Abstract
A study was conducted to determine the effects of lactic acid bacteria (LAB) on nutritive value and in vitro rumen digestibility of maize and rice straw silages. Two identical experiments were carried out for each of the two silages. A total of five treatments were used for each experiment: (1) negative control (NC); (2) positive control (PC); (3) Lactobacillus plantarum (LPL); (4) L. paracasei (LPA); and (5) L. acidophilus (LA). Each treatment was then divided into four ensiling periods: 3, 7, 20, and 40 days with three replications. The LPL treatment had significantly higher dry matter (DM), lower ammonia-N, and a lower number of fungi on maize silage after 40 days (p < 0.05). On the other hand, the LA treatment increased DM and CP content, reduced NDF and ADF contents compared to NC, and also produced more lactic acid compared to the other LAB-treated rice straw silages. Results of the in vitro rumen fermentation of maize silages showed no significant differences in DMD after LAB inoculation. However, higher DMD and ruminal ammonia-N were shown by rice straw ensiled with L. acidophilus. In conclusion, silage additives, which could improve the ensiling process of maize and rice straw, appeared to be different and substrate specific.
1. Introduction
Forage is an important source of nutrients in the diets for ruminants worldwide, providing energy and protein for maintenance, growth, and production [1,2]. Unreliable weather conditions make it difficult for farmers to produce good quality forage year-round [3] and, as a result, various methods have been used to preserve fresh forages (e.g., hay or silage).
Ensiling is one of the methods used to preserve fresh forages. Silage has several benefits which include low and stable pH (from the production of acids during the ensiling process) as well as improved dry matter intake (DMI) [4,5,6]. To facilitate the fermentation process during ensiling, silage additives such as lactic acid bacteria (LAB) have been added [4,7]. Inoculation of silage substrate with LAB results in a rapid decline in pH at the beginning of the silage fermentation [8,9] with a concomitant increase in lactic acid production [9]. Furthermore, there is a reduction in ammonia-N formation in LAB inoculated substrate compared with uninoculated substrates [4]. There are several different strains of LAB available for use during silage making, each with its own unique fermentation characteristics [10,11]. A number of silage additives are commercially developed with mixed strains of LAB, but they may be lacking in substrate specificity for certain roughages such as rice straw [12].
In the current study, two substrates with different nutritional characteristics were used to determine the effects of silage inoculants on their nutritive value. Maize (Zea mays L.), grown as a forage feed worldwide [13], is generally preserved as silage because of its low dry matter (DM) content (28–40%), low buffering capacity, and substantial water-soluble carbohydrates which produce organic acids, including lactic acid [11,14]. Feeding maize silage can increase the forage DM intake [15,16,17] and the milk yield as it is rich in metabolizable energy [16,17]. On the contrary, rice straw (Oryza sativa), an agricultural by-product, has relatively low digestibility due to a high level of cell wall components such as cellulose, hemicellulose, and lignin [18,19]. In Asian countries, ensiling rice straw was also one alternative to re-utilize this byproduct instead of disposing of it by burning and causing air pollution [20,21]. In the study by Pi et al. [22], feeding rice straw-based total mixed ration to Boer goats can produce favorable carcass characteristics and meat quality.
The fermentation characteristics of maize and rice straw differ due to their differing nutrient profiles and, as such, the application of appropriate additives for ensiling is essential to get the best out of each forage. The aim of the study was to examine how different LAB strains added during the ensiling process affect the nutritive value and in vitro digestibility of maize and rice straw silages.
2. Materials and Methods
2.1. Experimental Design and Ensiling Process
Two different silages were prepared using maize and rice straw. Experiment 1 was conducted in Gunwi-gun, Korea, in August 2013 using ripe, yellow maize (Zea mays L. cultivar Kwangpyeongok), while Experiment 2 was carried out in Cheongni-myeon, Sangju, Korea, in October 2013 using rice straw (Oryza sativa subsp. Japonica, cultivar Ilpum) as the substrate. Approximately 300 kg (fresh basis) of each substrate were harvested, chopped, and set aside for treatment.
Five treatments were applied to each of the maize and rice straw. Among the treatments there were two controls: a negative control, treatment of the forages with distilled water (NC) and a positive control, treatment of the forages with a commercial silage additive containing L. acidophilus (4.4 × 108 CFU/g), L. plantarum (9.5 × 108 CFU/g), and Pediococcus acidilactici (8.9 × 1010 CFU/g) (PC). The remaining three treatments were LAB treatments with the following species: L. plantarum (LPL), L. paracasei (LPA), and L. acidophilus (LA). All LAB treatments had 107 CFU/g of LAB in 50 mL of distilled water. After administering the treatments, the maize and rice straw were put into 10 L plastic containers, sealed, and ensiled in triplication at room temperature for 3, 7, 20, and 40 days before opening.
