Abstract
Increases in water scarcity due to climate change, especially in dry regions, can affect the dynamics of successional species. In view of the longest sequence of dry years (2010–2019) to have occurred in the Brazilian semi-arid region, with a consequent reduction in water availability, the influence of rainfall distribution on the production of above-ground plant biomass was investigated in a Dry Tropical Forest (DTF). This natural change monitoring experiment was conducted over 11 years (2009–2019) in a fragment of DTF under regeneration for 40 years, in the district of Iguatu, Ceará, Brazil. All living individuals of the woody component with a Diameter at Ground Level (DGL) ≥3 cm and a height (h) ≥100 cm were measured during 2009–2010, 2015–2016, 2018–2019. Biomass production was calculated using an allometric equation defined for DTF species. A mean mortality rate of 134 ind. ha−1 yr−1 was registered, with a recruitment of 39 ind. ha−1 yr−1, generating a mean deficit of 95 ind. ha−1 yr−1. The mean reduction in biomass was 3.26 Mg ha−1 yr−1. Climate conditions during consecutive dry years have a direct effect on the mortality and recruitment of woody species, with a recruitment/mortality ratio of 0.11. Shrubby-tree individuals of smaller diameter showed less resilience to the cumulative effect of drought.
1. Introduction
The dryland domain represents 41.5% of the world’s surface [1]. These regions are home to 14.4% of the world’s population and their sustainability depends on, among other factors, the scarce water availability [1]. The potential evapotranspiration to rainfall ratio is greater than one, with a five to eight month dry season and a Thornthwaite’s aridity index of 0.65 [1]. Vegetation is typically rangeland and savanna-steppe [2]. The most striking feature of dry regions is the water deficit that predominates for 8 to 10 months of the year [3].
According to the latest simulation models, climate change may increase this area by 11 to 23% by the end of the 21st century, with the largest increase occurring in semi-arid regions [3]. Dry Tropical Forests (DTFs) are part of this domain and account for 42% of tropical and subtropical forest resources, occupying around 2.7 million km2 distributed across the globe [4]. Tropical dry forests (TDFs) are located in regions of tropical dry climate, with average monthly temperatures above 18 °C, annual precipitation below the 1800 mm isohyet, and rainfall events concentrated within four to seven months characterizing a long period of continuous dry days (CDDs) [5]. This characteristic may be aggravated by possible changes in climate.
In Brazil, the DTF is known as Caatinga, and is located in the northeast of Brazil within the limits of the semi-arid region [6]. It has an area of approximately 844 thousand km2 and a population of 27 million people [6]. Areas of Caatinga are generally exploited for firewood, agriculture and pasture, and subsequently abandoned, starting the process of regeneration [7]. This process gives rise to new forest fragments that present a high rate of recruitment and high density of new individuals, with small individuals that show quick growth with a large increase in biomass [8,9]. In DTFs the biomass dynamics during the early processes of succession of succession are dominated by more tolerant species to drought that are better able to cope with the harsh environment during the initial stages of succession [10,11].
This dynamic of vegetation biomass depends on several factors, such as species composition and variability [12], the soil, the level and type of anthropogenic disturbance and the rainfall pattern [13,14,15]. Among the determinants of biomass production, changes in water availability are the most limiting on the dynamics of successional species [12,16].
Successive years of drought and the frequent occurrence of short dry spells impair the ability of plants to recover [13]. During drought, trees reduce their evapotranspiration through mechanisms of water-deficit tolerance, such as stomatal closure and deciduousness [17]. When climate conditions exceed the limits of plant tolerance due to an increase in temperature, vapor pressure and water deficit [10], the probability of tree mortality of some trees may increase. Long periods of drought can generate several problems, such as xylem hydraulic failure [18] embolisms that cut off the water supply, leading to tissue mortality [19].
However, a lack in long-term data on droughts caused by climate change limits the understanding on tree mortality patterns in TDFs. In general, studies have investigated forest mortality in the short term without assessing long-term mortality [15]. From 2010–2019, the Brazilian semi-arid region experienced the longest period of drought reported, and little is known about the biomass dynamics due to a reduction in water availability during this period. Based on this, this study assessed the biomass generated from this period by different species of plants in a Dry Tropical Forest in the Caatinga Phytogeographic Domain (DTF/CPD). Also, we determine the resilience of some species to this dry period. Specific aims were: (a) to evaluate how tree growth, recruitment and mortality have contributed to the accumulation of above-ground biomass; (b) the influence of the annual rainfall distribution on the production of above-ground woody biomass in a fragment of dry tropical forest under regeneration.
