photobiomodulation Potentiality on Human Osteoblasts and Mesenchymal Stromal Cells: A Morphological and Molecular In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1946. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Pierucci, F.; Tani, A.; Frati, A.; Chellini, F.; Matteini, F.; Vestri, A.; Anderloni, G.; Nosi, D.; Zecchi-Orlandini, S.; et al. Sphingosine 1-Phosphate Receptor 1 Is Required for MMP-2 Function in Bone Marrow Mesenchymal Stromal Cells: Implications for Cytoskeleton Assembly and Proliferation. Stem Cells Int. 2018, 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 81–93. [Google Scholar] [CrossRef]
- Hinz, B.; McCulloch, C.A.; Coelho, N.M. Mechanical regulation of myofibroblast phenoconversion and collagen contraction. Exp. Cell Res. 2019, 379, 119–128. [Google Scholar] [CrossRef]
- Squecco, R.; Chellini, F.; Idrizaj, E.; Tani, A.; Garella, R.; Pancani, S.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Sassoli, C. Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-β1–Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells 2020, 9, 1199. [Google Scholar] [CrossRef]
- Hawkins, D.H.; Abrahamse, H. The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg. Med. 2006, 38, 74–83. [Google Scholar] [CrossRef]
- Hawkins, D.; Abrahamse, H. Effect of Multiple Exposures of Low-Level Laser Therapy on the Cellular Responses of Wounded Human Skin Fibroblasts. Photomed. Laser Surg. 2006, 24, 705–714. [Google Scholar] [CrossRef]
- Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Irradiation at 636 nm Positively Affects Diabetic Wounded and Hypoxic Cellsin Vitro. Photomed. Laser Surg. 2011, 29, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-K.; Park, J.-K.; Song, C.; Kwon, S.-Y. Comparison of light-emitting diode wavelength on activity and migration of rabbit ACL cells. Lasers Med. Sci. 2013, 29, 245–255. [Google Scholar] [CrossRef]
- Esmaeelinejad, M.; Bayat, M.; Darbandi, H.; Bayat, M.; Mosaffa, N. The effects of low-level laser irradiation on cellular viability and proliferation of human skin fibroblasts cultured in high glucose mediums. Lasers Med. Sci. 2013, 29, 121–129. [Google Scholar] [CrossRef]
- Solmaz, H.; Ulgen, Y.; Gulsoy, M. Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med. Sci. 2017, 32, 903–910. [Google Scholar] [CrossRef]
- Lou, Z.; Gong, T.; Kang, J.; Xue, C.; Ulmschneider, C.; Jiang, J.J. The Effects of Photobiomodulation on Vocal Fold Wound Healing: In Vivo and In Vitro Studies. Photobiomodulation Photomed. Laser Surg. 2019, 37, 532–538. [Google Scholar] [CrossRef]
- Layegh, E.R.; Fathabadi, F.F.; Lotfinia, M.; Zare, F.; Tofigh, A.M.; Abrishami, S.; Piryaei, A. Photobiomodulation therapy improves the growth factor and cytokine secretory profile in human type 2 diabetic fibroblasts. J. Photochem. Photobiol. B Biol. 2020, 210, 111962. [Google Scholar] [CrossRef]
- Moore, P.; Ridgway, T.D.; Higbee, R.G.; Howard, E.W.; Lucroy, M.D. Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro. Lasers Surg. Med. 2005, 36, 8–12. [Google Scholar] [CrossRef]
- Opländer, C.; Hidding, S.; Werners, F.B.; Born, M.; Pallua, N.; Suschek, C.V. Effects of blue light irradiation on human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2011, 103, 118–125. [Google Scholar] [CrossRef]