2.2. Sampling Procedure
Samples of the silage were made using the coning and quartering method, and representative samples were placed in 35 × 76.5 cm net bags (1 mm2 pore size) and oven dried at 65 °C for three days to determine initial dry matter (DM) content. After drying, the samples were weighed and ground small enough to pass a 1-mm screen using an ultra-centrifugal mill ZM 2000 (Retch, Haan, Germany) in preparation for proximate analysis. A 200 g aliquot of fresh samples was stored in sealed plastic bags at −20 °C for further analyses.
2.3. Chemical Analyses
For the determination of the chemical composition of both silages, DM (AOAC: 934.01), organic matter (OM; AOAC: 942.05), crude protein (CP; AOAC: 984.13), and ether extract (EE; AOAC: 920.39A) were determined using the AOAC procedures [23]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) analyses were conducted based on the methods of Van Soest et al. [24] using an ANKOM2000 Fiber Analyzer (ANKOM Technology, Macedon, NY, USA). The frozen silage samples were thawed, and approximately 100 g of silage was mixed with 900 mL of distilled water and homogenized using a stomacher (Daihan Scientific, Wonju, Korea) for about 4 min [14,25]. An aliquot of 90 mL of the silage extracts was taken and pH was immediately measured by using probe (8157BNUMD ROSS Ultra pH/ATC Probe, Thermo Orion Inc., Massachusetts, United States) connected to pH meter (Orion 3 Star, Thermo Scientific, Waltham, MA, USA). The extracts were then used for ammonia-N and organic acids analyses. The ammonia-N concentration was determined according to the method of Chaney and Marbach [26]. The high-pressure liquid chromatography method, according to Canale et al. [27], was used to determine organic acid profiles. A 300 × 7.80 mm Rezex ROA-Organic Acid H+ (8%) column (Phenomex, Torrance, CA, USA) and a guard column (SecurityGuard, Cartridge Kit, Phenomenex, Torrance, CA, USA) were used with 0.005 N H2SO4 as the mobile phase with a 0.5 mL/min flow rate at room temperature.
2.4. Microbial Analyses
A sample of the silage (25 g) was chopped to a size of 2 cm and mixed with 225 mL saline solution (0.9% NaCl). This mixture was then homogenized for about 2 min. The homogenized silage–saline mixture was then serially diluted and spread on different types of media. For detection of Lactobacillus and Bacillus, Difco™ Lactobacillus MRS agar and Difco™ LB agar Miller (Becton, Dickinson and company, Sparks, MD, USA), respectively were used and samples were incubated for 24 h at 35 °C, while for yeast and fungi determination, Difco™ potato-dextrose agar (Becton, Dickinson and company, Sparks, MD, USA) was used and incubation was carried out for 36 h at 30 °C [28,29].
2.5. In Vitro Rumen Fermentation Study
In vitro rumen fermentation was conducted using the method by Tilley and Terry [30]. Half a gram of maize silage at 40 days of ensiling and rice straw silage at 40 days was weighed and put in incubation bottles. Rumen contents were obtained from two cannulated Hanwoo steers fed total mixed ration (TMR, dry matter 75.5%, crude protein 9.6%, crude fiber 15.82%, neutral detergent fiber 32.7% and acid detergent fiber 18.6%) and filtered using four layers of muslin. An aliquot 1 l of filtered rumen fluid was mixed with 4 l McDougall’s buffer [31] under anaerobic conditions and maintained at 39 °C using a water bath. About 50 mL of this rumen inoculum was added into the incubation bottles containing the maize and rice straw silages. The incubation bottles were sealed with butyl rubber stoppers and incubated at 39 °C for three incubation periods (0, 6, and 24 h) in triplicate. At each interval, samples were removed from the incubator for the determination of DM digestibility, total gas production, pH, and ammonia-N concentration. The rumen fluid for the in vitro ruminal fermentation characteristics was provided by the Department of Animal Resources, Daegu University, Korea. The management and care of animals were reviewed and approved by the Daegu University Institutional Animal Care and Use Committee (No. 2014-6).