2. Materials and Methods
2.1. Study Area
The study was carried out in a fragment of dry tropical forest in the Caatinga phytogeographic domain (DTF/CPD), in the district of Iguatu, in the south-central region of the state of Ceará. The experimental area belongs to the Ceará Federal Institute of Education Science and Technology (IFCE), located at UTM 047158 E and 9293148 S (Sirgas 2000) (Figure 1).
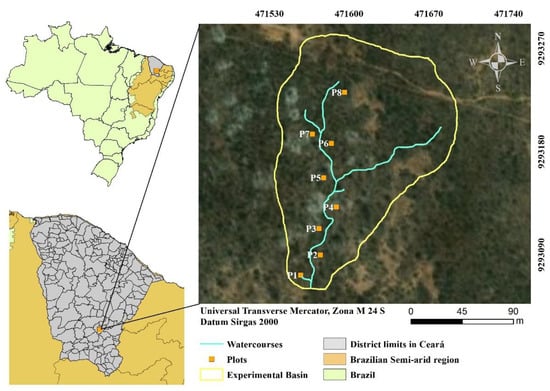
Figure 1.
Location of the experimental area in the district of Iguatu, Ceará, Brazil.
The climate in the region, according to the Köppen classification, is type BSh’ (hot semi-arid), the mean temperature is 28 °C, ranging from a minimum of 22.4 °C to a maximum of 33.5 °C (Figure 2). The mean potential evapotranspiration (PET) is 2000 ± 135 mm yr−1, with the highest rates between August and January. The mean historical rainfall is 994.2 ± 297.3 mm yr−1, with the rains concentrated between December and May, and 43% concentrated between March and April.
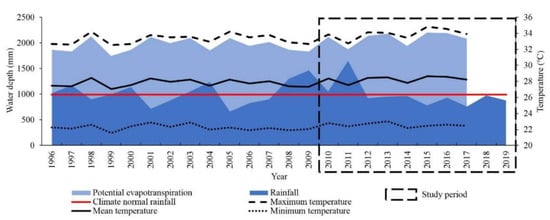
Figure 2.
Distribution of climate variables in the district of Iguatu, Ceará, Brazil.
The region is inserted in the basin of the Upper Jaguaribe in the Sertaneja Depression, at an altitude of 217.8 m, and has a gentle, flat relief. The soils are Alluvial, Litholic, Red-Yellow Podzolic and Vertisol. The predominant vegetation is low deciduous with steppe savannah physiognomy, with a mean height of 5 m, due to anthropogenic action, the tree density is highly variable (3.000–20,000 individuals ha−1) [7,12]. The DTF/CPD fragment has an area of 3.6 ha that has been under regeneration since 1980. Prior to this, the area was used for subsistence farming, i.e., corn farms (Zea mays L.) [20].
2.2. Shrub Layer
To investigate the effect of consecutive dry years on the potential for biomass production in the DTF/CPD, the functional composition of the vegetation was analyzed via a ten-year series (2009–2019). The study period corresponds to a transition between wet (2008–2011) and dry (2012–2019) years; during this period, the area was surveyed six times (September 2009, September 2010, March 2015, March 2016, March 2018 and February 2019).
In 2009, eight permanent and contiguous experimental plots of 10 × 10 m were installed along the watercourse [21]. All living individuals of the woody component with Diameter at Ground Level (DGL) ≥3 cm and height (h) ≥100 cm were marked with identification plates, measured and quantified in each plot [12]. The measurements were made 10 cm above the ground level through markings on the shafts. To characterize the structure of the woody community, the number of individuals and the absolute and relative density were calculated according to the methodology of Rodal et al. [22]. The allometric equation developed by Sampaio and Silva [23] for the Caatinga species was used to estimate the biomass of the woody stratum (Biomass (Mg ha−1) = 0.0644 × DGL2.3948), where DGL is the diameter at ground level.
All the plants that met the inclusion criteria in 2009 were surveyed between 2010–2019. Plants that had not been registered in previous surveys due to not meeting the inclusion criteria were identified, marked, measured, and considered recruits. Plants measured in previous years, which were not found in later surveys, were counted as dead, together with the dead trees, whether standing or on the ground.
2.3. Climate Variables
A time series of daily rainfall and temperature data for 2008 to 2018 was used. The data were obtained from the Meteorological Database for Teaching and Research (BDMEP/INMET) [24]. The annual climate normal (1974–2019) was calculated for the series of data available in the INMET system, in addition to consecutive dry days (CDDs) during the rainy season (December to May) from 2008 to 2018. A dry spell is considered as the occurrence of five CDDs with a rainfall of <2 mm [25]. The dry spells were later separated into classes: C1 (5–10 CDDs), C2 (11–20 CDDs) and C3 (>20 CDDs).