- McDonald, R.; MacGregor, S.J.; Anderson, J.G.; MacLean, M.; Grant, M.H. Effect of 405-nm high-intensity narrow-spectrum light on fibroblast-populated collagen lattices: An in vitro model of wound healing. J. Biomed. Opt. 2011, 16, 048003. [Google Scholar] [CrossRef]
- Mamalis, A.; Garcha, M.; Jagdeo, J. Light emitting diode-generated blue light modulates fibrosis characteristics: Fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers Surg. Med. 2015, 47, 210–215. [Google Scholar] [CrossRef]
- Feng, J.; Li, X.; Zhu, S.; Xie, Y.; Du, J.; Ge, H.; Bai, Y.; Liu, Y.; Guo, L. Photobiomodulation with 808-nm diode laser enhances gingival wound healing by promoting migration of human gingival mesenchymal stem cells via ROS/JNK/NF-κB/MMP-1 pathway. Lasers Med. Sci. 2020, 35, 1831–1839. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Lu, X.; Liu, J.; Chen, Z.; Zhou, X.; Liu, M.; Liu, S. RNA-Seq analysis revealed the molecular mechanisms of photobiomodulation effect on human fibroblasts. Photodermatol. Photoimmunol. Photomed. 2020, 36, 299–307. [Google Scholar] [CrossRef]
- Wright, J.; Schneider, B.L. Cell Growth: When Less Means More. Curr. Biol. 2014, 24, R283–R285. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pellegrin, S.; Mellor, H. Actin stress fibres. J. Cell Sci. 2007, 120, 3491–3499. [Google Scholar] [CrossRef]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin stress fibers-assembly, dynamics and biological roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef]
- De Magalhães, A.C.; Guimarães-Filho, Z.; Yoshimura, E.M.; Lilge, L. Photobiomodulation therapy can change actin filaments of 3T3 mouse fibroblast. Lasers Med. Sci. 2019, 35, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.; Georges, P.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Doss, B.L.; Pan, M.; Gupta, M.; Grenci, G.; Mège, R.-M.; Lim, C.T.; Sheetz, M.P.; Voituriez, R.; Ladoux, B. Cell response to substrate rigidity is regulated by active and passive cytoskeletal stress. Proc. Natl. Acad. Sci. USA 2020, 117, 12817–12825. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K. Activation of Rho-kinase and focal adhesion kinase regulates the organization of stress fibers and focal adhesions in the central part of fibroblasts. PeerJ 2017, 5, e4063. [Google Scholar] [CrossRef]
- Sandbo, N.; Smolyaninova, L.V.; Orlov, S.N.; Dulin, N.O. Control of myofibroblast differentiation and function by cytoskeletal signaling. Biochemistry (Moscow) 2016, 81, 1698–1708. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Abe, T.; Hara, Y. Roles of focal adhesions and fibronectin-mediated cohesion in proliferation of confluent fibroblasts. J. Cell. Physiol. 2009, 219, 194–201. [Google Scholar] [CrossRef]
- Koshimizu, T.; Kawai, M.; Kondou, H.; Tachikawa, K.; Sakai, N.; Ozono, K.; Michigami, T. Vinculin Functions as Regulator of Chondrogenesis. J. Biol. Chem. 2012, 287, 15760–15775. [Google Scholar] [CrossRef] [PubMed]
- Holle, A.W.; Tang, X.; VijayRaghavan, D.; Vincent, L.G.; Fuhrmann, A.; Choi, Y.S.; Del Álamo, J.C.; Engler, A.J. In situ mechanotransduction via vinculin regulates stem cell differentiation. STEM CELLS 2013, 31, 2467–2477. [Google Scholar] [CrossRef]
- Goldmann, W.H. Role of vinculin in cellular mechanotransduction. Cell Biol. Int. 2016, 40, 241–256. [Google Scholar] [CrossRef]