2.6. Statistical Analysis
Data were analyzed using General Linear Models (SPSS version 25, IBM, Madison, NY, USA). The effects of lactic acid bacteria (LAB) on maize and rice straw silages were examined using a one-way analysis of variance, and the means were further compared by the Duncan’s multiple range test. Principle component analysis (XLstat, Addinsoft, Long Island, NY, USA) was performed to visualize the effects of LAB and fermentation product variations of the maize and rice straw silages using biplots.
3. Results and Discussions
3.1. Effect of Lactic Acid Bacteria on Maize Silage
The chemical composition of maize silage after the different ensiling periods is presented in Table 1. After the three days ensiling period, there was no significant difference in the chemical compositions among treatments. However, at 40 days, the LAB treatments showed a significant difference (p < 0.05) in DM and OM. The highest DM contents were for the PC, LPL and LA treatments, 32.61%, 33.51% and 33.42%, respectively. The low DM content of the LPA treatment may be a consequence of the fermentation process, which causes DM losses whose magnitude is dependent on the inoculation level and the ensiling period [32].

Table 1.
Effect of different lactic acid bacteria on the chemical composition (%) of maize silage (dry matter basis unless otherwise stated).
The consumption of carbohydrates by LAB metabolism may be responsible for the DM loss seen in the study [5]. The observed DM loss in this study differed from the observations made by Santos et al. [11] which showed that DM content was not affected by LAB inoculation of maize silage. However, it was found that the utilization of additives was effective in reducing DM losses in only 35% of the studies [11]. DM content was highest in maize subjected to the PC, LPL, and LA treatments. This was expected as L. plantarum and L. acidophilus are commonly isolated from silage and are also preferred as silage additives [33].
The LPL treatment resulted in a numerically higher CP content in the maize silage compared to the other treatments after 40 days of ensiling (p = 0.081). In contrast, several studies have indicated that CP content is not affected by the addition of bacterial inoculant in the silage-making process [11,14,34]. The NDF and ADF contents showed a similar pattern across treatments being low in both the PC and LA treatments. The neutral detergent fiber in the LAB treatments was also not different from the NC treatment. This finding is similar to that reported by Contreras-Govea et al. [9], where no differences were observed between the control and LAB treated silages. Other studies are in agreement with the present finding, reporting no effect of bacterial inoculants on NDF and ADF [10,34,35,36]. However, there was an observable decrease in NDF, which can be attributed to the degradation of hemicellulose, which is relatively abundant in maize silage, under the acidic conditions of the ensiling process [37,38].
The pH of silage extracts at the end of the ensiling (40 days) was not affected by the inoculants used (Table 2). Keles and Demirci [39] and Kung Jr et al. [40] concluded that the addition of homofermentative bacteria results in a rapid drop in pH due to the increased production of lactic acid during fermentation. The results of the current study could not confirm this conclusion as all homofermentative bacteria used did not significantly reduce silage pH compared to the NC treatment. On the other hand, several studies seem to concur that bacterial inoculants do have a significant effect on the reduction of pH in silage. An accepted explanation as to why the addition of inoculants sometimes does not show an effect on pH reduction is competition with epiphytic LAB [8], and maize silage is known to have a high epiphytic LAB population [33]. During the ensiling process, homofermentative LAB mainly produce lactate as their primary product of sugar fermentation [41]. All treatments had numerically higher (p = 0.076) lactic acid production compared with NC. The LPA treatment numerically had the highest lactic acid production. The acetate present in the LPA and LA treated maize silages after 40 days of ensiling was higher compared to the NC treatment. The lactate to acetate ratios for the LAB treatments were 3.4, 3.5, and 3.0 for LPA, LPL, and LA treatment, respectively. These lactate to acetate ratios were higher than those reported by Huisden et al. [42], however similar to those found by Filya and Sucu [10] and Weinberg and Muck [33] in their studies on LAB-treated maize silage. The ammonia-N as a proportion of total-N was significantly lower (p < 0.05) in the NC treatment than the LPA and LA treatments and similar to the PC and LPL treatments. Among the LAB treatments, the LPL treatment recorded the lowest ammonia-N (Table 2). In the determination of spoilage of the silage, it was found that fungal spoilage was significantly lower in maize silage treated with LPL (p < 0.05) compared with the other LAB treatments but similar to the NC treatment. The number of lactobacilli was lower (p < 0.05) in LPA than that of the rest of the treatments.

Table 2.
Chemical and microbial analyses of maize silage extracts after 40 days of fermentation.