The Maximum Cumulative Water Deficit (MCWD) was calculated following the methodology described in Aragão et al. [26]. The MCWD is an annual estimate of the water deficit that considers both the duration and intensity of the dry season based solely on climate variables, i.e., the properties of the soil were not considered. The water year starting in December, at the start of the rainy season, was used to calculate the MCWD, as the beginning of the water year does not necessarily follow the calendar year [27]. Calculation of the water deficit used the potential evapotranspiration (PET), estimated as a function of temperature based on the Thornthwaite equation; whereas, Aragão et al. [26] determined a value derived from the mean evapotranspiration in different locations and seasons in the Amazon, of 100 mm mo−1.
2.4. Statistical Analysis
The annual biomass data were subjected to Friedman’s nonparametric test at a significance level of 5%, once it did not show normal distribution by the Shapiro–Wilk test. In addition to descriptive statistics, cluster analysis (CA) was used to assess the similarity of the climate indicators (Maximum Cumulative Water Deficit, Temperature, Precipitation, Potential Evapotranspiration and Classes of consecutive dry days). The IBM SPSS 16.0 software for Windows was used in the statistical analysis.
3. Results and Discussion
3.1. Similarity between the Years under Study
The cluster analysis showed three homogeneous groups (Figure 3).
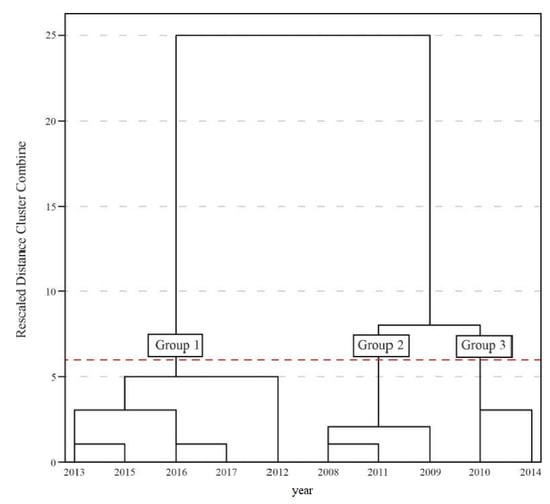
Figure 3.
Grouping dendrogram of homogeneous years for the district of Iguatu, Ceará, Brazil.
The criterion used to define the cut-off point, and consequently the group number, was the first jump of the rescaled distance [28]. The first group (G1) was defined by the years 2012, 2013, 2015, 2016 and 2017, which correspond to those with the largest annual water deficit (Table 1). G1 comprised the highest values for temperature and evapotranspiration, in addition to the lowest rainfall, and was characterized as the group with the driest years or with the largest water deficit.

Table 1.
Mean values and standard deviations of the climate variables for each group formed, for the district of Iguatu, Ceará, Brazil.
The second group (G2) was formed by 2008, 2009 and 2011, which correspond to the years with the highest water availability. The lowest temperatures and evapotranspiration were seen, in addition to the highest values for rainfall, characterizing G2 as the most humid among the three groups formed. The third and last group (G3) comprised 2010 and 2014, the two years with values closest to the mean, and considered as intermediate; 2010 had above-average values, while values for 2014 were below average, defining their similarity and placing them together as an intermediate group among the three formed.
3.2. Shrub Layer
For the diversity of woody cover in the area of DTF/CPD under regeneration for approximately 40 years, nine families, 14 genera and 15 species were identified in total which did not vary over the 11 years (Table 2), characterizing the area as a representative fragment of crystalline caatinga [15]. The number of families ranged from six to 26, while species from 12 to 64 [15]. The occurrence of families Anacardiaceae and Burseraceae, represented by the species Myracrodruon urundeuva and Commiphora leptophloeos respectively, show that the area is in an advanced process of regeneration, since these are indicators of more-protected or well-preserved areas of the Caatinga [9,29].

Table 2.
Botanical relationship for the mean number of individuals in a fragment of dry tropical forest under regeneration for approximately 40 years, in the district of Iguatu, Ceará, Brazil, from 2009 to 2019.
Araújo [7] found that after 25 years of fallow the density of the bushes begins to decrease to values below 5000 plants ha−1, and after 45 years there is a dominance of tree species. Shrub species are in the majority (1762 ind. ha−1), while tree species account for 921 ind. ha−1 (Table 2). It is therefore believed that the DTF fragment is in the process of transition between the third and fourth phase of secondary succession, due to the predominance of shrub species.