- Yamaba, H.; Haba, M.; Kunita, M.; Sakaida, T.; Tanaka, H.; Yashiro, Y.; Nakata, S. Morphological change of skin fibroblasts induced by UV Irradiation is involved in photoaging. Exp. Dermatol. 2016, 25, 45–51. [Google Scholar] [CrossRef]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Actin cytoskeleton assembly regulates collagen production via TGF-β type II receptor in human skin fibroblasts. J. Cell. Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef]
- Rajshankar, D.; Wang, Y.; McCulloch, C.A. Osteogenesis requires FAK-dependent collagen synthesis by fibroblasts and osteoblasts. FASEB J. 2016, 31, 937–953. [Google Scholar] [CrossRef]
- De Castro, I.C.V.; Rocha, C.A.G.; Henriques, Á.C.G.; De Sousa, A.P.C.; Lisboa, M.V.; Sotero, D.D.R.; Pinheiro, A.L.; Cury, P.R.; Dos Santos, J.N. Do laser and led phototherapies influence mast cells and myofibroblasts to produce collagen? Lasers Med. Sci. 2014, 29, 1405–1410. [Google Scholar] [CrossRef]
- Newby, A.C. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc. Res. 2006, 69, 614–624. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Hou, J.-F.; Zhang, H.; Yuan, X.; Li, J.; Wei, Y.-J.; Hu, S.-S. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: Proliferation, growth factors secretion and myogenic differentiation. Lasers Surg. Med. 2008, 40, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kreisler, M.; Christoffers, A.B.; Willershausen, B.; D’Hoedt, B. Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: An in vitro study. J. Clin. Periodontol. 2003, 30, 353–358. [Google Scholar] [CrossRef]
- Jampa-Ngern, S.; Viravaidya-Pasuwat, K.; Suvanasuthi, S.; Khantachawana, A. Effect of laser diode light irradiation on growth capability of human hair follicle dermal papilla cells. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 3592–3595. [Google Scholar] [CrossRef]
- Chang, L.; Fan, S.M.; Liao, Y.; Wang, W.; Chen, Y.; Lin, S.-J. Proteomic Analysis Reveals Anti-Fibrotic Effects of Blue Light Photobiomodulation on Fibroblasts. Lasers Surg. Med. 2020, 52, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Vedrenne, N.; Coulomb, B.; Danigo, A.; Bonté, F.; Desmoulière, A. The complex dialogue between (myo)fibroblasts and the extracellular matrix during skin repair processes and ageing. Pathol. Biol. 2012, 60, 20–27. [Google Scholar] [CrossRef]
- El-Mohri, H.; Wu, Y.; Mohanty, S.; Ghosh, G. Impact of matrix stiffness on fibroblast function. Mater. Sci. Eng. C 2017, 74, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Pierucci, F.; Zecchi-Orlandini, S.; Meacci, E. Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration. Int. J. Mol. Sci. 2019, 20, 5545. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Sarrafzadeh, A.; Kalhori, K.A.; Khan, I.; Arany, P.R.; Giubellino, A. Improved Wound Remodeling Correlates with Modulated TGF-beta Expression in Skin Diabetic Wounds Following Combined Red and Infrared Photobiomodulation Treatments. Photochem. Photobiol. 2018, 94, 775–779. [Google Scholar] [CrossRef]