The effects of LAB inoculated maize silage on in vitro rumen fermentation are shown in Table 3. There was no difference among treatments in dry matter digestibility (DMD). Similar observations were made by Muck et al. [43], where they did not report any differences between LAB-treated silage and the control on DMD after a 24 h in vitro rumen fermentation. Except for the LPA, LAB-treated silages showed numerically higher DMD compared with the NC treatment, although it was not statistically different. A Weinberg et al. [44] study showed that LAB inoculants could potentially improve DMD, which would, in turn, enhance animal performance. Gas production was significantly different (p < 0.05) across the treatments after a 24 h incubation period. The NC, LPL and LA treatments had higher gas production compared to the PC and LPA treatments whose gas production was similar. However, the values of PC and LPA seemed to erroneous relative to their DMDs and it was unclear why such values were obtained. After 6 h of incubation, the pH of treated maize was different (p < 0.05) across the treatment. The pH of the PC and LPA treatments was higher (p < 0.05) than the rest of the treatments. In the present study, a comparison of the fermentation characteristics during ensiling and in vitro rumen fermentation showed that all LAB used were marginally effective in producing quality maize silages. With lower DM loss and pH, and the lower counts of fungi and yeast, L. plantarum appeared to be better for maize silage. These findings are in agreement with previous studies [8,11].

Table 3.
Effects of LAB treated maize silage on in vitro rumen fermentation.
3.2. Effect of Lactic Acid Bacteria on Rice Straw Silage
Rice straw is an agricultural by-product with low nutritive value; it is low in CP but high in lignin and silica contents [45]. Due to its low nutritive value, it fails to meet energy and protein requirements of ruminants [46]. The addition of LAB in rice straw silage has been thought to improve the nutritional value of the rice straw. The chemical composition of rice straw after ensiling for 3, 7, 20, and 40 days is presented in Table 4. There was a significant difference (p < 0.05) in DM across the treatments after days 3, 20 and 40. At the end of the 40-day ensiling period, the LPA treatment had the lowest DM compared to the other treatments. The DM contents reported in the current study were similar in range to those reported by Li et al. [47], which were between 28.9 to 39.3%. CP content after 40 days of ensiling was higher (p < 0.05) in the LAB treated silages compared to NC treatment. Similar observations were made by Li et al. [47]. This was contrary to the findings of Gao et al. [48], who showed no effects of silage inoculation on CP content. The LA treatment had the least NDF content after 40 days, which was significantly lower than the other treatments except for the PC treatment, which had similar NDF content. This was in line with what was reported by Li et al. [47]. With regards to the difference in NDF content that exists between inoculated and uninoculated silage substrates. Neutral detergent fiber and ADF appear to have been degraded toward the end of the ensiling period. As was seen with NDF, the LA treatment had significantly lower ADF than the rest of the treatments (p < 0.05).

Table 4.
Effect of different starter cultures on chemical composition (%) of rice straw silage (dry matter basis unless otherwise stated).
The treatments significantly affected the pH of rice straw silage after 40 days (p < 0.05, Table 5.). Among the LAB treatments, the PC treatment resulted in the lowest pH, followed by LA and LPA treatments. The NC treatment resulted in a higher (p < 0.05) ammonia-N production than the other treatments, though this was similar to the LPL and LPA treatments. Fungi, yeast, and Lactobacillus as determinants of silage spoilage were not statistically significant. The proliferation of the genus Bacillus was significantly affected by the inoculation of the silage substrate (p < 0.05), with the LPL treatment having the least effect on Bacillus and the LA treatment having the most. The fermentation characteristics seen in the current study indicate that, except for the LPL treatment, rice straw treated by LAB inoculation produced silage that had lower pH compared to that which was not treated. The depressed pH in the case of LAB inoculated rice straw silage is associated with the production of lactic acid during fermentation. Several studies have also reported similar differences between LAB treated and untreated rice straw silage [47,48,49].

Table 5.
Chemical and microbial analyses of rice straw silage extracts after 40 days of fermentation.