The families with the highest number of identified species were represented by Fabaceae and Euphorbiaceae, with six and two species, respectively (Table 2). These two families are generally found in the floristic pattern of fragments of dry forest in the northeast of Brazil [15] and in other DTF across the world [2]; they are the most abundant woody families in terms of gender, species and plant abundance [22].
Among the identified species, Croton spp. (885 ind.), Aspidosperma spp. (538 ind.), Combretum spp. (279 ind.), Mimosa caesalpiniifolia Benth. (277 ind.) and Bauhinia cheilantha (Bong.) Steud. (217 ind.) are noteworthy, and together accounted for around 80% of the total density (Table 2). Croton spp. is a commonly reported species in floristic surveys due to its shrub habit and great capacity for regrowth and is considered the main colonizing shrub in successive DTF/CPD [7]. Aspidosperma spp. is present in more preserved environments with little anthropogenic intervention [12]. Mimosa caesalpiniifolia Benth. and Bauhinia cheilantha (Bong.) Steud are pioneer species, common in primary and secondary formations [11], and characterized by fast growth, high regenerative capacity and resistance to drought.
In the first survey (2010), with above average rainfall, the abundance of most species was slightly less in 2010 than in 2009 (Figure 4). However, it cannot be said whether there was any real significant change or simply random variation. This reduction in species abundance may be related to intra- and interspecific competition. The greatest increases in recruited individuals were seen in Ximenia americana L. (37.5 ind. ha−1), Aspidosperma spp. (37.5 ind. ha−1) and Jatropha mollissima (Pohl) Baill. (25 ind. ha−1). In the survey 2015 to 2019, a period below-average rainfall, there was a reduction in the number of individuals, especially for Croton spp., Aspidosperma spp. and Piptadenia stipulacea (Benth.) Duck., with a mean mortality rate of 45.0, 41.3 and 16.3 ind. ha−1, respectively.
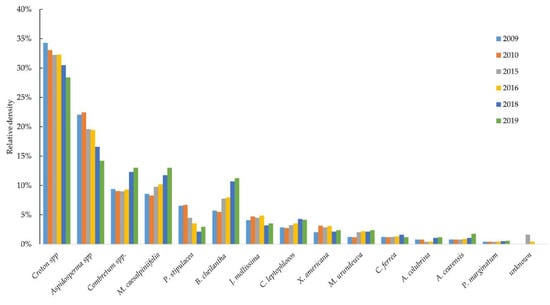
Figure 4.
Annual distribution of species density in a fragment of dry tropical forest under preservation for approximately 40 years, in the district of Iguatu, Ceará, Brazil.
Individuals that died during the study period had a wood density that was higher than the average for the area (0.660 g cm−3) (Table 2), suggesting that species with higher density, have less capacity to resist periods of prolonged drought. Tree species with highly dense wood may find it difficult to avoid a reduction in water potential compared to species whose wood is less dense [14]. Such a characteristic can result in smaller variations in leaf water potential over the year [30], in addition to affecting mechanical stability, inducing cavitation [14]. It is therefore important to conduct studies that relate wood density to periods of drought and rainfall.
Furthermore, when climate conditions exceed the plant tolerance limits (e.g., an increase in temperature and vapor pressure) [10], hydraulic failure can result in tree mortality due to [31] xylem embolism [18,31]. Another factor possibly causing mortality is an excess of water in soils that affects water potential in both soils and plants [32].
The study area is on a Vertisol, characterized by contraction and expansion of the soil mass due to expansive clays, showing cracks in the dry periods. Due to restrictions imposed by soil physics, plants need a large amount of energy to absorb water from the soil, increasing as the soil dries [32]. In times of drought, the trees approach a limit where it is no longer possible to extract enough water from the soil to meet the evapotranspiration losses, and irreversible and lethal damage to the system can occur [17].
Furthermore, the observed mortality, mainly between the years 2015 and 2016, may also be related to the process of ecological succession, also perceived by the reduction of individuals such as Aspidosperma spp. and Croton spp., which are secondary and pioneer species, respectively. It is noteworthy the recruitment of individuals such as M. urundeuva Allemao (2015 and 2016) and C. leptophloeos (Mart.) J.B.Gillett (2015), which are late secondary successional species.
The 3 to 12 cm diameter class concentrated the highest number of dead individuals in relation to the number of living individuals, followed by the 6 to 9 cm class (Figure 5). Annual mortality increased from 6% in 2015 to 12.8% in 2019. The effect of drought on plant mortality tends to accumulate, and there has been an increase in the number of dead individuals over the years. Sixty consecutive dry days (CDDs) were recorded in 2014; during the following years, the number of CDDs doubled: 2015 (120 days), 2016 (150 days) and 2017 (120 days).