- Xiao, H.; Li, C.; Zhou, X.; Wang, X.; Wu, Z.; Zhang, L.; Liu, C.; Wang, Z.; An, H.; Wang, Y.; et al. A new method of microskin autografting with a Vaseline-based moisture dressing on granulation tissue. Burns 2014, 40, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Kilik, R.; Lakyová, L.; Sabo, J.; Kruzliak, P.; Lacjaková, K.; Vasilenko, T.; Vidová, M.; Longauer, F.; Radoňak, J. Effect of Equal Daily Doses Achieved by Different Power Densities of Low-Level Laser Therapy at 635 nm on Open Skin Wound Healing in Normal and Diabetic Rats. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Nussbaum, E.L.; Heras, F.L.; Pritzker, K.P.; Mazzulli, T.; Lilge, L. Effects of low intensity laser irradiation during healing of infected skin wounds in the rat. Photon. Lasers Med. 2014, 3, 23–36. [Google Scholar] [CrossRef]
- Lim, W.; Choi, H.; Kim, J.; Kim, S.; Jeon, S.; Zheng, H.; Kim, D.; Ko, Y.; Kim, D.; Sohn, H.; et al. Anti-inflammatory effect of 635 nm irradiations onin vitrodirect/indirect irradiation model. J. Oral Pathol. Med. 2014, 44, 94–102. [Google Scholar] [CrossRef]

| Red Diode Laser | Near Infrared (NIR) GaAlAs Diode Laser | Violet-Blue LED | |
|---|---|---|---|
| Wavelength (λ) | 635 ± 5 nm | 808 ± 10 nm | 405 ± 5 nm |
| Handpiece type | Focalized zoom handpiece | Polymide-coated silica Fiber NA = 0.22 | Light pipe glass |
| Applicator Diameter | 6 mm | 0.6 mm | 10 mm |
| Distance from the target | 30 mm | 66 mm | 11 mm |
| Antibody Name | Company | Code | Dilution |
|---|---|---|---|
| Anti-Ki67 (rabbit polyclonal) | Abcam, Cambridge, UK | ab15580 | 1:100 (IF) |
| Anti-phospho-Focal adhesion Kinase (p-Tyr861) (rabbit polyclonal) | Sigma | F9176 | 1:50 (IF) |
| Anti-vinculin (mouse monoclonal, hVIN-1) | Sigma | V9131 | 1:100 (IF) |
| Anti-collagen 1 (rabbit polyclonal) | Abcam | ab34710 | 1:500 (IF) |
| Anti-α-smooth muscle actin (sma) (mouse monoclonal, 1A4) | Abcam | ab7817 | 1:100 (IF) |
| Anti-MMP-2 (rabbit polyclonal) | Abcam | ab37150 | 1:200 (IF) 1:2000 (WB) |
| Anti-MMP-9 (rabbit polyclonal) | Abcam | ab38898 | 1:100 (IF) 1:1000 (WB) |
| Anti-TIMP-1 (rabbit polyclonal) | Bioss, Inc., Woburn, MA | bs-0415R | 1:50 (IF) 1:500 (WB) |
| anti-TIMP-2 (mouse monoclonal, 3A4) | Abcam | ab1828 | 1:20 (IF) 1:200 (WB) |
| Anti β-actin (mouse monoclonal, AC-74) | Sigma | A2228 | 1:10000 (WB) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chellini, F.; Tani, A.; Zecchi-Orlandini, S.; Giannelli, M.; Sassoli, C. In Vitro Evidences of Different Fibroblast Morpho-Functional Responses to Red, Near-Infrared and Violet-Blue Photobiomodulation: Clues for Addressing Wound Healing. Appl. Sci. 2020, 10, 7878. https://doi.org/10.3390/app10217878
Chellini F, Tani A, Zecchi-Orlandini S, Giannelli M, Sassoli C. In Vitro Evidences of Different Fibroblast Morpho-Functional Responses to Red, Near-Infrared and Violet-Blue Photobiomodulation: Clues for Addressing Wound Healing. Applied Sciences. 2020; 10(21):7878. https://doi.org/10.3390/app10217878
Chicago/Turabian StyleChellini, Flaminia, Alessia Tani, Sandra Zecchi-Orlandini, Marco Giannelli, and Chiara Sassoli. 2020. "In Vitro Evidences of Different Fibroblast Morpho-Functional Responses to Red, Near-Infrared and Violet-Blue Photobiomodulation: Clues for Addressing Wound Healing" Applied Sciences 10, no. 21: 7878. https://doi.org/10.3390/app10217878
APA StyleChellini, F., Tani, A., Zecchi-Orlandini, S., Giannelli, M., & Sassoli, C. (2020). In Vitro Evidences of Different Fibroblast Morpho-Functional Responses to Red, Near-Infrared and Violet-Blue Photobiomodulation: Clues for Addressing Wound Healing. Applied Sciences, 10(21), 7878. https://doi.org/10.3390/app10217878