The in vitro rumen fermentation characteristics of ensiled rice straw are shown in Table 6. The DMD of rice straw silage was different (p < 0.05) at 6 and 24 h of incubation due to the treatments. At the end of the 24 h incubation, the PC and LA treatments had the highest DMD compared to other treatments. Our result was in contrast with the study conducted by Chen et al. [50], which had shown no differences in DMD of rice straw treated by L. acidophilus. The pH of the ensiled rice straw was significantly affected (p < 0.05) by treatment, with the pH of all LAB treatments being lower than that of NC. However, by 24 h of digestion, all treatments had similar pH. There was no significant difference in gas production across all treatments (p = 0.157). Ammonia-N differed significantly across the treatments at 6 h of incubation (p < 0.05). The LA and LPL treatments had higher concentration of ammonia-N compared to the rest of the treatments. The rice straw silage treated with the LA had the highest CP, and consequently, the amount of protein and amino acids available for deamination onto ammonia-N is likely to be increased. However, another factor that influences the degradation of protein is energy availability [51]. Rice straw has low amounts of energy due to the high level of cell wall content, with some indigestible components (i.e., lignin and silica). Such discrepancy in terms of energy and protein availability to ruminal microorganisms may lead to waste on ammonia-N.

Table 6.
Effects of LAB treated rice straw silage on in vitro rumen fermentation.
This study investigated cultures of LAB as inoculums for both maize and rice straw silage. These two silage materials are quite different in chemical composition and other characteristics. Generally, maize silage has relatively lower DM content and higher OM compared to rice straw silage. CP content was higher in maize silage compared to rice straw silage. On the other hand, rice straw silage had higher NDF and ADF values. Both silages showed declining pH after 40 days of fermentation by LAB. With the organic acid compounds, maize silage had higher lactate–acetate ratio than rice straw silage.
3.3. Principal Component Analysis
Principle component analysis was conducted to investigate the effects of LAB on the pattern of silage fermentation characteristics. Figure 1 shows the biplots for maize and rice straw silages based on fermentation products (lactic acid, acetic acid, and pH). The biplots divided the observed data into two clusters. The left cluster is mainly comprised of rice straw silage, and the right cluster maize silage. This indicates that the fermentation patterns of rice straw and maize were different. The differences in nutrient content between maize and rice straw silage are probably the reason for these apparent differences. Maize is likely to have a higher starch content compared to rice straw, which is rich in lignin and silica [45,52]. Consequently, maize produced higher amounts of fermentation products compared to rice straw during ensiling, as described in Figure 1.
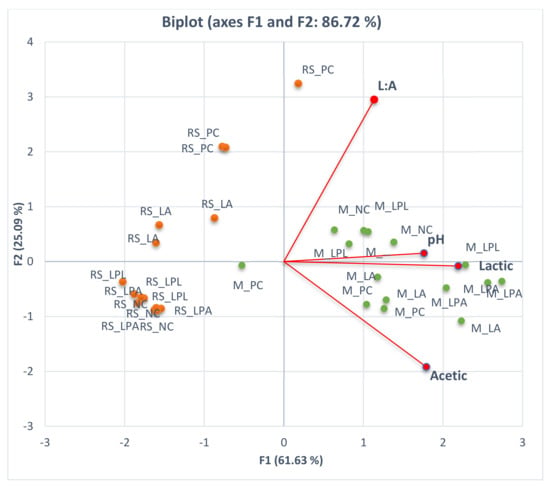
Figure 1.
Principle component analysis (PCA) of fermentation products (lactic acid, acetic acid, L: A ratio, and pH) in 40 days ensiled maize and rice straw silage. M: maize silage; RS: rice straw, NC: negative control; PC: positive control; LPA: Lactobacillus paracasei, LPL: Lactobacillus plantarum; LA: L. acidophilus.
Such contrast results (see Figure 1) seem to be associated with the nutritive values of maize and rice straw, which will lead to tremendous differences in animal performance. Nevertheless, maize silage is often lacking crude protein, so that either expensive plant protein such as soybean meal or a legume forage in the form of fresh or silage must be supplemented to improve animal performance [52,53]. On the other hand, rice straw as an agricultural byproduct with limited nutritive values plays an important role when forage sources are limited in a country like South Korea [18]. Therefore, improving the quality of either maize silage or rice straw silage, or both is imperative in the future for the ruminant industry.
Another point to highlight was the environmental and genetic variation of forages used. This was well-documented in the study by Jung and Buxtono [54], who reported that the genetic and environmental variation of maize affected the cell wall composition that would be related to the cell wall degradability. Similarly, Bainton et al. [55] reported differences in chemical composition and the degradability of rice straw according to the varieties and environmental conditions. Others [56,57] acknowledged genetic and/or environmental variation of such forages on digestibility in vitro. In this study, only a single cultivar from maize or rice straw was used. However, evidence concerning genetic and environmental factors provides more reason to continue this type of study, examining novel inoculants and their application to diverse varieties even within the same forage species.