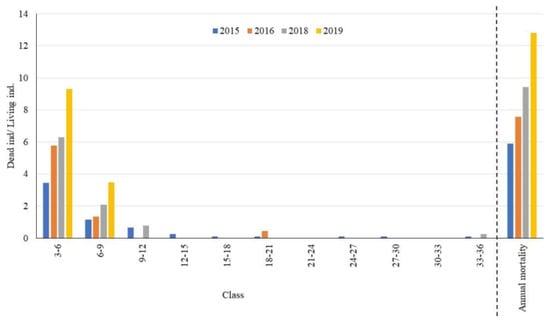
Figure 5.
Annual mortality proportion of individuals from the fragment of Dry Tropical Forest in the Caatinga Phytogeographic Domain (DTF/CPD).
Forest species with a smaller stem diameter have a lower amount of reserves and a root system that exploration a smaller area [13]. This situation hinders the effective exploitation of water in the deeper layers of the soil, as well as the maintenance of individuals through their reserves during periods of drought. The mortality was also found of larger-diameter individuals, Mimosa caesalpiniifolia Benth (2015), Aspidosperma spp. (2015), Bauhinia cheilantha (Bong.) Steud. (2016) and Anadenanthera colubrina (Vell.) Brenan (2018), probably related to the hydraulic failure that affects the wider ducts [8].
3.3. Biomass
Plant density in 2009 was 3063 ind. ha−1, increasing to 3175 ind. ha−1 in 2010, and decreasing to 3063 ind. ha−1 in 2015, 2825 ind. ha−1 in 2016, 2338 ind. ha−1 in 2018 and 2113 ind. ha−1 in 2019 (Figure 6). This reduction in plant density is the result of a higher mortality rate than recruitment rate, seen during the study period, where there was a mean mortality of 134 ind. ha−1 yr−1 and recruitment of 39 ind. ha−1 yr−1, generating a mean deficit of 95 ind. ha−1 yr−1. Probably, mortality rates that are higher than those of recruitment are strongly influenced by climate seasonality and consecutive years of drought. The reduction in plant density from 2015 to 2019, compared to the period from 2009 to 2010, is related to the droughts which have occurred in the region since 2012 [33].
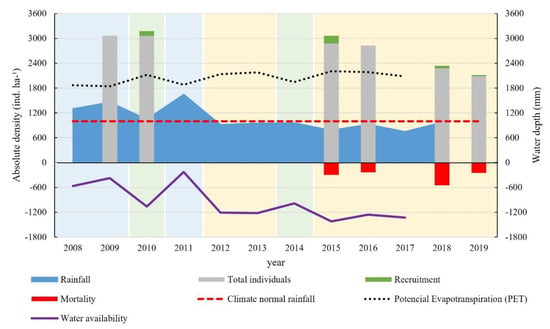
Figure 6.
Annual distribution of tree density, recruitment, and mortality in a fragment of dry forest in the northeast of Brazil.
Trees typically reduce evapotranspiration during drought by means of adaptive mechanisms, such as stomatal closure and the seasonal loss of leaves and canopy [14]. However, the intra- and interannual variation in rainfall accentuates the influence of water stress and can lead to plant mortality, which is strongly associated with drought [31]. In forested communities, the prolonged hydric stress reduces the number of trees, increasing the probability of survival of the remaining trees [10]. The reduction of the competition among vegetable individuals allows the hydric transportation systems to re-establish themselves, thus diminishing hydric stress [17].
Years of water deficit cause various problems to the development of newly recruited individuals, including delaying their development. As their roots are shallower than those of the older trees, during the dry season, they may suffer the negative effects of water stress in the upper layers of the soil [34]. Another important characteristic is the low capacity for accumulating reserves to maintain individuals during the dry season [13]. These reserves are influenced by phenological stage, genetic load, and the severity and intensity of the water stress [16].
Following the same trend as plant density, between 2009 and 2010, an increase of 7.26 Mg ha−1 was seen in the biomass stored in the above-ground woody compartment, going from 65.33 to 72.59 Mg ha−1 (Figure 7). In succeeding surveys, 2015 (55.20 Mg ha−1), 2016 (45.04 Mg ha−1) and 2018 (43.46 Mg ha−1), a decreasing sequence was identified for the estimated biomass. In 2019, there was an increase of 1.1 Mg ha−1, for a total stock of 44.47 Mg ha−1. As such, a mean increase of 1.33 Mg ha−1 yr−1 was seen in the above-ground woody biomass for the entire study period, with 0.92 Mg ha−1 yr−1 related to the increase and 0.41 Mg ha−1 yr−1 to recruitment. This was not enough to overcome the loss of biomass, which averaged 3.26 Mg ha−1 yr−1 due to the mortality of the woody individuals, giving a negative balance of 1.97 Mg ha−1 yr−1.
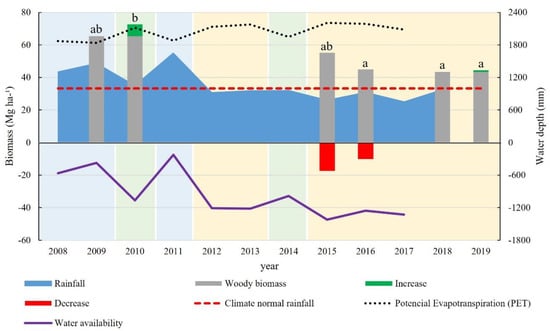
Figure 7.
Annual distribution of above-ground woody biomass and rainfall in a fragment of dry forest, Iguatu-Ceará. The same lowercase letters between years do not differ by the Friedman test (p ≤ 0.05).
The greatest production of biomass was seen 2010, which differed statistically by the Friedman test (p ≤ 0.05) from that of 2016, 2018 and 2019, but was like that of 2009 and 2015 (Figure 7). The increase in biomass from 2009 to 2010 is therefore related to the total rainfall between 2008 and 2009, which was above average, and resulted in greater water availability for the period. From 2008 to 2011, the total rainfall depth was 31% higher than the climate normal: 47%, 6% and 66% respectively, with a negative mean water balance of 558.4 mm yr−1.
Between 2012 and 2018, the total rainfall was below the climate normal by 7%, 4%, 4%, 21%, 7%, 24% and 2% respectively, with a negative mean water balance of 1237.0 mm yr−1, more than double the previous period. Thus, the biomass seen at the end of 2015 was influenced by the sequence of four dry years (2012 to 2015), and was worse during the final year (2015), which registered a water deficit of 1422.61 mm. The same was seen for 2016 and 2018.
In semi-arid regions, water is the main limiting factor to increases in biomass [12], since under conditions of consecutive periods of drought there is an increase in tree and shrub mortality due to the continuous scarcity of water [16]. Furthermore, the capacity of dry forests to produce biomass year after year is highly variable, precisely because they are influenced by consecutive years of drought, which can reduce this capacity [35], demonstrating the impact of climate conditions on biomass production in the DTF [16].
Therefore, limitations on water availability are not only the result of the total annual amount of rainfall, but also of the spatial and temporal distribution of the available water, in addition to solar energy being available for use in the process of evapotranspiration [33]. In addition to the existing limitations, recent simulation models suggest that semi-arid regions will suffer the greatest impact from climate change [3]. The mortality which took place demonstrates the reduction in total and potential biomass produced by the DTF/CPD over consecutive dry years, and which may worsen in the face of climate change. Climate change is expected to affect rainfall patterns and increase temperatures [36], culminating in droughts that, though previously tolerable, in the future may reach intolerable levels for plants, thereby reducing forest formations [10,36].
4. Conclusions
The sequence of consecutive dry years (2012 to 2017) showed the direct influence of climate conditions on the mortality and recruitment of woody species in a fragment of dry tropical forest in a phytogeographic domain of the Caatinga (DTF/CPD). After five years of consecutive drought, there was a reduction in the number of recruited individuals and an increase in the number of dead individuals, with a recruitment to mortality ratio of 0.11. The woody individuals of smaller diameter showed the least resilience, i.e., the mortality of individuals found in this study is more related to the stage of plant development than to species.
The least-resilient woody species were: Croton spp., Aspidosperma spp. and Piptadenia stipulacea (Benth.) Duck; while the most resilient to the consecutive dry years were: Bauhinia cheilantha (Bong.) Steud., Mimosa caesalpiniifolia Benth., Combretum spp. and Commiphora leptophloeos (Mart.) J.B.Gillett. Even with the long dry period (five years), there was no interruption in the recruitment of pioneer or secondary species, thereby demonstrating the resilience of these species to drought.
Author Contributions
D.A.C., Investigation, Formal analysis, Writing, Visualization; E.M.A., Supervision, Review, Project administration, Funding acquisition; A.D.A.C., Supervision, Review; R.C.F., Data Curation; H.Q.A.P.: Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq (Bolsa Jovens Talentos, process 400079/2013-5), the Universidade Federal do Ceará—UFC, the Programa de Pós-graduação em Engenharia Agrícola—PPGEA/UFC and the Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico—FUNCAP for the scientific and financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sorensen, L.A. A Spatial Analysis Approach to the Global Delineation of Dryland Areas of Relevance to the CBD Programme of Work on Dry and Subhumid Lands. Available online: https://www.unep-wcmc.org/system/dataset_file_fields/files/000/000/323/original/dryland_report_final_HR.pdf?1439378321 (accessed on 1 March 2019).