4. Conclusions
Based on these characteristics of maize and rice straw silage, the LAB, which could effectively ensile each of these substrates, was also different. L. plantarum was effective for ensiling maize. L. plantarum-treated maize silage had the highest DM and CP contents compared to the other treatments. It also resulted in low ammonia-N concentration compared to the other treatments. On the other hand, for ensiling rice straw, L. acidophilus had greater potential as a starter culture. Rice straw silage treated with L. acidophilus showed higher DM and CP contents as well as lower NDF and ADF compared to the other treatments. In in vitro rumen fermentation, it also showed a higher DMD compared to the other treatments.
Author Contributions
Conceptualization, T.D.M., K.L., J.S., C.H.K., D.Y., S.M.L., J.K., C.L., S.C. and E.J.K.; methodology, T.D.M., K.L., J.S., C.H.K., D.Y., S.M.L., J.K., C.L., S.C., and E.J.K..; software, T.D.M. and E.J.K..; validation, T.D.M. and E.J.K.; formal analysis, T.D.M., K.L., J.S. and E.J.K.; investigation, T.D.M. and E.J.K.; resources, C.H.K., D.Y., S.M.L. and E.K.; data curation, T.D.M. and E.J.K.; writing—original draft preparation, T.D.M. and E.J.K.; writing—review and editing, T.D.M., K.L., J.S., C.H.K., D.Y., S.M.L., J.K., C.L., S.C. and E.J.K.; visualization, T.D.M. and E.J.K.; supervision, E.J.K.; project administration, E.J.K.; funding acquisition, J.K., C.L. and E.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT) through the Research and Development for Regional Industry (No. R0002505).
Acknowledgments
The rumen fluid for the in vitro ruminal fermentation characteristics was provided by the Department of Animal Resources, Daegu University, Korea. The management and care of animals were reviewed and approved by the Daegu University Institutional Animal Care and Use Committee (No. 2014-6). We greatly appreciate the technical support of Daegu University (Chang Weon Choi and his staff).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caton, J.; Bauer, M.; Hidari, H. Metabolic components of energy expenditure in growing beef cattle-review. Asian Australas. J. Anim. Sci. 2000, 13, 702–710. [Google Scholar] [CrossRef]
- Lalman, D.; Williams, J.; Hess, B.; Thomas, M.; Keisler, D. Effect of dietary energy on milk production and metabolic hormones in thin, primiparous beef heifers. Anim. Sci. J. 2000, 78, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, G.; Castonguay, Y.; Bertrand, A.; Dhont, C.; Rochette, P.; Couture, L.; Drapeau, R.; Mongrain, D.; Chalifour, F.-P.; Michaud, R. Winter damage to perennial forage crops in eastern Canada: Causes, mitigation, and prediction. Can. J. Plant Sci. 2006, 86, 33–47. [Google Scholar] [CrossRef]
- Henderson, N. Silage additives. Anim. Feed Sci. Technol. 1993, 45, 35–56. [Google Scholar] [CrossRef]
- Winters, A.L.; Fychan, R.; Jones, R. Effect of formic acid and a bacterial inoculant on the amino acid composition of grass silage and on animal performance. Grass Forage Sci. 2001, 56, 181–192. [Google Scholar] [CrossRef]
- Sun, Q.; Gao, F.; Yu, Z.; Tao, Y.; Zhao, S.; Cai, Y. Fermentation quality and chemical composition of shrub silage treated with lactic acid bacteria inoculants and cellulase additives. Anim. Sci. J. 2012, 83, 305–309. [Google Scholar] [CrossRef]
- Filya, I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J. Dairy Sci. 2003, 86, 3575–3581. [Google Scholar] [CrossRef]
- Contreras-Govea, F.E.; Muck, R.E.; Broderick, G.A.; Weimer, P.J. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 2013, 179, 61–68. [Google Scholar] [CrossRef]
- Contreras-Govea, F.E.; Muck, R.E.; Mertens, D.R.; Weimer, P.J. Microbial inoculant effects on silage and in vitro ruminal fermentation, and microbial biomass estimation for alfalfa, bmr corn, and corn silages. Anim. Feed Sci. Technol. 2011, 163, 2–10. [Google Scholar] [CrossRef]
- Filya, I.; Sucu, E. The effects of lactic acid bacteria on the fermentation, aerobic stability and nutritive value of maize silage. Grass Forage Sci. 2010, 65, 446–455. [Google Scholar] [CrossRef]