- Pennington, R.T.; Lavin, M.; Oliveira-Filho, A. Woody Plant Diversity, Evolution, and Ecology in the Tropics: Perspectives from Seasonally Dry Tropical Forests. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 437–457. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated dryland expansion under climate change. Nat. Clim. Chang. 2015, 6, 166–171. [Google Scholar] [CrossRef]
- Bastin, J.F.; Berrahmouni, N.; Grainger, A.; Maniatis, D.; Mollicone, D.; Moore, R.; Patriarca, C.; Picard, N.; Sparrow, B.; Abraham, E.M.; et al. The extent of forest in dryland biomes. For. Ecol. Sci. 2017, 356, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.J.S.; de Andrade, E.M.; Abreu, I.; Lajinha, T. Long-term variation of precipitation indices in Ceará State, Northeast Brazil. Int. J. Climatol. 2013, 33, 2929–2939. [Google Scholar] [CrossRef]
- Ministério do Meio Ambiente. Caatinga. Available online: http://www.mma.gov.br/biomas/caatinga (accessed on 1 March 2019).
- Araújo Filho, J. Manejo Pastoril Sustentável da Caatinga, 22nd ed.; Projeto Dom Helder Camara: Recife, Brazil, 2013; pp. 45–47. ISBN 978-85-64154-04-9. [Google Scholar]
- Aryal, D.R.; de Jong, B.H.J.; Ochoa-Gaona, S.; Esparza-Olguin, L.; Mendoza-Vega, J. Carbon stocks and changes in tropical secondary forests of southern Mexico. Agric. Ecosyst. Environ. 2014, 195, 220–230. [Google Scholar] [CrossRef]
- Cabral, G.A.L.; Sampaio, E.V.S.B.; Almeida-Cortez, J. Estrutura Espacial e Biomassa da Parte Aérea em Diferentes Estádios Sucessionais de Caatinga, em Santa Terezinha, Paraíba. Rev. Bras. Geogr. Física 2013, 6, 566–574. [Google Scholar] [CrossRef]
- Mcdowell, N.G.; Allen, C.D. Darcys law predicts widespread forest mortality under climate warming. Nat. Clim. Chang. 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Silva, A.C.C.; Oliveira, D.G. Population Structure and Spatial Distribution of Bauhinia cheilantha (Bong.) Steud. in Two Fragments at Different Regeneration Stages in the Caatinga, in Sergipe, Brazil. Rev. Árvore 2015, 39, 431–437. [Google Scholar] [CrossRef]
- Pereira, L.R., Jr.; de Andrade, E.M.; Palácio, H.A.Q.; Raymer, P.C.L.; Ribeiro Filho, J.C.; Pereira, F.J.S. Carbon stocks in a tropical dry forest in Brazil. Rev. Ciência Agronômica 2016, 47, 32–40. [Google Scholar] [CrossRef]
- Feitosa, R.C. Estoque de Carbono em Floresta Tropical Sazonalmente Seca no Nordeste do Brasil: Uma Comparação Entre Dois usos do Solo. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2017. Available online: http://www.repositorio.ufc.br/handle/riufc/28686 (accessed on 21 May 2019).
- Greenwood, S.; Ruiz-Benito, P.; Martínez-Vilalta, J.; Lloret, F.; Kitzberger, T.; Allen, C.D.; Fensham, R.; Laughlin, D.C.; Kattge, J.; Bönisch, G.; et al. Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 2017, 20, 539–553. [Google Scholar] [CrossRef]
- Moro, M.F.; Lughadha, E.N.; de Araújo, F.S.; Martins, F.R. A Phytogeographical Metaanalysis of the Semiarid Caatinga Domain in Brazil. Bot. Rev. 2016, 82, 91–148. [Google Scholar] [CrossRef]
- Spannl, S.; Volland, F.; Pucha, D.; Peters, T.; Cueva, E.; Bräuning, A. Climate variability, tree increment patterns and ENSO-related carbon sequestration reduction of the tropical dry forest species Loxopterygium huasango of Southern Ecuador. Trees 2016, 30, 1245–1258. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2012, 3, 30–36. [Google Scholar] [CrossRef]
- Sperry, J.S.; Wang, Y.; Wolfe, B.T.; Mackay, D.S.; Anderegg, W.R.L.; McDowell, N.G.; Pockman, W.T. Pragmatic hydraulic theory predicts stomatal responses to climatic water deficits. New Phytol. 2016, 212, 577–589. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Bienaimé, D.; Marmottant, P. Revealing catastrophic failure of leaf networks under stress. Proc. Natl. Acad. Sci. USA 2016, 113, 4865–4869. [Google Scholar] [CrossRef]
- Aquino, D.N.; de Andrade, E.M.; Castanho, A.D.A.; Pereira, L.R., Jr.; Palácio, H.A.Q. Belowground Carbon and Nitrogen on a Thinned and UnThinned Seasonally Dry Tropical Forest. Am. J. Plant Sci. 2017, 8, 2083–2100. [Google Scholar] [CrossRef][Green Version]
- Rodal, M.J.N.; Martins, F.R.; Sampaio, E.V.S.B. Levantamento quantitativo das plantas lenhosas em trechos de vegetação de Caatinga em Pernambuco. Rev. Caatinga 2008, 21, 192–205. [Google Scholar]
- Rodal, M.J.N.; Sampaio, E.V.S.B.; Figueiredo, M.A. Manual Sobre Métodos de Estudos Florístico e Fitossiciológico: Ecossistema Caatinga; SB: Brasília, Brazil, 2013; p. 24. ISBN 978-85-60428-03-8. [Google Scholar]
- Sampaio, E.V.; Silva, G.C. Biomass equations for Brazilian semiarid caatinga plants. Acta Bot. Bras. 2005, 19, 935–943. [Google Scholar] [CrossRef]
- Instituto Nacional de Meteorologia/Banco de Dados Meteorológicos para Ensino e Pesquisa (INMET/BDMEP). Available online: http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep (accessed on 1 March 2019).
- Fernandes, F.B.P. Disponibilidade Hídrica para a Cultura do Feijão-de-Corda em Função do Manejo de Solo no Semiárido Cearense. Ph.D. Thsis, Universidade Federal do Ceará, Fortaleza, Brazil, 2014. Available online: http://www.repositorio.ufc.br/handle/riufc/10570 (accessed on 21 April 2019).
- Aragão, L.E.O.C.; Malhi, Y.; Roman-Cuesta, R.M.; Saatchi, S.; Anderson, L.O.; Shimabukuro, Y.E. Spatial patterns and fire response of recent Amazonian droughts. Geophys. Res. Lett. 2007, 34, L070701:1–L070701:5. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E. Compositional response of Amazon forests to climate change. Glob. Chang. Biol. 2018, 25, 39–56. [Google Scholar] [CrossRef]
- Corrar, L.J.; Paulo, E.; Dias Filho, J.M. Análise Multivariada para os Cursos de Administração, Ciências Contábeis e Economia, 1st ed.; Atlas: São Paulo, Brazil, 2007; p. 568. ISBN 978-8522447077. [Google Scholar]
- de Andrade, L.A.; Pereira, I.M.; Leite, U.T.; Barbosa, M.R.V. Análise da cobertura de duas fitofisionomias de caatinga, com diferentes históricos de uso, no município de São João do Cariri, Estado da Paraíba. Rev. Cerne 2005, 11, 253–262. [Google Scholar]
- Santos, M.G.; Oliveira, M.T.; Figueiredo, K.V.; Falcão, H.M.; Arruda, E.C.P.; Almeida-Cortez, J.; Sampaio, E.V.S.B.; Ometto, J.P.H.B.; Menezes, R.S.C.; Oliveira, A.F.M.; et al. Caatinga, the Brazilian dry tropical forest: Can it tolerate climate changes? Theor. Exp. Plant Physiol. 2014, 26, 83–99. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Klein, V.A. Estratégias para potencializar a retenção e disponibilidade de água no solo. Rev. Eletrônica Gestão Educ. Tecnol. Ambient. 2015, 19, 21–29. [Google Scholar] [CrossRef]
- Brito, S.S.B.; Cunha, A.P.M.A.; Cunningham, C.C.; Alvalá, R.C.; Marengo, J.A.; Carvalho, M.A. Frequency, duration and severity of drought in the Semiarid Northeast Brazil region. Int. J. Climatol. 2017, 38, 517–529. [Google Scholar] [CrossRef]
- Van der Sande, M.T.; Zuidema, P.A.; Sterck, F. Explaining biomass growth of tropical canopy trees: The importance of sapwood. Oecologia 2015, 117, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Mitchard, E.T.A. The tropical forest carbon cycle and climate change. Nature 2018, 559, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—Past, present, and future. Theor. Appl. Climatol. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).