- Santos, A.; Ávila, C.; Schwan, R.F. Selection of tropical lactic acid bacteria for enhancing the quality of maize silage. J. Dairy Sci. 2013, 96, 7777–7789. [Google Scholar] [CrossRef]
- McAllister, T.; Selinger, L.; McMahon, L.; Bae, H.; Lysyk, T.; Oosting, S.; Cheng, K.-J. Intake, digestibility and aerobic stability of barley silage inoculated with mixtures of Lactobacillus plantarum and Enterococcus faecium. Can. J. Anim. Sci. 1995, 75, 425–432. [Google Scholar] [CrossRef]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307. [Google Scholar] [CrossRef]
- Meeske, R.; Basson, H. The effect of a lactic acid bacterial inoculant on maize silage. Anim. Feed Sci. Technol. 1998, 70, 239–247. [Google Scholar] [CrossRef]
- O’Mara, F.P.; Fitzgerald, J.J.; Murphy, J.J.; Rath, M. The effect on milk production of replacing grass silage with maize silage in the diet of dairy cows. Livest. Prod. Sci. 1998, 55, 79–87. [Google Scholar] [CrossRef]
- Khan, N.A.; Yu, P.; Ali, M.; Cone, J.W.; Hendriks, W.H. Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 2015, 95, 238–252. [Google Scholar] [CrossRef]
- Shaver, R.; Erdman, R.; O’connor, A.; Vandersall, J. Effects of silage pH on voluntary intake of corn silage and alfalfa haylage. J. Dairy Sci. 1985, 68, 338–346. [Google Scholar] [CrossRef]
- Jackson, M. Review article: The alkali treatment of straws. Anim. Feed Sci. Technol. 1977, 2, 105–130. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Ahmad, B.; Ahmad, W. Why do farmers burn rice residue? Examining farmers’ choices in Punjab, Pakistan. Land Use Policy 2015, 47, 448–458. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Gao, S.; Liao, Z.; Lai, T.; Zhou, H.; Chen, Q.; Li, L.; Gao, H.; Lu, W. The effect of a diet based on rice straw co-fermented with probiotics and enzymes versus a fresh corn Stover-based diet on the rumen bacterial community and metabolites of beef cattle. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Pi, Z.K.; Wu, Y.M.; Liu, J.X. Effect of pretreatment and pelletization on nutritive value of rice straw-based total mixed ration, and growth performance and meat quality of growing Boer goats fed on TMR. Small Rumin. Res. 2005, 56, 81–88. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.v.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed Sci. Technol. 2008, 145, 122–135. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Canale, A.; Valente, M.E.; Ciotti, A. Determination of volatile carboxylic acids (C1–C5i) and lactic acid in aqueous acid extracts of silage by high performance liquid chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage 1. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Tilley, J.; Terry, R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- McDougall, E. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99. [Google Scholar] [CrossRef]
- Driehuis, F.; Elferink, S.J.W.H.O.; Spoelstra, S.F. Anaerobic lactic acid degradation during ensilage of whole crop maize inoculated with Lactobacillus buchneri inhibits yeast growth and improves aerobic stability. J. Appl. Microbiol. 1999, 87, 583–594. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Bernardi, A.; Härter, C.J.; Silva, A.W.L.; Reis, R.A.; Rabelo, C.H.S. A meta-analysis examining lactic acid bacteria inoculants for maize silage: Effects on fermentation, aerobic stability, nutritive value and livestock production. Grass Forage Sci. 2019, 74, 596–612. [Google Scholar] [CrossRef]
- Nadeau, E.; Buxton, D.; Russell, J.; Allison, M.; Young, J. Enzyme, bacterial inoculant, and formic acid effects on silage composition of orchardgrass and alfalfa. J. Dairy Sci. 2000, 83, 1487–1502. [Google Scholar] [CrossRef]
- Meeske, R.; Van der Merwe, G.; Greyling, J.; Cruywagen, C. The effect of adding an enzyme containing lactic acid bacterial inoculant to big round bale oat silage on intake, milk production and milk composition of Jersey cows. Anim. Feed Sci. Technol. 2002, 97, 159–167. [Google Scholar] [CrossRef]
- Filya, I. Nutritive value and aerobic stability of whole crop maize silage harvested at four stages of maturity. Anim. Feed Sci. Technol. 2004, 116, 141–150. [Google Scholar] [CrossRef]
- Morrison, I.M. Changes in the cell wall components of laboratory silages and the effect of various additives on these changes. J. Agric. Sci. 1979, 93, 581–586. [Google Scholar] [CrossRef]
- Keles, G.; Demirci, U. The effect of homofermentative and heterofermentative lactic acid bacteria on conservation characteristics of baled triticale–Hungarian vetch silage and lamb performance. Anim. Feed Sci. Technol. 2011, 164, 21–28. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Stokes, M.R.; Lin, C. Silage additives. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2003; Volume 42, pp. 305–360. [Google Scholar]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef]
- Huisden, C.; Adesogan, A.; Kim, S.; Ososanya, T. Effect of applying molasses or inoculants containing homofermentative or heterofermentative bacteria at two rates on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2009, 92, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.; Filya, I.; Contreras-Govea, F. Inoculant effects on alfalfa silage: In vitro gas and volatile fatty acid production. J. Dairy Sci. 2007, 90, 5115–5125. [Google Scholar] [CrossRef]
- Weinberg, Z.; Shatz, O.; Chen, Y.; Yosef, E.; Nikbahat, M.; Ben-Ghedalia, D.; Miron, J. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J. Dairy Sci. 2007, 90, 4754–4762. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.J.; Nader, G.; Forero, L. Feeding rice straw to cattle. In ANR UC Davis; ANR: Davis, CA, USA, 2002; p. 8079. [Google Scholar]
- Rahal, A.; Singh, A.; Singh, M. Effect of urea treatment and diet composition on, and prediction of nutritive value of rice straw of different cultivars. Anim. Feed Sci. Technol. 1997, 68, 165–182. [Google Scholar] [CrossRef]
- Li, J.; Shen, Y.; Cai, Y. Improvement of fermentation quality of rice straw silage by application of a bacterial inoculant and glucose. Asian Australas. J. Anim. Sci. 2010, 23, 901–906. [Google Scholar] [CrossRef]
- Gao, L.; Yang, H.; Wang, X.; Huang, Z.; Ishii, M.; Igarashi, Y.; Cui, Z. Rice straw fermentation using lactic acid bacteria. Bioresour. Technol. 2008, 99, 2742–2748. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Matsuzaki, M.; Suzuki, H.; Cai, Y.; Horiguchi, K.i.; Takahashi, T. Effects of lactic acid bacteria and urea treatment on fermentation quality, digestibility and ruminal fermentation of roll bale rice straw silage in wethers. Grassl. Sci. 2012, 58, 73–78. [Google Scholar] [CrossRef]
- Chen, L.; Ren, A.; Zhou, C.; Tan, Z. Effects of Lactobacillus acidophilus supplementation for improving in vitro rumen fermentation characteristics of cereal straws. Ital. J. Anim. Sci. 2017, 16, 52–60. [Google Scholar] [CrossRef]
- Russell, J.B.; Sniffen, C.; Van Soest, P. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J. Dairy Sci. 1983, 66, 763–775. [Google Scholar] [CrossRef]
- Kolver, E.; Roche, J.; Miller, D.; Densley, R. Maize silage for dairy cows. In Proceedings of the Conference-New Zealand Grassland Association; Wellington, New Zealand Grassland Association: Hamilton, New Zealand, 2001; pp. 195–202. [Google Scholar]
- Titterton, M.; Maasdorp, B.V. Nutritional improvement of maize silage for dairying: Mixed crop silages from sole and intercropped legumes and a long season variety of maize. 2. Ensilage. Anim. Feed Sci. Technol. 1997, 69, 263–270. [Google Scholar] [CrossRef]
- Jung, H.J.G.; Buxtono, D.R. Forage quality variation among maize inbreds: Relationships of cell-wall composition and in-vitro degradability for stem internodes. J. Sci. Food Agric. 1994, 66, 313–322. [Google Scholar] [CrossRef]
- Bainton, S.J.; Plumb, V.E.; Juliano, B.O.; Perez, C.M.; Roxas, D.B.; Khush, G.S.; de Jesus, J.C.; Gomez, K.A. Variation in the nutritional value of rice straw. Anim. Feed Sci. Technol. 1991, 34, 261–277. [Google Scholar] [CrossRef]
- Vadiveloo, J. Varietal differences in the chemical composition and in vitro digestibility of rice straw. J. Agric. Sci. 1992, 119, 27–33. [Google Scholar] [CrossRef]
- Capper, B. Genetic variation in the feeding value of cereal straw. Anim. Feed Sci. Technol. 1988, 21, 127–140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).